Communication and Therapy Planning for Patients of Reproductive Age Under Immunomodulatory Treatments for Psoriasis or Psoriatic Arthritis—Survey of the German National Psoriasis Registry PsoBest
Abstract
1. Introduction
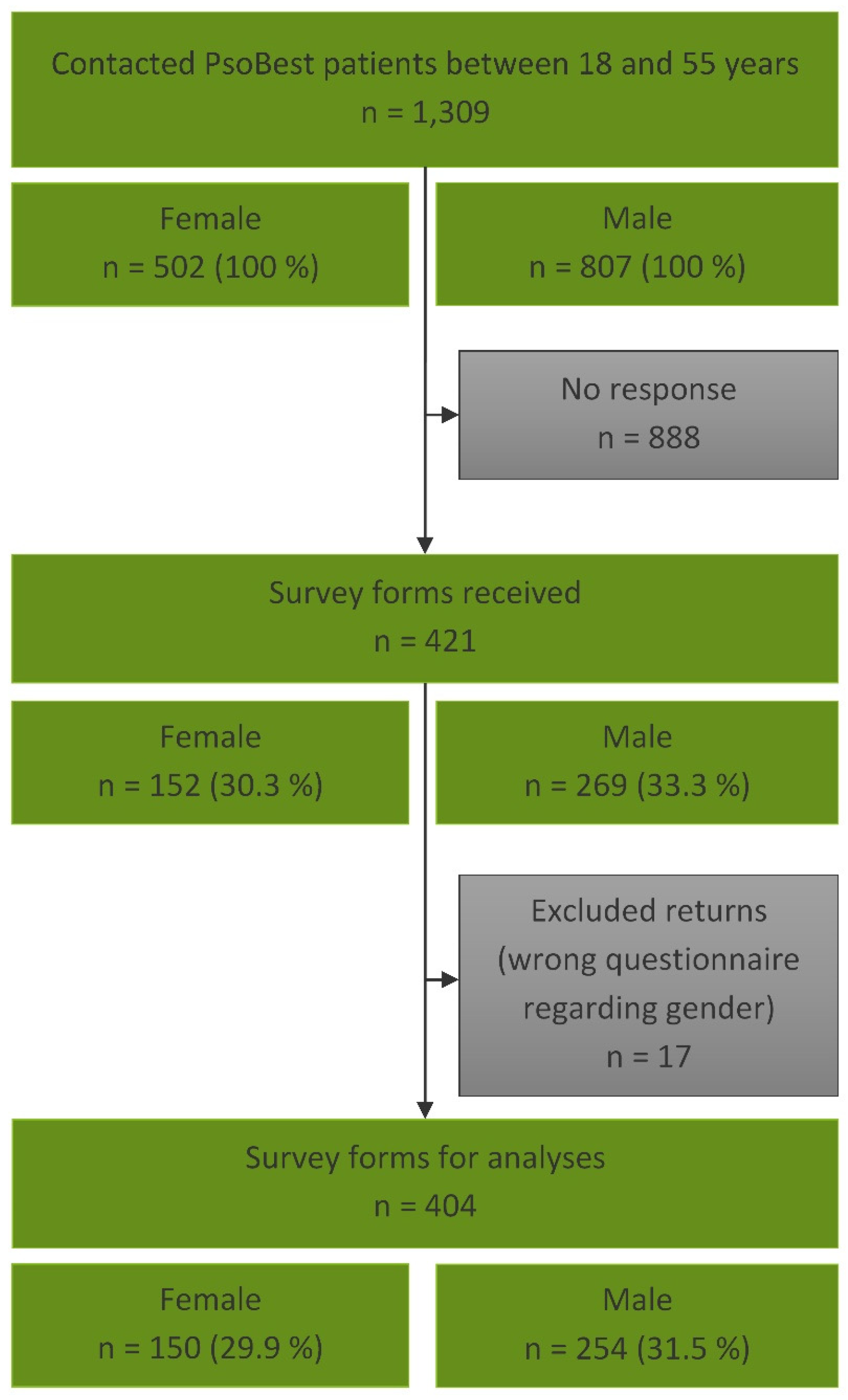
2. Materials and Methods
3. Results
3.1. Reproductive Intentions in Patients with Psoriasis or Psoriatic Arthritis Under Immunomodulatory Treatment
3.2. Patient Counselling on Reproductive Health During Immunomodulatory Treatment
3.3. Perceived Disease Severity in Women During Pregnancy
3.4. Perceived Impact of Psoriasis on Conception
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Augustin, M.; Reich, K.; Glaeske, G.; Schaefer, I.; Radtke, M. Co-morbidity and age-related prevalence of psoriasis: Analysis of health insurance data in Germany. Acta Derm. Venereol. 2010, 90, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Mrowietz, U.; Steinz, K.; Gerdes, S. Psoriasis: To treat or to manage? Exp. Dermatol. 2014, 23, 705–709. [Google Scholar] [CrossRef] [PubMed]
- D’Haens, G.; Baert, F.; van Assche, G.; Caenepeel, P.; Vergauwe, P.; Tuynman, H.; de Vos, M.; van Deventer, S.; Stitt, L.; Donner, A.; et al. Early combined immunosuppression or conventional management in patients with newly diagnosed Crohn’s disease: An open randomised trial. Lancet 2008, 371, 660–667. [Google Scholar] [CrossRef]
- Storre, M.-L. “Hit hard and early” bei Psoriasis. Ästhet. Dermatol. Kosmetol. 2023, 15, 17. [Google Scholar] [CrossRef]
- Nast, A.; Altenburg, A.; Augustin, M.; Boehncke, W.-H.; Härle, P.; Klaus, J.; Koza, J.; Mrowietz, U.; Ockenfels, H.-M.; Philipp, S.; et al. German S3-Guideline on the treatment of Psoriasis vulgaris, adapted from EuroGuiDerm—Part 1: Treatment goals and treatment recommendations. J. Dtsch. Dermatol. Ges. 2021, 19, 934–950. [Google Scholar] [CrossRef] [PubMed]
- Nast, A.; Smith, C.; Spuls, P.I.; Avila Valle, G.; Bata-Csörgö, Z.; Boonen, H.; de Jong, E.; Garcia-Doval, I.; Gisondi, P.; Kaur-Knudsen, D.; et al. EuroGuiDerm Guideline on the systemic treatment of Psoriasis vulgaris—Part 2: Specific clinical and comorbid situations. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 281–317. [Google Scholar] [CrossRef]
- Nast, A.; Altenburg, A.; Augustin, M.; Boehncke, W.-H.; Härle, P.; Klaus, J.; Koza, J.; Mrowietz, U.; Ockenfels, H.-M.; Philipp, S.; et al. German S3-Guideline on the treatment of Psoriasis vulgaris, adapted from EuroGuiDerm—Part 2: Treatment monitoring and specific clinical or comorbid situations. J. Dtsch. Dermatol. Ges. 2021, 19, 1092–1115. [Google Scholar] [CrossRef]
- Sbidian, E.; Chaimani, A.; Guelimi, R.; Garcia-Doval, I.; Hua, C.; Hughes, C.; Naldi, L.; Kinberger, M.; Afach, S.; Le Cleach, L. Systemic pharmacological treatments for chronic plaque psoriasis: A network meta-analysis. Cochrane Database Syst. Rev. 2023, 7, CD011535. [Google Scholar] [CrossRef]
- Ding, W.; Yao, M.; Wang, Y.; Wang, M.; Zhu, Y.; Li, Y.; Li, Z.; Li, L.; Ma, W.; Liu, M.; et al. Patient Needs in Psoriasis Treatment and their Influencing Factors: A Nationwide Multicentre Cross-Sectional Study in China. Indian J. Dermatol. 2023, 68, 587. [Google Scholar] [CrossRef]
- Augustin, M.; Radtke, M.A. Quality of life in psoriasis patients. Expert Rev. Pharmacoecon. Outcomes Res. 2014, 14, 559–568. [Google Scholar] [CrossRef]
- Eijkemans, M.J.C.; van Poppel, F.; Habbema, D.F.; Smith, K.R.; Leridon, H.; te Velde, E.R. Too old to have children? Lessons from natural fertility populations. Hum. Reprod. 2014, 29, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Huang, H.; Ji, L.; Zhang, Z. Maternal and neonatal outcomes in pregnant women with psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Rheumatology 2021, 60, 4018–4028. [Google Scholar] [CrossRef]
- Bröms, G.; Haerskjold, A.; Granath, F.; Kieler, H.; Pedersen, L.; Berglind, I.A. Effect of Maternal Psoriasis on Pregnancy and Birth Outcomes: A Population-based Cohort Study from Denmark and Sweden. Acta Derm. Venereol. 2018, 98, 728–734. [Google Scholar] [CrossRef]
- Bobotsis, R.; Gulliver, W.P.; Monaghan, K.; Lynde, C.; Fleming, P. Psoriasis and adverse pregnancy outcomes: A systematic review of observational studies. Br. J. Dermatol. 2016, 175, 464–472. [Google Scholar] [CrossRef]
- Luna, P.C.; Chu, C.-Y.; Fatani, M.; Borlenghi, C.; Adora, A.; Llamado, L.Q.; Wee, J. Psychosocial Burden of Psoriasis: A Systematic Literature Review of Depression Among Patients with Psoriasis. Dermatol. Ther. 2023, 13, 3043–3055. [Google Scholar] [CrossRef]
- da Silva, N.; Augustin, M.; Hilbring, C.; Braren-von Stülpnagel, C.C.; Sommer, R. Psychological (co)morbidity in patients with psoriasis: The impact of pruritus and anogenital involvement on symptoms of depression and anxiety and on body dysmorphic concerns—A cross-sectional study. BMJ Open 2022, 12, e055477. [Google Scholar] [CrossRef]
- da Silva, N.; Augustin, M.; Langenbruch, A.; Mrowietz, U.; Reich, K.; Thaçi, D.; Boehncke, W.-H.; Kirsten, N.; Danckworth, A.; Sommer, R. Sex-related impairment and patient needs/benefits in anogenital psoriasis: Difficult-to-communicate topics and their impact on patient-centred care. PLoS ONE 2020, 15, e0235091. [Google Scholar] [CrossRef]
- Sommer, R.; Mrowietz, U.; Du Gaarn Jardin, K.; Kasujee, I.; Martini, E.; Daudén, E.; Fabbrocini, G.; Zink, A.; Griffiths, C.E.M.; Augustin, M. Implementing well-being in the management of psoriasis: An expert recommendation. J. Eur. Acad. Dermatol. Venereol. 2024, 38, 302–310. [Google Scholar] [CrossRef]
- Dalgard, F.J.; Bewley, A.; Evers, A.W.; Gieler, U.; Lien, L.; Sampogna, F.; Ständer, S.; Tomas-Aragones, L.; Vulink, N.; Kupfer, J. Stigmatisation and body image impairment in dermatological patients: Protocol for an observational multicentre study in 16 European countries. BMJ Open 2018, 8, e024877. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-C.; Iskandar, I.Y.K.; Parisi, R.; Pierce, M.; Tower, C.; Kleyn, C.E.; Griffiths, C.E.M.; Ashcroft, D.M. Fertility Trends and Adverse Pregnancy Outcomes in Female Patients with Psoriasis in the UK. JAMA Dermatol. 2023, 159, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Rademaker, M.; Agnew, K.; Andrews, M.; Armour, K.; Baker, C.; Foley, P.; Frew, J.; Gebauer, K.; Gupta, M.; Kennedy, D.; et al. Psoriasis in those planning a family, pregnant or breast-feeding. The Australasian Psoriasis Collaboration. Australas. J. Dermatol. 2018, 59, 86–100. [Google Scholar] [CrossRef] [PubMed]
- Kiran, Z.; Sheikh, A.; Malik, S.; Meraj, A.; Masood, M.; Ismail, S.; Rashid, M.O.; Shaikh, Q.; Majeed, N.; Sheikh, L.; et al. Maternal characteristics and outcomes affected by hypothyroidism during pregnancy (maternal hypothyroidism on pregnancy outcomes, MHPO-1). BMC Pregnancy Childbirth 2019, 19, 476. [Google Scholar] [CrossRef]
- Scher, J.; Dabao, C.; Rukat, C. Thyroid Autoimmunity: The Treatment of Hashimoto’s Disease, or the Presence of Antithyroid Antibodies Alone, in Pregnancy. Am. J. Reprod. Immunol. 2025, 93, e70042. [Google Scholar] [CrossRef] [PubMed]
- Sim, B.L.; Daniel, R.S.; Hong, S.S.; Matar, R.H.; Ganiel, I.; Nakanishi, H.; Mansour, R.; Than, C.A.; Alrahmani, L. Pregnancy Outcomes in Women with Rheumatoid Arthritis: A Systematic Review and Meta-analysis. J. Clin. Rheumatol. 2023, 29, 36–42. [Google Scholar] [CrossRef]
- Pina Vegas, L.; Drouin, J.; Weill, A.; Dray-Spira, R. Pregnancy outcomes in women with rheumatoid arthritis: An 11-year French nationwide study. RMD Open 2024, 10, e003762. [Google Scholar] [CrossRef]
- Tondreau, A.; Breuval, C.; Gondry, J.; Fumery, M.; Foulon, A. Obstetric outcomes of patients with inflammatory bowel disease. Arch. Gynecol. Obstet. 2024, 310, 943–951. [Google Scholar] [CrossRef]
- Hoffmann, P.; Krueger, J.; Bashlekova, T.; Rupp, C.; Baumann, L.; Gauss, A. Pregnancy with inflammatory bowel disease: Outcomes for mothers and their children at a European tertiary care center. J. Obstet. Gynaecol. Res. 2022, 48, 621–633. [Google Scholar] [CrossRef]
- Cornish, J.; Tan, E.; Teare, J.; Teoh, T.G.; Rai, R.; Clark, S.K.; Tekkis, P.P. A meta-analysis on the influence of inflammatory bowel disease on pregnancy. Gut 2007, 56, 830–837. [Google Scholar] [CrossRef]
- Kuhnt, A.-K.; Minkus, L.; Buhr, P. Uncertainty in fertility intentions from a life course perspective: Which life course markers matter? JFamRes 2021, 33, 184–208. [Google Scholar] [CrossRef]
- Aghajani, M.J.; Aghajani, R. TikTok and #psoriasis: A cross-sectional study of content quality. Australas. J. Dermatol. 2023, 64, e369–e371. [Google Scholar] [CrossRef] [PubMed]
- Charnock, D.; Shepperd, S.; Needham, G.; Gann, R. DISCERN: An instrument for judging the quality of written consumer health information on treatment choices. J. Epidemiol. Community Health 1999, 53, 105–111. [Google Scholar] [CrossRef]
- Gorrepati, P.L.; Smith, G.P. Evaluating social media as a source of patient information regarding psoriasis treatments using the DISCERN instrument. J. Dermatolog. Treat. 2022, 33, 2685–2686. [Google Scholar] [CrossRef]
- Schuster, B.; Ziehfreund, S.; Biedermann, T.; Zink, A. Psoriasis 2.0: Facebook as a source of disease-related information for patients with psoriasis. J. Dtsch. Dermatol. Ges. 2020, 18, 571–581. [Google Scholar] [CrossRef]
- Peltier, M.R. Immunology of term and preterm labor. Reprod. Biol. Endocrinol. 2003, 1, 122. [Google Scholar] [CrossRef]
- Hwang, Y.M.; Wei, Q.; Piekos, S.N.; Vemuri, B.; Molani, S.; Mease, P.; Hood, L.; Hadlock, J.J. Maternal-fetal outcomes in patients with immune mediated inflammatory diseases, with consideration of comorbidities: A retrospective cohort study in a large U.S. healthcare system. EClinicalMedicine 2024, 68, 102435. [Google Scholar] [CrossRef]
- Johansen, C.B.; Egeberg, A.; Jimenez-Solem, E.; Skov, L.; Thomsen, S.F. Psoriasis and adverse pregnancy outcomes: A nationwide case-control study in 491,274 women in Denmark. JAAD Int. 2022, 7, 146–155. [Google Scholar] [CrossRef]
- Kimball, A.B.; Guenther, L.; Kalia, S.; de Jong, E.M.G.J.; Lafferty, K.P.; Chen, D.Y.; Langholff, W.; Shear, N.H. Pregnancy Outcomes in Women with Moderate-to-Severe Psoriasis from the Psoriasis Longitudinal Assessment and Registry (PSOLAR). JAMA Dermatol. 2021, 157, 301–306. [Google Scholar] [CrossRef]
- Chambers, C.D.; Johnson, D.L.; Xu, R.; Luo, Y.; Lopez-Jimenez, J.; Adam, M.P.; Braddock, S.R.; Robinson, L.K.; Vaux, K.; Lyons Jones, K. Birth outcomes in women who have taken adalimumab in pregnancy: A prospective cohort study. PLoS ONE 2019, 14, e0223603. [Google Scholar] [CrossRef]
- Clowse, M.E.B.; Wolf, D.C.; Förger, F.; Cush, J.J.; Golembesky, A.; Shaughnessy, L.; de Cuyper, D.; Mahadevan, U. Pregnancy Outcomes in Subjects Exposed to Certolizumab Pegol. J. Rheumatol. 2015, 42, 2270–2278. [Google Scholar] [CrossRef] [PubMed]
- Clowse, M.E.B.; Scheuerle, A.E.; Chambers, C.; Afzali, A.; Kimball, A.B.; Cush, J.J.; Cooney, M.; Shaughnessy, L.; Vanderkelen, M.; Förger, F. Pregnancy Outcomes After Exposure to Certolizumab Pegol: Updated Results from a Pharmacovigilance Safety Database. Arthritis Rheumatol. 2018, 70, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Chugh, R.; Long, M.D.; Jiang, Y.; Weaver, K.N.; Beaulieu, D.B.; Scherl, E.J.; Mahadevan, U. Maternal and Neonatal Outcomes in Vedolizumab- and Ustekinumab-Exposed Pregnancies: Results from the PIANO Registry. Am. J. Gastroenterol. 2024, 119, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Ghalandari, N.; Crijns, H.J.M.J.; Bergman, J.E.H.; Dolhain, R.J.E.M.; van Puijenbroek, E.P.; Hazes, J.M.W. Reported congenital malformations after exposure to non-tumour necrosis factor inhibitor biologics: A retrospective comparative study in EudraVigilance. Br. J. Clin. Pharmacol. 2022, 88, 5378–5388. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.D.; Jo, H.; Cho, H.; Jang, W.; Park, J.; Lee, S.; Lee, H.; Lee, K.; Oh, J.; Wen, X.; et al. Biologics Use for Psoriasis during Pregnancy and Its Related Adverse Outcomes in Pregnant Women and Newborns: Findings from WHO Pharmacovigilance Study. Int. Arch. Allergy Immunol. 2024, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.; Azevedo, A.; Nogueira, M.; Torres, T. Management of psoriasis in pregnancy—A review of the evidence to date. Drugs Context 2020, 9, 2019-11-6. [Google Scholar] [CrossRef]
- Dathe, K.; Schaefer, C. Drug safety in pregnancy: The German Embryotox institute. Eur. J. Clin. Pharmacol. 2018, 74, 171–179. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Gubatan, J.M.; Juhl, C.B.; Streett, S.E.; Maxwell, C. Biologics for Inflammatory Bowel Disease and Their Safety in Pregnancy: A Systematic Review and Meta-analysis. Clin. Gastroenterol. Hepatol. 2022, 20, 74–87.e3. [Google Scholar] [CrossRef]
- D’Gama, J.D.; Bermas, B.L. Safety of biologic agents for the management of rheumatic diseases during pregnancy. Curr. Opin. Rheumatol. 2024, 36, 184–190. [Google Scholar] [CrossRef]
- Russell, M.D.; Dey, M.; Flint, J.; Davie, P.; Allen, A.; Crossley, A.; Frishman, M.; Gayed, M.; Hodson, K.; Khamashta, M.; et al. Executive Summary: British Society for Rheumatology guideline on prescribing drugs in pregnancy and breastfeeding: Immunomodulatory anti-rheumatic drugs and corticosteroids. Rheumatology 2023, 62, 1370–1387. [Google Scholar] [CrossRef]
- Murase, J.E.; Chan, K.K.; Garite, T.J.; Cooper, D.M.; Weinstein, G.D. Hormonal effect on psoriasis in pregnancy and post-partum. Arch. Dermatol. 2005, 141, 601–606. [Google Scholar] [CrossRef]

| Men | |||||||||||
| Responder to survey | Non-Responder to survey | ||||||||||
| N | Min | Max | Mean/% | SD | N | Min | Max | Mean/% | SD | p-value | |
| Age | 248 | 18.0 | 49.0 | 38.4 | 7.4 | 548 | 18.0 | 52.0 | 36.9 | 8.3 | 0.014 |
| BMI | 244 | 19.8 | 54.9 | 28.4 | 5.3 | 538 | 17.2 | 56.3 | 28.4 | 5.4 | 0.970 |
| PsA (%) | 248 | n.a | n.a | 19.4 | n.a | 548 | n.a | n.a | 18.6 | n.a | 0.804 |
| PASI | 241 | 0.0 | 59.4 | 16.4 | 11.0 | 533 | 0.0 | 57.6 | 16.3 | 10.7 | 0.990 |
| BSA | 236 | 0.0 | 100.0 | 25.6 | 20.1 | 525 | 0.0 | 100.0 | 26.4 | 21.2 | 0.604 |
| DLQI | 229 | 0.0 | 28.0 | 10.5 | 6.8 | 510 | 0.0 | 30.0 | 12.1 | 7.6 | 0.005 |
| Women | |||||||||||
| Responder to survey | Non-Responder to survey | ||||||||||
| N | Min | Max | Mean/% | SD | N | Min | Max | Mean/% | SD | p-value | |
| Age | 150 | 21.0 | 51.0 | 37.6 | 7.7 | 348 | 18.0 | 52.0 | 35.8 | 8.4 | 0.026 |
| BMI | 147 | 18.4 | 52.1 | 28.3 | 7.2 | 345 | 15.6 | 57.9 | 28.1 | 7.2 | 0.798 |
| PsA (%) | 150 | n.a | n.a | 22.7 | n.a | 348 | n.a | n.a | 21.3 | n.a | 0.728 |
| PASI | 146 | 0.6 | 57.6 | 14.4 | 9.0 | 344 | 0.0 | 63.6 | 14.7 | 10.2 | 0.747 |
| BSA | 142 | 1.0 | 90.0 | 25.4 | 20.9 | 332 | 0.0 | 90.0 | 24.3 | 19.9 | 0.585 |
| DLQI | 145 | 0.0 | 29.0 | 14.3 | 7.4 | 316 | 0.0 | 30.0 | 14.1 | 7.4 | 0.780 |
| Men Surveyed with Wish to Conceive (n = 20) | Men Surveyed Without Wish to Conceive (n = 229) | p | Women Surveyed with Wish to Conceive (n = 19) | Women Surveyed Without Wish to Conceive (n = 125) | p | |
|---|---|---|---|---|---|---|
| Biological treatment | 17 (85.0) | 190 (82.9) | >0.999 | 13 (68.4) | 81 (64.8) | >0.999 |
| TNF-alpha inhibitors (other than Certolizumab) | 4 (20.0) | 28 (12.2) | >0.999 | 1 (5.3) | 6 (4.8) | >0.999 |
| Certolizumab | 0 (0.0) | 0 (0.0) | n.a | 3 (15.8) | 1 (0.8) | 0.113 |
| IL-17 inhibitors | 7 (35.0) | 73 (31.9) | >0.999 | 4 (21.1) | 29 (23.2) | >0.999 |
| IL-23 inhibitors or IL-12/23 inhibitors | 6 (30.0) | 89 (38.8) | >0.999 | 5 (26.3) | 45 (36.0) | >0.999 |
| Other biological treatment | 0 (0.0) | 0 (0.0) | n.a | 0 (0.0) | 0 (0.0) | n.a |
| Non biological treatment | 2 (10.0) | 20 (8.7) | >0.999 | 1 (5.3) | 15 (12.0) | >0.999 |
| PDE-4 inhibitors (Apremilast) | 0 (0.0) | 2 (0.9) | >0.999 | 0 (0.0) | 0 (0.0) | n.a |
| Ciclosporin | 0 (0.0) | 0 (0.0) | n.a | 0 (0.0) | 0 (0.0) | n.a |
| Methotrexate (MTX) | 2 (10.0) | 13 (5.7) | >0.999 | 0 (0.0) | 10 (8.0) | >0.999 |
| Fumaric acid esters | 0 (0.0) | 5 (2.2) | >0.999 | 1 (5.3) | 3 (2.4) | >0.999 |
| JAK inhibitors | 0 (0.0) | 0 (0.0) | n.a | 0 (0.0) | 2 (1.6) | >0.999 |
| Other non-biological treatment | 0 (0.0) | 0 (0.0) | n.a | 0 (0.0) | 0 (0.0) | n.a |
| No regular systemic therapy/unknown | 2 (10.0) | 23 (10.0) | >0.999 | 5 (26.3) | 31 (24.8) | >0.999 |
| Men: Systemic Therapy at the Time of Fathering (n = 47 Events) | Women: Systemic Therapy at the Time of Pregnancy (n = 20 Events) | Male PsoBest Patients Registered in 2018/2019 (n = 2730) | Female PsoBest Patients Registered in 2018/2019 (n = 1888) | |
|---|---|---|---|---|
| Biological treatment | 31 (65.9) | 8 (40.0) | 1317 (48.2) | 849 (44.9) |
| TNF-alpha inhibitors (other than Certolizumab) | 9 (19.1) | 2 (10.0) | 235 (8.6) | 160 (8.5) |
| Certolizumab | 0 (0.0) | 3 (15.0) | 12 (0.4) | 54 (2.9) |
| IL-17 inhibitors | 12 (25.5) | 3 (15.0) | 566 (20.7) | 341 (18.1) |
| IL-23 inhibitors or IL-12/23 inhibitors | 10 (21.3) | 0 (0.0) | 504 (18.5) | 294 (15.6) |
| Other biological treatment | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Non biological treatment | 6 (12.8) | 1 (5.0) | 1464 (53.6) | 1083 (57.4) |
| PDE-4 inhibitors (Apremilast) | 0 (0.0) | 0 (0.0) | 80 (2.9) | 93 (4.9) |
| Ciclosporin | 1 (2.1) | 0 (0.0) | 29 (1.1) | 19 (1.0) |
| Methotrexate | 1 (2.1) | 0 (0.0) | 543 (19.9) | 385 (20.4) |
| Fumaric acid esters | 4 (8.5) | 1 (5.0) | 765 (28.0) | 546 (28.9) |
| JAK inhibitors | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other non-biological treatment | 0 (0.0) | 0 (0.0) | 47 (1.7) | 40 (2.1) |
| No regular systemic therapy/unknown | 10 (21.3) | 11 (55.0) | 0 (0.0) | 0 (0.0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephan, B.; Sorbe, C.; Zyriax, B.-C.; Schmittinger, J.; Augustin, M.; Sommer, R.; da Silva Burger, N.M.B.; Weyergraf, A.; von Kiedrowski, R.; Kühl, L. Communication and Therapy Planning for Patients of Reproductive Age Under Immunomodulatory Treatments for Psoriasis or Psoriatic Arthritis—Survey of the German National Psoriasis Registry PsoBest. Healthcare 2025, 13, 1017. https://doi.org/10.3390/healthcare13091017
Stephan B, Sorbe C, Zyriax B-C, Schmittinger J, Augustin M, Sommer R, da Silva Burger NMB, Weyergraf A, von Kiedrowski R, Kühl L. Communication and Therapy Planning for Patients of Reproductive Age Under Immunomodulatory Treatments for Psoriasis or Psoriatic Arthritis—Survey of the German National Psoriasis Registry PsoBest. Healthcare. 2025; 13(9):1017. https://doi.org/10.3390/healthcare13091017
Chicago/Turabian StyleStephan, Brigitte, Christina Sorbe, Birgit-Christiane Zyriax, Janne Schmittinger, Matthias Augustin, Rachel Sommer, Neuza Maria Bernardino da Silva Burger, Ansgar Weyergraf, Ralph von Kiedrowski, and Laura Kühl. 2025. "Communication and Therapy Planning for Patients of Reproductive Age Under Immunomodulatory Treatments for Psoriasis or Psoriatic Arthritis—Survey of the German National Psoriasis Registry PsoBest" Healthcare 13, no. 9: 1017. https://doi.org/10.3390/healthcare13091017
APA StyleStephan, B., Sorbe, C., Zyriax, B.-C., Schmittinger, J., Augustin, M., Sommer, R., da Silva Burger, N. M. B., Weyergraf, A., von Kiedrowski, R., & Kühl, L. (2025). Communication and Therapy Planning for Patients of Reproductive Age Under Immunomodulatory Treatments for Psoriasis or Psoriatic Arthritis—Survey of the German National Psoriasis Registry PsoBest. Healthcare, 13(9), 1017. https://doi.org/10.3390/healthcare13091017






