Effects of High-Resistance Elastic Band Training and a Curcumin-Based Formulation on Neuro-Oxidative and Functional Health in Sedentary Older Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
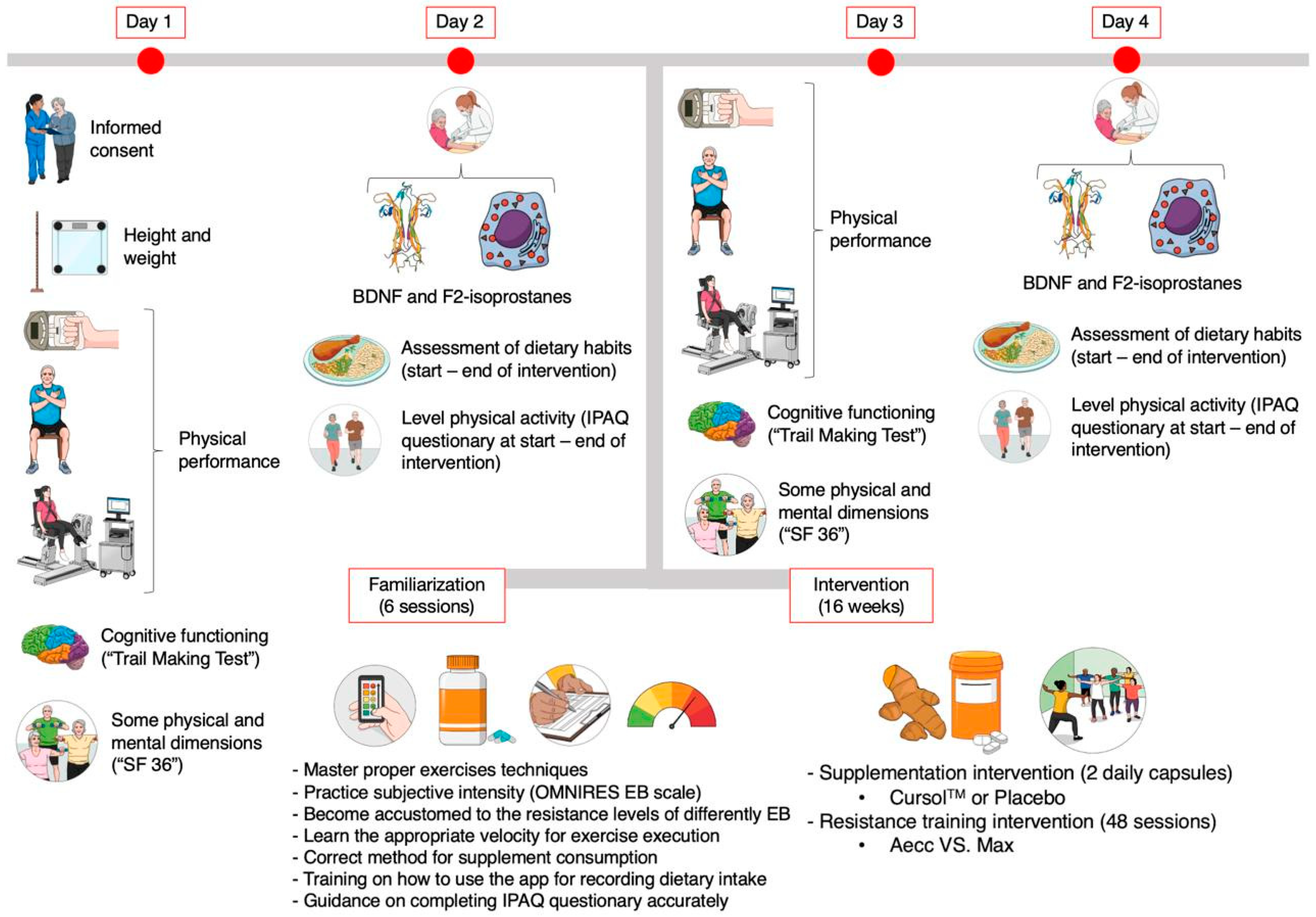
2.2. Study Design
2.3. Testing Procedures
2.4. Training Procedures
2.5. Procedures for Monitoring Supplementation, Diet, and Physical Activity
2.6. Statistical Analyses
3. Results
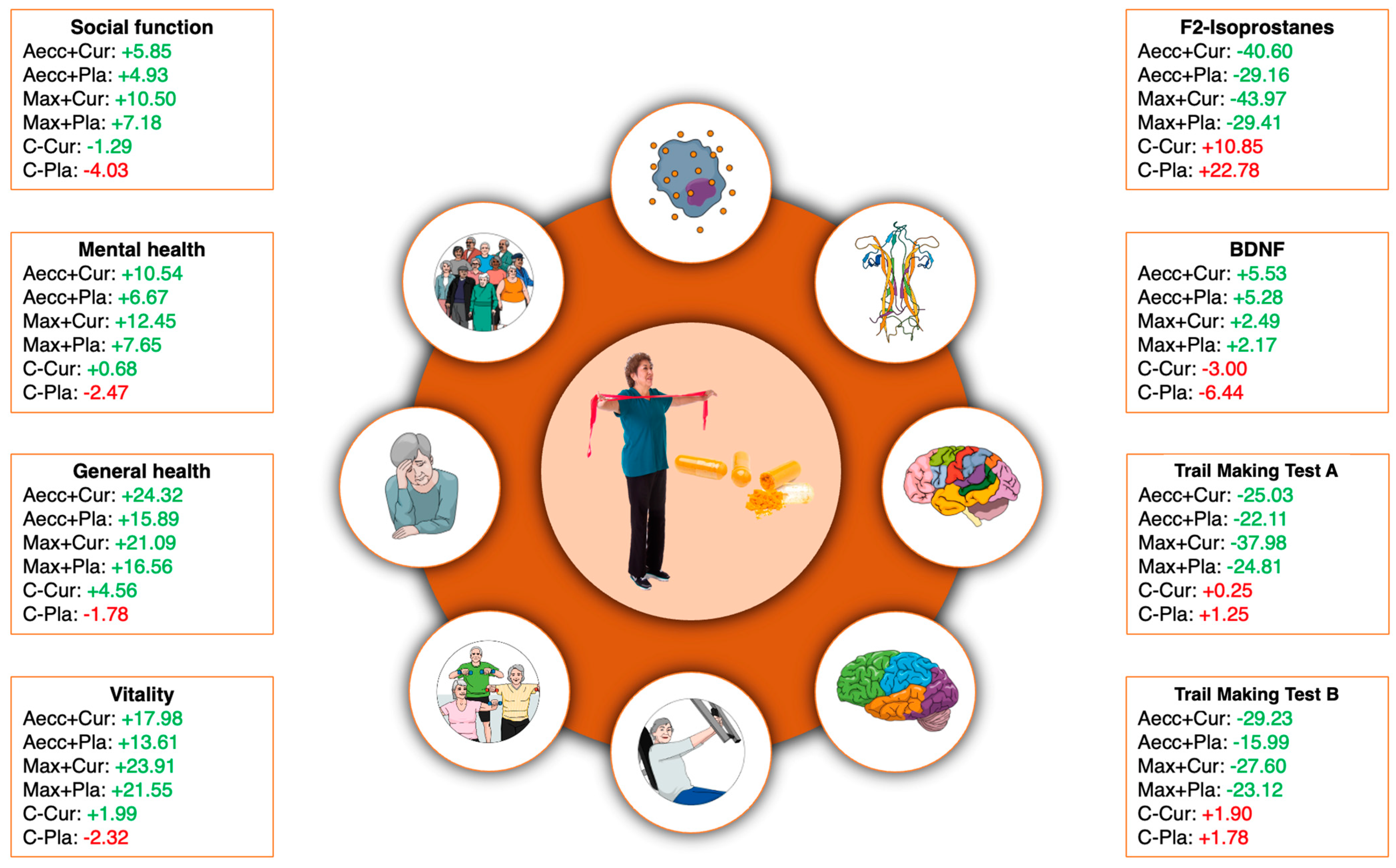
3.1. Neurogenesis and Oxidative Stress Parameters
3.2. Cognitive Functioning
3.3. Health-Related Quality of Life Dimensions
3.4. Physical Performance
3.5. Bivariate Correlation Analysis (Spearman’s ρ)
3.6. Clinical Relevance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Aecc | Accentuated eccentric |
| ANCOVA | Analysis of covariance |
| ANOVA | Analysis of variance |
| BDNF | Brain-derived neurotrophic factor |
| C | Control |
| CLSI | Clinical and laboratory standards institute |
| Cur | Curcumin |
| ELISA | Enzyme-linked immunosorbent assay |
| ES | Effect size |
| IGF-1 | Insulin-like growth factor 1 |
| LSD | Least significant difference |
| Max | Maximal strength |
| MCID | Minimum clinically important difference |
| METS | Metabolic equivalents |
| PGF2α | Prostaglandin F2 alpha |
| Pla | Placebo |
| RPE | Rating of perceived exertion |
| RPM | Revolutions per minute |
| RT | Resistance training |
| SD | Standard deviations |
| SF 36 | 36-Item short-form survey |
| SST | Serum-separating tube |
| TMT | Trail Making Test |
| 1RM | One-repetition maximum |
References
- Michel, J.P.; Sadana, R. “Healthy Aging” Concepts and Measures. J. Am. Med. Dir. Assoc. 2017, 18, 460–464. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a Risk Factor for Neurodegenerative Disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as Therapeutic Options for Neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Colucci-D’amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, L.M.; Meng, Y.; Xhima, K.; Lipsman, N.; Hamani, C.; Aubert, I. The Neuroprotective Effects of Exercise: Maintaining a Healthy Brain Throughout Aging. Brain Plast. 2018, 4, 17. [Google Scholar] [CrossRef]
- Greco, A.; Minghetti, L.; Levi, G. Isoprostanes, Novel Markers of Oxidative Injury, Help Understanding the Pathogenesis of Neurodegenerative Diseases. Neurochem. Res. 2000, 25, 1357–1364. [Google Scholar] [CrossRef]
- Miller, E.; Morel, A.; Saso, L.; Saluk, J. Isoprostanes and Neuroprostanes as Biomarkers of Oxidative Stress in Neurodegenerative Diseases. Oxid. Med. Cell. Longev. 2014, 2014, 572491. [Google Scholar] [CrossRef]
- Fuentes-Claramonte, P.; Ávila, C.; Rodríguez-Pujadas, A.; Costumero, V.; Ventura-Campos, N.; Bustamante, J.C.; Rosell-Negre, P.; Barrós-Loscertales, A. Characterizing Individual Differences in Reward Sensitivity from the Brain Networks Involved in Response Inhibition. Neuroimage 2016, 124, 287–299. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Haidar, E.A.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise Promotes the Expression of Brain Derived Neurotrophic Factor (BDNF) through the Action of the Ketone Body β-Hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef]
- Santiago, J.A.; Quinn, J.P.; Potashkin, J.A. Physical Activity Rewires the Human Brain against Neurodegeneration. Int. J. Mol. Sci. 2022, 23, 6223. [Google Scholar] [CrossRef]
- Elhampour, L.; Azarbayjani, M.A.; Nasehi, M.; Peeri, M. Concurrent Effects of Exercise and Curcumin on Spatial Learning and Memory in Sensitized Male Mice Following Morphine Administration. Galen Med. J. 2019, 8, e1072. [Google Scholar] [CrossRef]
- Çakir-Atabek, H.; Demir, S.; Pinarbaşili, R.D.; Gündüz, N. Effects of Different Resistance Training Intensity on Indices of Oxidative Stress. J. Strength Cond. Res. 2010, 24, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2015, 53, 4094–4125. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Vondrakova, D.; Lawson, M.; Valko, M. Metals, Oxidative Stress and Neurodegenerative Disorders. Mol. Cell. Biochem. 2010, 345, 91–104. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, A.; Solana, E.; Corpas, R.; Bartrés-Faz, D.; Pallàs, M.; Vina, J.; Sanfeliu, C.; Gomez-Cabrera, M.C. Long-Term Exercise Training Improves Memory in Middle-Aged Men and Modulates Peripheral Levels of BDNF and Cathepsin B. Sci. Rep. 2019, 9, 3337. [Google Scholar] [CrossRef]
- Singh, B.; Olds, T.; Curtis, R.; Dumuid, D.; Virgara, R.; Watson, A.; Szeto, K.; O’Connor, E.; Ferguson, T.; Eglitis, E.; et al. Effectiveness of Physical Activity Interventions for Improving Depression, Anxiety and Distress: An Overview of Systematic Reviews. Br. J. Sports Med. 2023, 57, 1203–1209. [Google Scholar] [CrossRef]
- Noetel, M.; Sanders, T.; Gallardo-Gómez, D.; Taylor, P.; Del Pozo Cruz, B.; Van Den Hoek, D.; Smith, J.J.; Mahoney, J.; Spathis, J.; Moresi, M.; et al. Effect of Exercise for Depression: Systematic Review and Network Meta-Analysis of Randomised Controlled Trials. BMJ 2024, 384, e075847. [Google Scholar] [CrossRef]
- Huang, T.; Larsen, K.T.; Ried-Larsen, M.; Møller, N.C.; Andersen, L.B. The Effects of Physical Activity and Exercise on Brain-Derived Neurotrophic Factor in Healthy Humans: A Review. Scand. J. Med. Sci. Sports 2014, 24, 1–10. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, R.; Álvarez-Bueno, C.; Martínez-Ortega, I.A.; Martínez-Vizcaíno, V.; Mesas, A.E.; Notario-Pacheco, B. Immediate Effect of High-Intensity Exercise on Brain-Derived Neurotrophic Factor in Healthy Young Adults: A Systematic Review and Meta-Analysis. J. Sport Health Sci. 2022, 11, 367–375. [Google Scholar] [CrossRef]
- Ferrer-Uris, B.; Ramos, M.A.; Busquets, A.; Angulo-Barroso, R. Can Exercise Shape Your Brain? A Review of Aerobic Exercise Effects on Cognitive Function and Neuro-Physiological Underpinning Mechanisms. AIMS Neurosci. 2022, 9, 150. [Google Scholar] [CrossRef] [PubMed]
- Herold, F.; Törpel, A.; Schega, L.; Müller, N.G. Functional and/or Structural Brain Changes in Response to Resistance Exercises and Resistance Training Lead to Cognitive Improvements—A Systematic Review. Eur. Rev. Aging Phys. Act. 2019, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Sanabria, L.C.; Cavero-Redondo, I.; Martínez-Vizcaino, V.; Cano-Gutierrez, C.A.; Álvarez-Bueno, C. Effect of Multicomponent Exercise in Cognitive Impairment: A Systematic Review and Meta-Analysis. BMC Geriatr. 2022, 22, 617. [Google Scholar] [CrossRef]
- Ruscheweyh, R.; Willemer, C.; Krüger, K.; Duning, T.; Warnecke, T.; Sommer, J.; Völker, K.; Ho, H.V.; Mooren, F.; Knecht, S.; et al. Physical Activity and Memory Functions: An Interventional Study. Neurobiol. Aging 2011, 32, 1304–1319. [Google Scholar] [CrossRef]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M.; et al. Exercise Training Increases Size of Hippocampus and Improves Memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef]
- Furrer, R.; Hawley, J.A.; Handschin, C. The Molecular Athlete: Exercise Physiology from Mechanisms to Medals. Physiol. Rev. 2023, 103, 1693–1787. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.P.; Rahman, H.S. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 402374. [Google Scholar] [CrossRef] [PubMed]
- Traustadóttir, T.; Davies, S.S.; Su, Y.; Choi, L.; Brown-Borg, H.M.; Roberts, L.J.; Harman, S.M. Oxidative Stress in Older Adults: Effects of Physical Fitness. Age 2012, 34, 969–982. [Google Scholar] [CrossRef]
- Kozakiewicz, M.; Rowiński, R.; Kornatowski, M.; Dabrowski, A.; Kȩdziora-Kornatowska, K.; Strachecka, A. Relation of Moderate Physical Activity to Blood Markers of Oxidative Stress and Antioxidant Defense in the Elderly. Oxid. Med. Cell. Longev. 2019, 2019, 5123628. [Google Scholar] [CrossRef]
- Alikhani, S.; Sheikholeslami-Vatani, D. Oxidative Stress and Anti-Oxidant Responses to Regular Resistance Training in Young and Older Adult Women. Geriatr. Gerontol. Int. 2019, 19, 419–422. [Google Scholar] [CrossRef]
- Ribeiro, A.S.; Deminice, R.; Schoenfeld, B.J.; Tomeleri, C.M.; Padilha, C.S.; Venturini, D.; Barbosa, D.S.; Sardinha, L.B.; Cyrino, E.S. Effect of Resistance Training Systems on Oxidative Stress in Older Women. Int. J. Sport Nutr. Exerc. Metab. 2017, 27, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Elosua, R.; Molina, L.; Fito, M.; Arquer, A.; Sanchez-Quesada, J.L.; Covas, M.I.; Ordoñez-Llanos, J.; Marrugat, J. Response of Oxidative Stress Biomarkers to a 16-Week Aerobic Physical Activity Program, and to Acute Physical Activity, in Healthy Young Men and Women. Atherosclerosis 2003, 167, 327–334. [Google Scholar] [CrossRef]
- Karolkiewicz, J.; Michalak, E.; Pospieszna, B.; Deskur-Śmielecka, E.; Nowak, A.; Pilaczyńska-Szcześniak, Ł. Response of Oxidative Stress Markers and Antioxidant Parameters to an 8-Week Aerobic Physical Activity Program in Healthy, Postmenopausal Women. Arch. Gerontol. Geriatr. 2009, 49, e67–e71. [Google Scholar] [CrossRef] [PubMed]
- Hunter, G.R.; McCarthy, J.P.; Bamman, M.M. Effects of Resistance Training on Older Adults. Sport. Med. 2004, 34, 329–348. [Google Scholar] [CrossRef]
- Osali, A. Aerobic Exercise and Nano-Curcumin Supplementation Improve Inflammation in Elderly Females with Metabolic Syndrome. Diabetol. Metab. Syndr. 2020, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Tabrizi, R.; Vakili, S.; Akbari, M.; Mirhosseini, N.; Lankarani, K.B.; Rahimi, M.; Mobini, M.; Jafarnejad, S.; Vahedpoor, Z.; Asemi, Z. The Effects of Curcumin-Containing Supplements on Biomarkers of Inflammation and Oxidative Stress: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Phytother. Res. 2019, 33, 253–262. [Google Scholar] [CrossRef]
- Rainey-Smith, S.R.; Brown, B.M.; Sohrabi, H.R.; Shah, T.; Goozee, K.G.; Gupta, V.B.; Martins, R.N. Curcumin and Cognition: A Randomised, Placebo-Controlled, Double-Blind Study of Community-Dwelling Older Adults. Br. J. Nutr. 2016, 115, 2106–2113. [Google Scholar] [CrossRef]
- Yu, J.J.; Pei, L.B.; Zhang, Y.; Wen, Z.Y.; Yang, J.L. Chronic Supplementation of Curcumin Enhances the Efficacy of Antidepressants in Major Depressive Disorder: A Randomized, Double-Blind, Placebo-Controlled Pilot Study. J. Clin. Psychopharmacol. 2015, 35, 406–410. [Google Scholar] [CrossRef]
- Benameur, T.; Giacomucci, G.; Panaro, M.A.; Ruggiero, M.; Trotta, T.; Monda, V.; Pizzolorusso, I.; Lofrumento, D.D.; Porro, C.; Messina, G. New Promising Therapeutic Avenues of Curcumin in Brain Diseases. Molecules 2021, 27, 236. [Google Scholar] [CrossRef]
- Namgyal, D.; Ali, S.; Mehta, R.; Sarwat, M. The Neuroprotective Effect of Curcumin against Cd-Induced Neurotoxicity and Hippocampal Neurogenesis Promotion through CREB-BDNF Signaling Pathway. Toxicology 2020, 442, 152542. [Google Scholar] [CrossRef]
- Reitan, R.M. Validity of the Trial Making Test as an Indicator of Organic Brain Damage. Percept. Mot. Ski. 1958, 8, 276. [Google Scholar] [CrossRef]
- Vilagut, G.; Valderas, J.M.; Ferrer, M.; Garin, O.; López-García, E.; Alonso, J. Interpretación de Los Cuestionarios de Salud SF-36 y SF-12 En España: Componentes Físico y Mental. Med. Clin. 2008, 130, 726–735. [Google Scholar] [CrossRef] [PubMed]
- López-García, E.; Banegas, J.R.; Pérez-Regadera, A.G.; Gutiérrez-Fisac, J.L.; Alonso, J.; Rodríguez-Artalejo, F. Population-Based Reference Values for the Spanish Version of the SF-36 Health Survey in the Elderly. Med. Clin. 2003, 120, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Roberts, H.C.; Denison, H.J.; Martin, H.J.; Patel, H.P.; Syddall, H.; Cooper, C.; Sayer, A.A. A Review of the Measurement of Grip Strength in Clinical and Epidemiological Studies: Towards a Standardised Approach. Age Ageing 2011, 40, 423–429. [Google Scholar] [CrossRef]
- Rikli, R.E.; Jones, C.J. Senior Fitness Test Manual; Human Kinetics: Champaign, IL, USA, 2013; ISBN 1-4504-1118-5. [Google Scholar]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P. Quantity and Quality of Exercise for Developing and Maintaining Cardiorespiratory, Musculoskeletal, and Neuromotor Fitness in Apparently Healthy Adults: Guidance for Prescribing Exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Colado, J.C.; Gené-Morales, J.; Jiménez-Martínez, P.; Flandez, J.; Ferri-Caruana, A.M.; Babiloni-Lopez, C. Rating of Perceived Exertion in the First Repetition Is Related to the Total Repetitions Performed in Elastic Bands Training. Mot. Control 2023, 27, 830–843. [Google Scholar] [CrossRef]
- Vieira, E.R.; Palmer, R.C.; Chaves, P.H.M. Prevention of Falls in Older People Living in the Community. BMJ 2016, 353, i1419. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; Furtado, G.E.; Teixeira, A.M.; Flandez, J.; Naclerio, F. Concurrent and Construct Validation of a New Scale for Rating Perceived Exertion during Elastic Resistance Training in The Elderly. J. Sports Sci. Med. 2020, 19, 175. [Google Scholar] [PubMed]
- Saraf-Bank, S.; Ahmadi, A.; Paknahad, Z.; Maracy, M.; Nourian, M. Effects of Curcumin on Cardiovascular Risk Factors in Obese and Overweight Adolescent Girls: A Randomized Clinical Trial. Sao Paulo Med. J. 2019, 137, 414–422. [Google Scholar] [CrossRef]
- Shep, D.; Khanwelkar, C.; Gade, P.; Karad, S. Safety and Efficacy of Curcumin versus Diclofenac in Knee Osteoarthritis: A Randomized Open-Label Parallel-Arm Study. Trials 2019, 20, 214. [Google Scholar] [CrossRef]
- Pivari, F.; Mingione, A.; Piazzini, G.; Ceccarani, C.; Ottaviano, E.; Brasacchio, C.; Dei Cas, M.; Vischi, M.; Cozzolino, M.G.; Fogagnolo, P.; et al. Curcumin Supplementation (Meriva®) Modulates Inflammation, Lipid Peroxidation and Gut Microbiota Composition in Chronic Kidney Disease. Nutrients 2022, 14, 231. [Google Scholar] [CrossRef]
- Corder, G.W.; Foreman, D.I. Non-Parametric Statistics: A Step-by-Step Approach; Wiley: Hoboken, NJ, USA, 2014; Volume 2, pp. 1–283. [Google Scholar]
- Quade, D. Rank Analysis of Covariance. J. Am. Stat. Assoc. 1967, 62, 1187–1200. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; ISBN 0805802835. [Google Scholar]
- Draak, T.H.P.; de Greef, B.T.A.; Faber, C.G.; Merkies, I.S.J. The Minimum Clinically Important Difference: Which Direction to Take. Eur. J. Neurol. 2019, 26, 850–855. [Google Scholar] [CrossRef] [PubMed]
- Beaton, D.E.; Boers, M.; Wells, G.A. Many Faces of the Minimal Clinically Important Difference (MCID): A Literature Review and Directions for Future Research. Curr. Opin. Rheumatol. 2002, 14, 109–114. [Google Scholar] [CrossRef]
- King, M.T. A Point of Minimal Important Difference (MID): A Critique of Terminology and Methods. Expert Rev. Pharmacoecon. Outcomes Res. 2011, 11, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Schober, P.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Walsh, E.I.; Smith, L.; Northey, J.; Rattray, B.; Cherbuin, N. Towards an Understanding of the Physical Activity-BDNF-Cognition Triumvirate: A Review of Associations and Dosage. Ageing Res. Rev. 2020, 60, 101044. [Google Scholar] [CrossRef]
- van ’t Erve, T.J.; Kadiiska, M.B.; London, S.J.; Mason, R.P. Classifying Oxidative Stress by F2-Isoprostane Levels across Human Diseases: A Meta-Analysis. Redox Biol. 2017, 12, 582. [Google Scholar] [CrossRef]
- Devries, M.C.; Hamadeh, M.J.; Glover, A.W.; Raha, S.; Samjoo, I.A.; Tarnopolsky, M.A. Endurance Training without Weight Loss Lowers Systemic, but Not Muscle, Oxidative Stress with No Effect on Inflammation in Lean and Obese Women. Free Radic. Biol. Med. 2008, 45, 503–511. [Google Scholar] [CrossRef]
- Margaritelis, N.V.; Nastos, G.G.; Vasileiadou, O.; Chatzinikolaou, P.N.; Theodorou, A.A.; Paschalis, V.; Vrabas, I.S.; Kyparos, A.; Fatouros, I.G.; Nikolaidis, M.G. Inter-Individual Variability in Redox and Performance Responses after Antioxidant Supplementation: A Randomized Double Blind Crossover Study. Acta Physiol. 2023, 238, e14017. [Google Scholar] [CrossRef]
- Ciolek, C.H.; Lee, S.Y. Cognitive Issues in the Older Adult. In Guccione’s Geriatric Physical Therapy E-Book, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 425–452. [Google Scholar] [CrossRef]
- Bruun, I.H.; Mogensen, C.B.; Nørgaard, B.; Schiøttz-Christensen, B.; Maribo, T. Validity and Responsiveness to Change of the 30-Second Chair-Stand Test in Older Adults Admitted to an Emergency Department. J. Geriatr. Phys. Ther. 2019, 42, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Kojima, G.; Masud, T.; Kendrick, D.; Morris, R.; Gawler, S.; Treml, J.; Iliffe, S. Does the Timed up and Go Test Predict Future Falls among British Community-Dwelling Older People? Prospective Cohort Study Nested within a Randomised Controlled Trial. BMC Geriatr. 2015, 15, 38. [Google Scholar] [CrossRef]
- Kline Mangione, K.; Craik, R.L.; McCormick, A.A.; Blevins, H.L.; White, M.B.; Sullivan-Marx, E.M.; Tomlinson, J.D. Detectable Changes in Physical Performance Measures in Elderly African Americans. Phys. Ther. 2010, 90, 921–927. [Google Scholar] [CrossRef]
- Oksuzyan, A.; Demakakos, P.; Shkolnikova, M.; Thinggaard, M.; Vaupel, J.W.; Christensen, K.; Shkolnikov, V.M. Handgrip Strength and Its Prognostic Value for Mortality in Moscow, Denmark, and England. PLoS ONE 2017, 12, e0182684. [Google Scholar] [CrossRef] [PubMed]
- Fragala, M.S.; Alley, D.E.; Shardell, M.D.; Harris, T.B.; McLean, R.R.; Kiel, D.P.; Cawthon, P.M.; Dam, T.T.L.; Ferrucci, L.; Guralnik, J.M.; et al. Comparison of Handgrip to Leg Extension Strength for Predicting Slow Gait Speed in Older Adults. J. Am. Geriatr. Soc. 2016, 64, 144. [Google Scholar] [CrossRef] [PubMed]
- Steffl, M.; Stastny, P. Isokinetic Testing of Muscle Strength of Older Individuals with Sarcopenia or Frailty: A Systematic Review. Isokinet. Exerc. Sci. 2020, 28, 291–301. [Google Scholar] [CrossRef]
- Setayesh, S.; Mohammad Rahimi, G.R. The Impact of Resistance Training on Brain-Derived Neurotrophic Factor and Depression among Older Adults Aged 60 Years or Older: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Geriatr. Nurs. 2023, 54, 23–31. [Google Scholar] [CrossRef]
- Schiffer, T.; Schulte, S.; Sperlich, B.; Achtzehn, S.; Fricke, H.; Strüder, H.K. Lactate Infusion at Rest Increases BDNF Blood Concentration in Humans. Neurosci. Lett. 2011, 488, 234–237. [Google Scholar] [CrossRef]
- Müller, P.; Duderstadt, Y.; Lessmann, V.; Müller, N.G. Lactate and BDNF: Key Mediators of Exercise Induced Neuroplasticity? J. Clin. Med. 2020, 9, 1136. [Google Scholar] [CrossRef]
- Radak, Z.; Zhao, Z.; Koltai, E.; Ohno, H.; Atalay, M. Oxygen Consumption and Usage During Physical Exercise: The Balance Between Oxidative Stress and ROS-Dependent Adaptive Signaling. Antioxid. Redox Signal. 2013, 18, 1208–1246. [Google Scholar] [CrossRef]
- Mueller, M.; Breil, F.A.; Lurman, G.; Klossner, S.; Flück, M.; Billeter, R.; Däpp, C.; Hoppeler, H. Different Molecular and Structural Adaptations with Eccentric and Conventional Strength Training in Elderly Men and Women. Gerontology 2011, 57, 528–538. [Google Scholar] [CrossRef] [PubMed]
- Simioni, C.; Zauli, G.; Martelli, A.M.; Vitale, M.; Sacchetti, G.; Gonelli, A.; Neri, L.M. Oxidative Stress: Role of Physical Exercise and Antioxidant Nutraceuticals in Adulthood and Aging. Oncotarget 2018, 9, 17181. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, J.F.; Bell, T.; Crowe, M.; Clay, O.J.; Mirman, D. Lifting Cognition: A Meta-Analysis of Effects of Resistance Exercise on Cognition. Psychol. Res. 2020, 84, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, K.P.M.; de Oliveira, V.H.; de Medeiros, G.C.B.S.; Mata, Á.N.D.S.; García, D.Á.; Martínez, D.G.; Leitão, J.C.; Knackfuss, M.I.; Piuvezam, G. The Effects of Exercise on BDNF Levels in Adolescents: A Systematic Review with Meta-Analysis. Int. J. Environ. Res. Public Health 2020, 17, 6056. [Google Scholar] [CrossRef]
- Piepmeier, A.T.; Etnier, J.L. Brain-Derived Neurotrophic Factor (BDNF) as a Potential Mechanism of the Effects of Acute Exercise on Cognitive Performance. J. Sport Health Sci. 2015, 4, 14–23. [Google Scholar] [CrossRef]
- Park, H.; Poo, M.-M. Neurotrophin Regulation of Neural Circuit Development and Function. Nat. Rev. Neurosci. 2013, 14, 7–23. [Google Scholar] [CrossRef]
- Yu, H.; Chen, Z.Y. The Role of BDNF in Depression on the Basis of Its Location in the Neural Circuitry. Acta Pharmacol. Sin. 2010, 32, 3–11. [Google Scholar] [CrossRef]
- Polyakova, M.; Stuke, K.; Schuemberg, K.; Mueller, K.; Schoenknecht, P.; Schroeter, M.L. BDNF as a Biomarker for Successful Treatment of Mood Disorders: A Systematic & Quantitative Meta-Analysis. J. Affect. Disord. 2015, 174, 432–440. [Google Scholar] [CrossRef]
- Khodadad Kashi, S.; Mirzazadeh, Z.S.; Saatchian, V. A Systematic Review and Meta-Analysis of Resistance Training on Quality of Life, Depression, Muscle Strength, and Functional Exercise Capacity in Older Adults Aged 60 Years or More. Biol. Res. Nurs. 2023, 25, 88–106. [Google Scholar] [CrossRef]
- Ramos-Junior, O.J.F.; Pinheiro, V.D.S.; Souza, T.G.D.S.D.; Alvares, T.S. Effect of Curcumin Intake on Skeletal Muscle Oxygen Saturation Parameters in Older Participants. Antioxidants 2024, 13, 1175. [Google Scholar] [CrossRef]
- Jin, T.; Zhang, Y.; Botchway, B.O.A.; Zhang, J.; Fan, R.; Zhang, Y.; Liu, X. Curcumin Can Improve Parkinson’s Disease via Activating BDNF/PI3k/Akt Signaling Pathways. Food Chem. Toxicol. 2022, 164, 113091. [Google Scholar] [CrossRef] [PubMed]
- Kavyani, Z.; Najafi, K.; Naghsh, N.; Karvane, H.B.; Musazadeh, V. The Effects of Curcumin Supplementation on Biomarkers of Inflammation, Oxidative Stress, and Endothelial Function: A Meta-Analysis of Meta-Analyses. Prostaglandins Other Lipid Mediat. 2024, 174, 106867. [Google Scholar] [CrossRef] [PubMed]




| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| Brain-derived neurotrophic factor (pg/mL) | (1) Aecc-Cur | 38,738 ± 7834 | 40,881 ± 6523 | 5.5 | 0.006 | 0.29 | 1−5 | <0.001 | 0.28 |
| 1−6 | 0.017 | 0.59 | |||||||
| (2) Aecc-Pla | 39,222 ± 8742 | 41,296 ± 8936 | 5.3 | 0.020 | 0.23 | 2−5 | <0.001 | 0.21 | |
| 2−6 | 0.025 | 0.46 | |||||||
| (3) Max-Cur | 43,737 ± 9565 | 44,828 ± 8377 | 2.5 | 0.256 | 0.12 | 3−5 | <0.001 | 0.21 | |
| 3−6 | 0.026 | 0.07 | |||||||
| (4) Max-Pla | 47,309 ± 9208 | 48,341 ± 8019 | 2.2 | 0.282 | 0.12 | 4−5 | 0.001 | 0.61 | |
| (5) C-Pla | 46,016 ± 9587 | 43,052 ± 9042 | −6.4 | <0.001 | 0.32 | ||||
| (6) C-Cur | 46,685 ± 8268 | 45,284 ± 8590 | −3.0 | 0.075 | 0.17 | ||||
| F2-Isoprostanes (pg/mL) | (1) Aecc-Cur | 4486 ± 1744 | 2664 ± 1243 | −40.6 | 0.003 | 1.20 | 1−5 | <0.001 | 1.83 |
| 1−6 | <0.001 | 1.82 | |||||||
| (2) Aecc-Pla | 4497 ± 1647 | 3185 ± 1027 | −29.2 | 0.007 | 0.95 | 2−3 | 0.006 | 0.97 | |
| 2−5 | <0.001 | 1.52 | |||||||
| (3) Max-Cur | 3640 ± 1784 | 2039 ± 1350 | −44.0 | 0.007 | 10.01 | 2−6 | <0.001 | 1.52 | |
| 3−5 | <0.001 | 2.35 | |||||||
| (4) Max-Pla | 4694 ± 1937 | 3313 ± 1878 | −29.4 | 0.017 | 0.72 | 3−6 | <0.001 | 2.21 | |
| 4−5 | 0.006 | 1.02 | |||||||
| (5) C-Pla | 3858 ± 1292 | 4738 ± 1012 | 22.8 | <0.001 | 0.75 | 4−6 | 0.019 | 1.08 | |
| (6) C-Cur | 4539 ± 940 | 5032 ± 1353 | 10.9 | 0.078 | 0.42 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| Trail Making Test A (seconds) | (1) Aecc-Cur | 47.9 ± 11.7 | 35.9 ± 13.0 | −25.03 | 0.001 | 0.88 | 1–5 | 0.000 | 1.46 |
| 1–6 | 0.001 | 0.03 | |||||||
| (2) Aecc-Pla | 39.3 ± 9.1 | 30.6 ± 9.0 | −22.11 | 0.002 | 0.91 | 2–5 | 0.000 | 3.24 | |
| 2–6 | 0.003 | 1.31 | |||||||
| (3) Max-Cur | 52.4 ± 18.1 | 32.5 ± 6.8 | −37.98 | 0.005 | 2.66 | 3–5 | 0.000 | 2.77 | |
| 3–6 | 0.000 | 0.85 | |||||||
| (4) Max-Pla | 39.1 ± 8.3 | 29.4 ± 6.7 | −24.81 | 0.005 | 1.33 | 4–5 | 0.000 | 3.61 | |
| 4–6 | 0.002 | 1.62 | |||||||
| (5) C-Pla | 42.5 ± 13.3 | 43.1 ± 12.4 | 1.25 | 0.928 | 0.04 | ||||
| (6) C-Cur | 35.9 ± 12.1 | 36.0 ± 12.9 | 0.25 | 0.395 | 0.01 | ||||
| Trail Making Test B (seconds) | (1) Aecc-Cur | 98.4 ± 32.2 | 69.6 ± 21.2 | −29.23 | 0.003 | 1.27 | 1–5 | 0.026 | 1.21 |
| 1–6 | 0.024 | 1.13 | |||||||
| (2) Aecc-Pla | 82.1 ± 21.2 | 68.9 ± 18.6 | −15.99 | 0.001 | 0.67 | 2–5 | 0.030 | 0.62 | |
| 2–6 | 0.037 | 0.50 | |||||||
| (3) Max-Cur | 94.2 ± 28.7 | 68.2 ± 15.7 | −27.60 | 0.007 | 1.51 | 3–5 | 0.043 | 1.17 | |
| 3–6 | 0.035 | 1.10 | |||||||
| (4) Max-Pla | 90.4 ± 25.9 | 69.5 ± 12.7 | −23.12 | 0.007 | 1.50 | 4–5 | 0.030 | 0.95 | |
| 4–6 | 0.024 | 0.87 | |||||||
| (5) C-Pla | 94.5 ± 30.7 | 96.2 ± 27.2 | 1.78 | 0.065 | 0.06 | ||||
| (6) C-Cur | 87.8 ± 17.2 | 86.1 ± 25.4 | 1.90 | 0.656 | 0.07 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| General health (score) | (1) Aecc-Cur | 70.6 ± 11.8 | 87.8 ± 6.0 | 24.3 | <0.001 | 1.59 | 1–5 | <0.001 | 0.27 |
| 1–6 | <0.001 | 0.20 | |||||||
| (2) Aecc-Pla | 75.0 ± 12.7 | 86.9 ± 6.9 | 15.9 | 0.002 | 1.01 | 2–5 | <0.001 | 0.53 | |
| 2–6 | 0.004 | 0.16 | |||||||
| (3) Max-Cur | 73.5 ± 12.7 | 89.0 ± 4.6 | 21.1 | 0.007 | 1.41 | 3–5 | <0.001 | 0.44 | |
| 3–6 | 0.003 | 0.05 | |||||||
| (4) Max-Pla | 75.5 ± 13.4 | 88.0 ± 7.9 | 16.6 | 0.005 | 1.00 | 4–5 | <0.001 | 0.55 | |
| 4–6 | 0.004 | 0.20 | |||||||
| (5) C-Pla | 66.2 ± 18.6 | 65.0 ± 17.1 | −1.8 | 0.194 | 0.06 | 5–6 | 0.050 | 0.43 | |
| (6) C-Cur | 73.0 ± 10.5 | 76.3 ± 9.2 | 4.6 | 0.490 | 0.30 | ||||
| Social functioning (score) | (1) Aecc-Cur | 91.1 ± 8.8 | 96.5 ± 7.0 | 5.9 | 0.010 | 0.58 | 1–5 | <0.001 | 0.24 |
| 2–5 | <0.001 | 0.63 | |||||||
| (2) Aecc-Pla | 95.3 ± 8.0 | 100.0 ± 0.0 | 4.9 | 0.050 | 0.72 | 3–5 | 0.013 | 0.20 | |
| 5–6 | 0.049 | 0.02 | |||||||
| (3) Max-Cur | 90.5 ± 9.5 | 100.0 ± 8.8 | 10.5 | 0.003 | 0.90 | ||||
| (4) Max-Pla | 86.4 ± 18.1 | 92.6 ± 10.5 | 7.2 | 0.066 | 0.37 | ||||
| (5) C-Pla | 88.3 ± 12.8 | 84.8 ± 11.3 | −4.0 | 0.040 | 0.26 | ||||
| (6) C-Cur | 88.4 ± 21.4 | 87.2 ± 17.3 | −1.3 | 0.170 | 0.05 | ||||
| Vitality (score) | (1) Aecc-Cur | 67.8 ± 12.6 | 80.0 ± 7.3 | 18.0 | <0.001 | 1.03 | 1–5 | 0.012 | 0.10 |
| 1–6 | 0.013 | 0.05 | |||||||
| (2) Aecc-Pla | 73.4 ± 14.3 | 83.5 ± 10.1 | 13.6 | <0.001 | 0.70 | 2–5 | 0.002 | 0.47 | |
| 2–6 | 0.003 | 0.37 | |||||||
| (3) Max-Cur | 64.0 ± 18.8 | 79.3 ± 13.3 | 23.9 | <0.001 | 0.82 | 3–5 | 0.038 | 0.14 | |
| 3–6 | 0.039 | 0.15 | |||||||
| (4) Max-Pla | 71.0 ± 21.2 | 86.3 ± 9.7 | 21.6 | <0.001 | 0.82 | 4–5 | <0.001 | 0.24 | |
| 4–6 | <0.001 | 0.20 | |||||||
| (5) C-Pla | 66.5 ± 14.3 | 64.9 ± 14.2 | −2.3 | 0.038 | 0.09 | ||||
| (6) C-Cur | 67.0 ± 19.0 | 68.3 ± 18.2 | 2.0 | 0.556 | 0.06 | ||||
| Mental health (score) | (1) Aecc-Cur | 78.3 ± 11.5 | 86.5 ± 8.4 | 10.5 | 0.001 | 0.71 | 1–5 | 0.003 | 0.13 |
| 2–5 | <0.001 | 0.54 | |||||||
| (2) Aecc-Pla | 83.1 ± 10.5 | 88.6 ± 8.8 | 6.7 | 0.018 | 0.50 | 3–5 | 0.008 | 0.05 | |
| 4–5 | 0.009 | 0.14 | |||||||
| (3) Max-Cur | 77.1 ± 12.1 | 86.7 ± 7.3 | 12.5 | 0.017 | 0.84 | ||||
| (4) Max-Pla | 78.4 ± 11.2 | 84.4 ± 6.4 | 7.7 | 0.042 | 0.57 | ||||
| (5) C-Pla | 76.7 ± 12.1 | 74.8 ± 11.5 | −2.5 | 0.114 | 0.14 | ||||
| (6) C-Cur | 77.6 ± 15.8 | 78.1 ± 17.0 | 0.7 | 0.480 | 0.03 |
| Variable | Group | Mean ± SD (Pre-Test) | Mean ± SD (Post-Test) | Δ% | p-Value (Time) | ES (Time) | Comparison (Group) | p-Value (Group) | ES (Group) |
|---|---|---|---|---|---|---|---|---|---|
| 30-Second Chair Stand (repetitions) | (1) Aecc-Cur | 15.6 ± 3.7 | 19.8 ± 3.4 | 26.4 | <0.001 | 1.12 | 1–5 | <0.001 | 1.09 |
| 1–6 | <0.001 | 1.43 | |||||||
| (2) Aecc-Pla | 16.2 ± 3.7 | 18.8 ± 3.3 | 15.7 | 0.001 | 0.69 | 2–5 | 0.006 | 0.70 | |
| 2–6 | <0.001 | 0.95 | |||||||
| (3) Max-Cur | 15.0 ± 2.8 | 19.4 ± 2.8 | 29.3 | 0.004 | 1.48 | 3–5 | 0.004 | 1.18 | |
| 3–6 | <0.001 | 1.77 | |||||||
| (4) Max-Pla | 13.9 ± 3.0 | 17.1 ± 2.9 | 23.0 | 0.005 | 1.01 | 4–5 | 0.039 | 0.87 | |
| 4–6 | 0.016 | 1.28 | |||||||
| (5) C-Pla | 15.3 ± 4.8 | 15.1 ± 4.3 | −1.7 | 0.248 | 0.05 | ||||
| (6) C-Cur | 15.9 ± 2.4 | 15.6 ± 2.4 | −1.5 | 0.563 | 0.10 | ||||
| Timed Up And Go Test (seconds) | (1) Aecc-Cur | 5.4 ± 0.7 | 4.7 ± 0.5 | −13.5 | <0.001 | 1.26 | 1–5 | <0.001 | 0.96 |
| 1–6 | <0.001 | 1.06 | |||||||
| (2) Aecc-Pla | 5.6 ± 0.6 | 5.0 ± 0.6 | −11.1 | 0.001 | 0.99 | 2–5 | 0.005 | 0.77 | |
| 2–6 | 0.041 | 0.62 | |||||||
| (3) Max-Cur | 5.5 ± 0.4 | 4.8 ± 0.4 | −13.6 | 0.005 | 1.90 | 3–5 | <0.001 | 0.92 | |
| 3–6 | <0.001 | 1.02 | |||||||
| (4) Max-Pla | 5.6 ± 0.9 | 5.0 ± 0.7 | −11.5 | 0.005 | 0.73 | 4–5 | 0.008 | 0.74 | |
| 4–6 | 0.031 | 0.80 | |||||||
| (5) C-Pla | 5.9 ± 1.0 | 6.0 ± 1.0 | 1.0 | 0.272 | 0.07 | ||||
| (6) C-Cur | 5.4 ± 0.9 | 5.5 ± 0.9 | 1.7 | 0.265 | 0.11 | ||||
| Six-Minute Walk Test (meters) | (1) Aecc-Cur | 605 ± 62 | 661 ± 36 | 9.2 | <0.001 | 1.06 | 1–2 | 0.002 | 0.46 |
| 1–4 | 0.003 | 0.50 | |||||||
| (2) Aecc-Pla | 597 ± 71 | 628 ± 69 | 5.1 | 0.001 | 0.42 | 1–5 | 0.000 | 0.70 | |
| 1–6 | 0.000 | 0.83 | |||||||
| (3) Max-Cur | 575 ± 69 | 628 ± 72 | 9.2 | 0.005 | 0.70 | 2–5 | 0.004 | 0.35 | |
| 2–6 | 0.000 | 0.40 | |||||||
| (4) Max-Pla | 597 ± 67 | 627 ± 66 | 5.1 | 0.005 | 0.42 | 3–4 | 0.036 | 0.41 | |
| 3–5 | 0.000 | 0.56 | |||||||
| (5) C-Pla | 549 ± 108 | 545 ± 110 | −0.7 | 0.136 | 0.03 | 3–6 | 0.000 | 0.64 | |
| 4–5 | 0.013 | 0.34 | |||||||
| (6) C-Cur | 599 ± 98 | 595 ± 94 | −0.7 | 0.112 | 0.04 | 4–6 | 0.000 | 0.39 | |
| 5–6 | 0.002 | 0.08 | |||||||
| Manual dynamometry (kg) | (1) Aecc-Cur | 30.1 ± 9.3 | 34.9 ± 9.2 | 16.1 | <0.001 | 0.51 | 1–5 | 0.034 | 0.80 |
| 1–6 | 0.021 | 0.82 | |||||||
| (2) Aecc-Pla | 32.8 ± 8.2 | 36.5 ± 8.4 | 11.1 | 0.001 | 0.42 | 2–5 | 0.009 | 1.02 | |
| 2–6 | 0.010 | 1.07 | |||||||
| (3) Max-Cur | 28.2 ± 8.4 | 34.8 ± 8.8 | 23.2 | 0.005 | 0.71 | 3–5 | 0.050 | 0.84 | |
| (4) Max-Pla | 28.8 ± 9.8 | 33.6 ± 10.1 | 16.7 | 0.005 | 0.46 | ||||
| (5) C-Pla | 28.4 ± 7.3 | 27.9 ± 7.4 | −1.8 | 0.130 | 0.07 | ||||
| (6) C-Cur | 28.5 ± 8.0 | 27.6 ± 8.4 | −3.2 | 0.444 | 0.12 | ||||
| Knee flexion 60° /s (N·m) | (1) Aecc-Cur | 48.3 ± 15.5 | 78.7 ± 17.8 | 63.03 | <0.001 | 1.82 | 1–3 | 0.047 | 0.54 |
| 1–5 | <0.001 | 1.42 | |||||||
| (2) Aecc-Pla | 49.1 ± 14.8 | 72.3 ± 12.3 | 47.31 | 0.001 | 1.71 | 1–6 | <0.001 | 1.43 | |
| 2–3 | 0.018 | 1.23 | |||||||
| (3) Max-Cur | 49.4 ± 14.3 | 86.6 ± 10.5 | 75.40 | 0.005 | 2.97 | 2–5 | 0.001 | 1.25 | |
| 2–6 | 0.007 | 1.25 | |||||||
| (4) Max-Pla | 52.5 ± 24.3 | 84.2 ± 24.0 | 60.30 | 0.005 | 1.31 | 3–5 | <0.001 | 2.06 | |
| 3–6 | <0.001 | 2.06 | |||||||
| (5) C-Pla | 50.7 ± 18.4 | 48.8 ± 17.5 | −3.63 | 0.026 | 0.01 | 4–5 | <0.001 | 1.47 | |
| 4–6 | <0.001 | 1.47 | |||||||
| (6) C-Cur | 52.0 ± 22.3 | 51.0 ± 21.0 | −1.98 | 0.139 | 0.05 | ||||
| Knee extension 60° /s (N·m) | (1) Aecc-Cur | 82.6 ± 22.4 | 127.4 ± 22.4 | 54.34 | <0.001 | 1.95 | 1–5 | 0.004 | 1.18 |
| 1–6 | 0.038 | 0.99 | |||||||
| (2) Aecc -Pla | 93.1 ± 27.4 | 130.9 ± 27.4 | 40.61 | 0.001 | 1.33 | 2–5 | 0.005 | 1.19 | |
| 2–6 | 0.029 | 1.00 | |||||||
| (3) Max-Cur | 80.8 ± 28.1 | 126.7 ± 26.4 | 56.85 | 0.005 | 1.61 | 3–5 | 0.013 | 1.05 | |
| 3–6 | 0.044 | 0.87 | |||||||
| (4) Max-Pla | 90.9 ± 41.2 | 129.9 ± 42.5 | 42.96 | 0.005 | 0.90 | 4–5 | 0.005 | 0.99 | |
| 4–6 | 0.031 | 0.82 | |||||||
| (5) C-Pla | 91.1 ± 39.0 | 88.7 ± 39.1 | −2.56 | 0.338 | 0.07 | ||||
| (6) C-Cur | 96.1 ± 42.5 | 95.5 ± 39.1 | −0.60 | 0.826 | 0.04 |
| Variable | Endpoint ^/Cutoff Point * | Benefits/Risks | Aecc-Cur (n = 16) | Aecc-Pla (n = 13) | Max-Cur (n = 10) | Max-Pla (n = 10) | C-Cur (n = 15) | C-Pla (n = 17) |
|---|---|---|---|---|---|---|---|---|
| BDNF | <20–30 ng/mL [60] * | Relevant clinical relationship between lower scores and brain shrinkage (8% by age 60) | 100% | 100% | 100% | 100% | 92% | 94% |
| F2-isoprostanes | 12.98 pg/mL decrease [61,62,63] ^ | High values raise coronary calcification risk by 23% and acute syndrome risk by 42% | 56% | 38% | 44% | 40% | 0% | 0% |
| TMT-A | 11.70 s decrease [64] ^ | High values are associated with a 9% higher prevalence of Alzheimer’s disease | 44% | 39% | 60% | 40% | 0% | 0% |
| TMT-B | 24.40 s decrease [64] ^ | 44% | 15% | 40% | 40% | 6% | 6% | |
| 30 s Chair Stand Test | ≤8 repetitions [65] * | Relevant clinical changes in the appearance of sarcopenia | 100% | 100% | 100% | 100% | 100% | 88% |
| Timed-Up and Go Test | 1.7 s decrease [66,67] ^ | Each second decrease reduces fall risk by 9% | 50% | 46% | 60% | 50% | 0% | 0% |
| 6-min Walk Test | 28 m increase [67] ^ | Relevant changes in the prevention of falls | 69% | 46% | 100% | 60% | 6% | 0% |
| Grip strength | 1 kg increase [68] ^ | Decrease in mortality (from 3 to 10%) | 100% | 100% | 100% | 100% | 0% | 0% |
| Knee extension 60° /s | <94.50 Nm ♂ * <62.30 Nm ♀ [69] * | Relevant clinical changes in the appearance of leg muscle weakness and slow walking speed | 100% | 100% | 100% | 100% | 66% | 53% |
| Knee flexion 60° /s | <47.00 Nm ♂ * <36.00 Nm ♀ [70] * | 100% | 100% | 100% | 100% | 60% | 58% | |
| General Health | <55.90 points [43] * | Relevant clinical relationship between lower scores and risk of death (SF-36 mental scores: estimates of mortality at 8 years are 7.8%; SF-36 physical scores: estimates of mortality at 8 years are 15.4%) | 100% | 100% | 100% | 100% | 100% | 65% |
| Social Functioning | <79.20 points [43] * | 100% | 100% | 95% | 90% | 86% | 59% | |
| Vitality | <60.50 points [43] * | 100% | 100% | 100% | 90% | 73% | 70% | |
| Mental Health | <68.30 points [43] * | 100% | 100% | 100% | 100% | 88% | 80% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juesas, A.; Saez-Berlanga, A.; Gene-Morales, J.; Jiménez-Martínez, P.; Alix-Fages, C.; Fernandez-Garrido, J.; Caballero, O.; Janicijevic, D.; Zarza, V.; Colado, J.C. Effects of High-Resistance Elastic Band Training and a Curcumin-Based Formulation on Neuro-Oxidative and Functional Health in Sedentary Older Adults. Healthcare 2025, 13, 1055. https://doi.org/10.3390/healthcare13091055
Juesas A, Saez-Berlanga A, Gene-Morales J, Jiménez-Martínez P, Alix-Fages C, Fernandez-Garrido J, Caballero O, Janicijevic D, Zarza V, Colado JC. Effects of High-Resistance Elastic Band Training and a Curcumin-Based Formulation on Neuro-Oxidative and Functional Health in Sedentary Older Adults. Healthcare. 2025; 13(9):1055. https://doi.org/10.3390/healthcare13091055
Chicago/Turabian StyleJuesas, Alvaro, Angel Saez-Berlanga, Javier Gene-Morales, Pablo Jiménez-Martínez, Carlos Alix-Fages, Julio Fernandez-Garrido, Oscar Caballero, Danica Janicijevic, Virginia Zarza, and Juan C. Colado. 2025. "Effects of High-Resistance Elastic Band Training and a Curcumin-Based Formulation on Neuro-Oxidative and Functional Health in Sedentary Older Adults" Healthcare 13, no. 9: 1055. https://doi.org/10.3390/healthcare13091055
APA StyleJuesas, A., Saez-Berlanga, A., Gene-Morales, J., Jiménez-Martínez, P., Alix-Fages, C., Fernandez-Garrido, J., Caballero, O., Janicijevic, D., Zarza, V., & Colado, J. C. (2025). Effects of High-Resistance Elastic Band Training and a Curcumin-Based Formulation on Neuro-Oxidative and Functional Health in Sedentary Older Adults. Healthcare, 13(9), 1055. https://doi.org/10.3390/healthcare13091055









