Associations Between Physical Capability Markers and Risk of Coronary Artery Disease: A Prospective Study of 439,295 UK Biobank Participants
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Assessment of Sarcopenia
2.3. Outcome
2.4. Covariates
2.5. Genetic Risk Score for Coronary Artery Disease
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Crea, F. The burden of cardiovascular risk factors: A global perspective. Eur. Heart J. 2022, 43, 2817–2820. [Google Scholar] [CrossRef]
- Vaduganathan, M.; Mensah, G.A.; Turco, J.V.; Fuster, V.; Roth, G.A. The Global Burden of Cardiovascular Diseases and Risk: A Compass for Future Health. J. Am. Coll. Cardiol. 2022, 80, 2361–2371. [Google Scholar] [CrossRef] [PubMed]
- Tcheandjieu, C.; Zhu, X.; Hilliard, A.T.; Clarke, S.L.; Napolioni, V.; Ma, S.; Lee, K.M.; Fang, H.; Chen, F.; Lu, Y.; et al. Large-scale genome-wide association study of coronary artery disease in genetically diverse populations. Nat. Med. 2022, 28, 1679–1692. [Google Scholar] [CrossRef]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Lu, X.; Liu, Z.; Cui, Q.; Liu, F.; Li, J.; Niu, X.; Shen, C.; Hu, D.; Huang, K.; Chen, J.; et al. A polygenic risk score improves risk stratification of coronary artery disease: A large-scale prospective Chinese cohort study. Eur. Heart J. 2022, 43, 1702–1711. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E.; Kulkarni, A.; German, C.; Satish, P.; Iluyomade, A.; Dudum, R.; Thakkar, A.; Rifai, M.A.; Mehta, A.; Thobani, A.; et al. Ten things to know about ten cardiovascular disease risk factors—2022. Am. J. Prev. Cardiol. 2022, 10, 100342. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Sayer, A.A.; Cruz-Jentoft, A. Sarcopenia definition, diagnosis and treatment: Consensus is growing. Age Ageing 2022, 51, afac220. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Sayer, A.A. Sarcopenia. Lancet 2019, 393, 2636–2646. [Google Scholar] [CrossRef]
- Gielen, E.; O’Neill, T.W.; Pye, S.R.; Adams, J.E.; Wu, F.C.; Laurent, M.R.; Claessens, F.; Ward, K.A.; Boonen, S.; Bouillon, R.; et al. Endocrine determinants of incident sarcopenia in middle-aged and elderly European men. J. Cachexia Sarcopenia Muscle 2015, 6, 242–252. [Google Scholar] [CrossRef]
- Dodds, R.M.; Granic, A.; Davies, K.; Kirkwood, T.B.; Jagger, C.; Sayer, A.A. Prevalence and incidence of sarcopenia in the very old: Findings from the Newcastle 85+ Study. J. Cachexia Sarcopenia Muscle 2017, 8, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Wong, M.; Leung, J.; Lee, J.; Auyeung, T.W.; Woo, J. Incidence, reversibility, risk factors and the protective effect of high body mass index against sarcopenia in community-dwelling older Chinese adults. Geriatr. Gerontol. Int. 2014, 14 (Suppl. S1), 15–28. [Google Scholar] [CrossRef]
- Zuo, X.; Li, X.; Tang, K.; Zhao, R.; Wu, M.; Wang, Y.; Li, T. Sarcopenia and cardiovascular diseases: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2023, 14, 1183–1198. [Google Scholar] [CrossRef]
- Xia, M.F.; Chen, L.Y.; Wu, L.; Ma, H.; Li, X.M.; Li, Q.; Aleteng, Q.; Hu, Y.; He, W.Y.; Gao, J.; et al. Sarcopenia, sarcopenic overweight/obesity and risk of cardiovascular disease and cardiac arrhythmia: A cross-sectional study. Clin. Nutr. 2021, 40, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.E.; Kang, M.; Jin, S.M.; Kim, K.; Hwang, Y.C.; Jeong, I.K.; Kim, J.H. Additive effect of low skeletal muscle mass and abdominal obesity on coronary artery calcification. Eur. J. Endocrinol. 2021, 184, 867–877. [Google Scholar] [CrossRef]
- Campos, A.M.; Moura, F.A.; Santos, S.N.; Freitas, W.M.; Sposito, A.C. Sarcopenia, but not excess weight or increased caloric intake, is associated with coronary subclinical atherosclerosis in the very elderly. Atherosclerosis 2017, 258, 138–144. [Google Scholar] [CrossRef]
- Jun, J.E.; Choi, M.S.; Park, S.W.; Kim, G.; Jin, S.M.; Kim, K.; Hwang, Y.C.; Ahn, K.J.; Chung, H.Y.; Jeong, I.K.; et al. Low Skeletal Muscle Mass Is Associated with the Presence, Incidence, and Progression of Coronary Artery Calcification. Can. J. Cardiol. 2021, 37, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Ko, B.J.; Chang, Y.; Jung, H.S.; Yun, K.E.; Kim, C.W.; Park, H.S.; Chung, E.C.; Shin, H.; Ryu, S. Relationship Between Low Relative Muscle Mass and Coronary Artery Calcification in Healthy Adults. Arterioscler. Thromb. Vasc. Biol. 2016, 36, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Kang, D.O.; Park, S.Y.; Choi, B.G.; Na, J.O.; Choi, C.U.; Kim, E.J.; Rha, S.W.; Park, C.G.; Hong, S.J.; Seo, H.S. Prognostic Impact of Low Skeletal Muscle Mass on Major Adverse Cardiovascular Events in Coronary Artery Disease: A Propensity Score-Matched Analysis of a Single Center All-Comer Cohort. J. Clin. Med. 2019, 8, 712. [Google Scholar] [CrossRef]
- Liu, H.M.; Zhang, Q.; Shen, W.D.; Li, B.Y.; Lv, W.Q.; Xiao, H.M.; Deng, H.W. Sarcopenia-related traits and coronary artery disease: A bi-directional Mendelian randomization study. Aging 2020, 12, 3340–3353. [Google Scholar] [CrossRef]
- Tikkanen, E.; Gustafsson, S.; Ingelsson, E. Associations of Fitness, Physical Activity, Strength, and Genetic Risk With Cardiovascular Disease: Longitudinal Analyses in the UK Biobank Study. Circulation 2018, 137, 2583–2591. [Google Scholar] [CrossRef] [PubMed]
- Hadley, T.D.; Agha, A.M.; Ballantyne, C.M. How Do We Incorporate Polygenic Risk Scores in Cardiovascular Disease Risk Assessment and Management? Curr. Atheroscler. Rep. 2021, 23, 28. [Google Scholar] [CrossRef] [PubMed]
- Collins, R. What makes UK Biobank special? Lancet 2012, 379, 1173–1174. [Google Scholar] [CrossRef]
- Sudlow, C.; Gallacher, J.; Allen, N.; Beral, V.; Burton, P.; Danesh, J.; Downey, P.; Elliott, P.; Green, J.; Landray, M.; et al. UK biobank: An open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015, 12, e1001779. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyère, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Gray, S.R.; Forrest, E.; Welsh, P.; Sattar, N.; Celis-Morales, C.; Ho, F.K.; Pell, J.P. Associations of muscle mass and grip strength with severe NAFLD: A prospective study of 333,295 UK Biobank participants. J. Hepatol. 2022, 76, 1021–1029. [Google Scholar] [CrossRef]
- Esteban-Cornejo, I.; Ho, F.K.; Petermann-Rocha, F.; Lyall, D.M.; Martinez-Gomez, D.; Cabanas-Sánchez, V.; Ortega, F.B.; Hillman, C.H.; Gill, J.M.R.; Quinn, T.J.; et al. Handgrip strength and all-cause dementia incidence and mortality: Findings from the UK Biobank prospective cohort study. J. Cachexia Sarcopenia Muscle 2022, 13, 1514–1525. [Google Scholar] [CrossRef]
- Ho, F.K.W.; Celis-Morales, C.A.; Petermann-Rocha, F.; Sillars, A.; Welsh, P.; Welsh, C.; Anderson, J.; Lyall, D.M.; Mackay, D.F.; Sattar, N.; et al. The association of grip strength with health outcomes does not differ if grip strength is used in absolute or relative terms: A prospective cohort study. Age Ageing 2019, 48, 684–691. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Ho, F.K.; Foster, H.; Boopor, J.; Parra-Soto, S.; Gray, S.R.; Mathers, J.C.; Celis-Morales, C.; Pell, J.P. Nonlinear Associations Between Cumulative Dietary Risk Factors and Cardiovascular Diseases, Cancer, and All-Cause Mortality: A Prospective Cohort Study from UK Biobank. Mayo Clin. Proc. 2021, 96, 2418–2431. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Wirth, M.D.; Boonpor, J.; Parra-Soto, S.; Zhou, Z.; Mathers, J.C.; Livingstone, K.; Forrest, E.; Pell, J.P.; Ho, F.K.; et al. Associations between an inflammatory diet index and severe non-alcoholic fatty liver disease: A prospective study of 171,544 UK Biobank participants. BMC Med. 2023, 21, 123. [Google Scholar] [CrossRef]
- Kassi, E.; Pervanidou, P.; Kaltsas, G.; Chrousos, G. Metabolic syndrome: Definitions and controversies. BMC Med. 2011, 9, 48. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef] [PubMed]
- Bycroft, C.; Freeman, C.; Petkova, D.; Band, G.; Elliott, L.T.; Sharp, K.; Motyer, A.; Vukcevic, D.; Delaneau, O.; O’Connell, J.; et al. The UK Biobank resource with deep phenotyping and genomic data. Nature 2018, 562, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Thompson, D.J.; Wells, D.; Selzam, S.; Peneva, I.; Moore, R.; Sharp, K.; Tarran, W.A.; Beard, E.J.; Riveros-Mckay, F.; Palmer, D.; et al. UK Biobank release and systematic evaluation of optimised polygenic risk scores for 53 diseases and quantitative traits. medRxiv 2022. [Google Scholar] [CrossRef]
- Welsh, C.E.; Celis-Morales, C.A.; Ho, F.K.; Brown, R.; Mackay, D.F.; Lyall, D.M.; Anderson, J.J.; Pell, J.P.; Gill, J.M.R.; Sattar, N.; et al. Grip Strength and Walking Pace and Cardiovascular Disease Risk Prediction in 406,834 UK Biobank Participants. Mayo Clin. Proc. 2020, 95, 879–888. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Gray, S.R.; Pell, J.P.; Ho, F.K.; Celis-Morales, C. The joint association of sarcopenia and frailty with incidence and mortality health outcomes: A prospective study. Clin. Nutr. 2021, 40, 2427–2434. [Google Scholar] [CrossRef]
- Petermann-Rocha, F.; Ho, F.K.; Welsh, P.; Mackay, D.; Brown, R.; Gill, J.M.R.; Sattar, N.; Gray, S.R.; Pell, J.P.; Celis-Morales, C.A. Physical capability markers used to define sarcopenia and their association with cardiovascular and respiratory outcomes and all-cause mortality: A prospective study from UK Biobank. Maturitas 2020, 138, 69–75. [Google Scholar] [CrossRef]
- Yates, T.; Zaccardi, F.; Dhalwani, N.N.; Davies, M.J.; Bakrania, K.; Celis-Morales, C.A.; Gill, J.M.R.; Franks, P.W.; Khunti, K. Association of walking pace and handgrip strength with all-cause, cardiovascular, and cancer mortality: A UK Biobank observational study. Eur. Heart J. 2017, 38, 3232–3240. [Google Scholar] [CrossRef]
- Celis-Morales, C.A.; Petermann, F.; Hui, L.; Lyall, D.M.; Iliodromiti, S.; McLaren, J.; Anderson, J.; Welsh, P.; Mackay, D.F.; Pell, J.P.; et al. Associations Between Diabetes and Both Cardiovascular Disease and All-Cause Mortality Are Modified by Grip Strength: Evidence From UK Biobank, a Prospective Population-Based Cohort Study. Diabetes Care 2017, 40, 1710–1718. [Google Scholar] [CrossRef]
- Wei, L.; Zeng, J.; Fan, M.; Chen, B.; Li, X.; Li, Y.; Xu, S. Associations between handgrip strength and skeletal muscle mass with all-cause mortality and cardiovascular mortality in people with type 2 diabetes: A prospective cohort study of the UK Biobank. J. Diabetes 2023, 16, e13464. [Google Scholar] [CrossRef]
- Knowles, R.; Carter, J.; Jebb, S.A.; Bennett, D.; Lewington, S.; Piernas, C. Associations of Skeletal Muscle Mass and Fat Mass With Incident Cardiovascular Disease and All-Cause Mortality: A Prospective Cohort Study of UK Biobank Participants. J. Am. Heart Assoc. 2021, 10, e019337. [Google Scholar] [CrossRef]
- Silventoinen, K.; Magnusson, P.K.; Tynelius, P.; Batty, G.D.; Rasmussen, F. Association of body size and muscle strength with incidence of coronary heart disease and cerebrovascular diseases: A population-based cohort study of one million Swedish men. Int. J. Epidemiol. 2009, 38, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Baskin, K.K.; Winders, B.R.; Olson, E.N. Muscle as a “mediator” of systemic metabolism. Cell Metab. 2015, 21, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Gallagher, D.; Belmonte, D.; Deurenberg, P.; Wang, Z.; Krasnow, N.; Pi-Sunyer, F.X.; Heymsfield, S.B. Organ-tissue mass measurement allows modeling of REE and metabolically active tissue mass. Am. J. Physiol. 1998, 275, E249–E258. [Google Scholar] [CrossRef]
- Shoemaker, M.E.; Pereira, S.L.; Mustad, V.A.; Gillen, Z.M.; McKay, B.D.; Lopez-Pedrosa, J.M.; Rueda, R.; Cramer, J.T. Differences in muscle energy metabolism and metabolic flexibility between sarcopenic and nonsarcopenic older adults. J. Cachexia Sarcopenia Muscle 2022, 13, 1224–1237. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.F.; Befroy, D.; Dufour, S.; Dziura, J.; Ariyan, C.; Rothman, D.L.; DiPietro, L.; Cline, G.W.; Shulman, G.I. Mitochondrial dysfunction in the elderly: Possible role in insulin resistance. Science 2003, 300, 1140–1142. [Google Scholar] [CrossRef]
- Henriksen, T.; Green, C.; Pedersen, B.K. Myokines in myogenesis and health. Recent Pat. Biotechnol. 2012, 6, 167–171. [Google Scholar] [CrossRef]
- Barros, D.; Marques, E.A.; Magalhães, J.; Carvalho, J. Energy metabolism and frailty: The potential role of exercise-induced myokines—A narrative review. Ageing Res. Rev. 2022, 82, 101780. [Google Scholar] [CrossRef]
- Huh, J.Y.; Mougios, V.; Kabasakalis, A.; Fatouros, I.; Siopi, A.; Douroudos, I.I.; Filippaios, A.; Panagiotou, G.; Park, K.H.; Mantzoros, C.S. Exercise-induced irisin secretion is independent of age or fitness level and increased irisin may directly modulate muscle metabolism through AMPK activation. J. Clin. Endocrinol. Metab. 2014, 99, E2154–E2161. [Google Scholar] [CrossRef]
- Zheng, S.; Chen, N.; Kang, X.; Hu, Y.; Shi, S. Irisin alleviates FFA induced β-cell insulin resistance and inflammatory response through activating PI3K/AKT/FOXO1 signaling pathway. Endocrine 2022, 75, 740–751. [Google Scholar] [CrossRef]
- Ye, W.; Wang, J.; Lin, D.; Ding, Z. The immunomodulatory role of irisin on osteogenesis via AMPK-mediated macrophage polarization. Int. J. Biol. Macromol. 2020, 146, 25–35. [Google Scholar] [CrossRef] [PubMed]
- Koeth, R.A.; Wang, Z.; Levison, B.S.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Shi, Y.; Wiklund, P.; Tan, X.; Wu, N.; Zhang, X.; Tikkanen, O.; Zhang, C.; Munukka, E.; Cheng, S. The Association between Cardiorespiratory Fitness and Gut Microbiota Composition in Premenopausal Women. Nutrients 2017, 9, 792. [Google Scholar] [CrossRef]
- Fiuza-Luces, C.; Santos-Lozano, A.; Joyner, M.; Carrera-Bastos, P.; Picazo, O.; Zugaza, J.L.; Izquierdo, M.; Ruilope, L.M.; Lucia, A. Exercise benefits in cardiovascular disease: Beyond attenuation of traditional risk factors. Nat. Rev. Cardiol. 2018, 15, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Fülster, S.; Tacke, M.; Sandek, A.; Ebner, N.; Tschöpe, C.; Doehner, W.; Anker, S.D.; von Haehling, S. Muscle wasting in patients with chronic heart failure: Results from the studies investigating co-morbidities aggravating heart failure (SICA-HF). Eur. Heart J. 2013, 34, 512–519. [Google Scholar] [CrossRef]
- Hajahmadi, M.; Shemshadi, S.; Khalilipur, E.; Amin, A.; Taghavi, S.; Maleki, M.; Malek, H.; Naderi, N. Muscle wasting in young patients with dilated cardiomyopathy. J. Cachexia Sarcopenia Muscle 2017, 8, 542–548. [Google Scholar] [CrossRef]
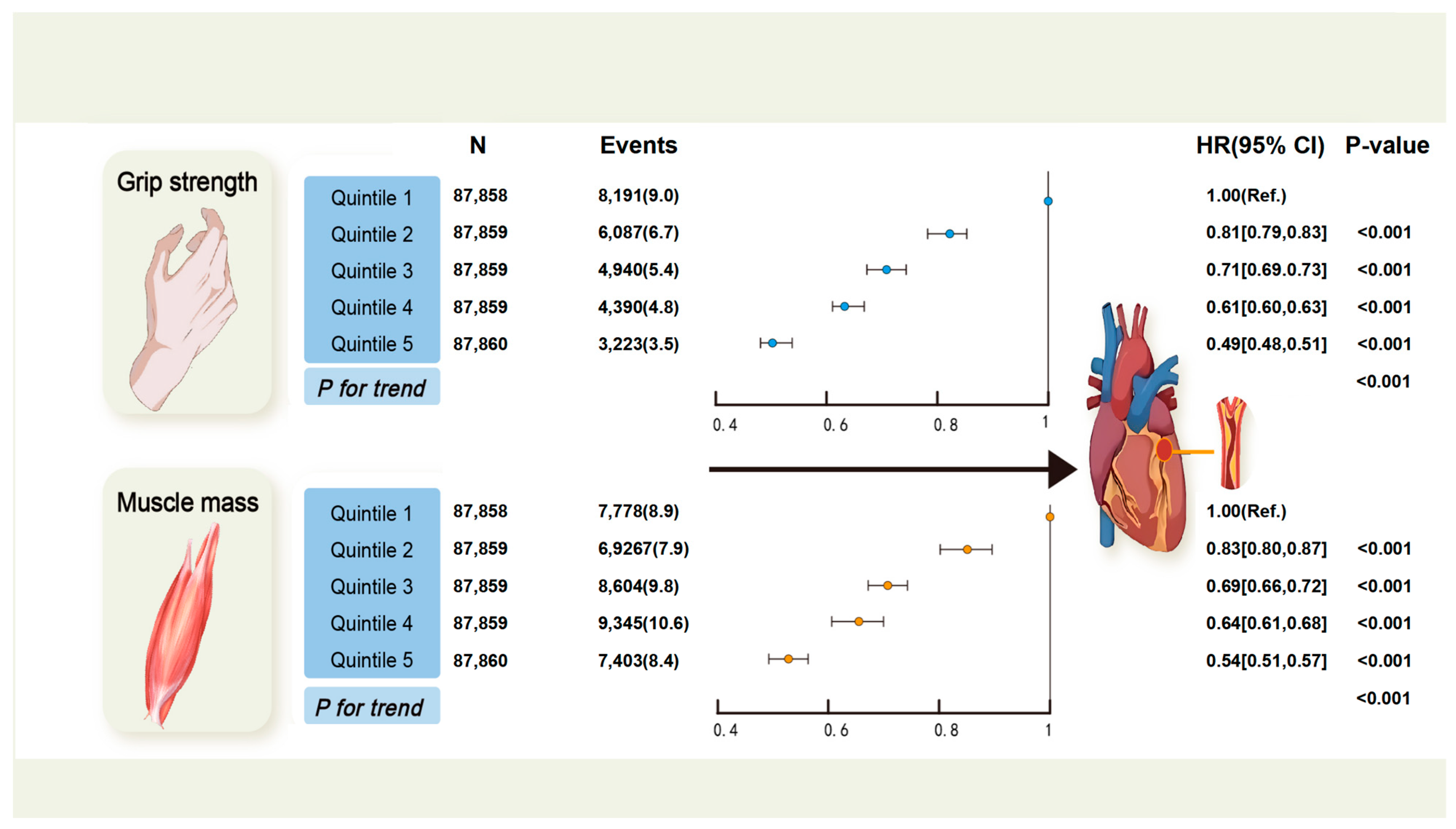
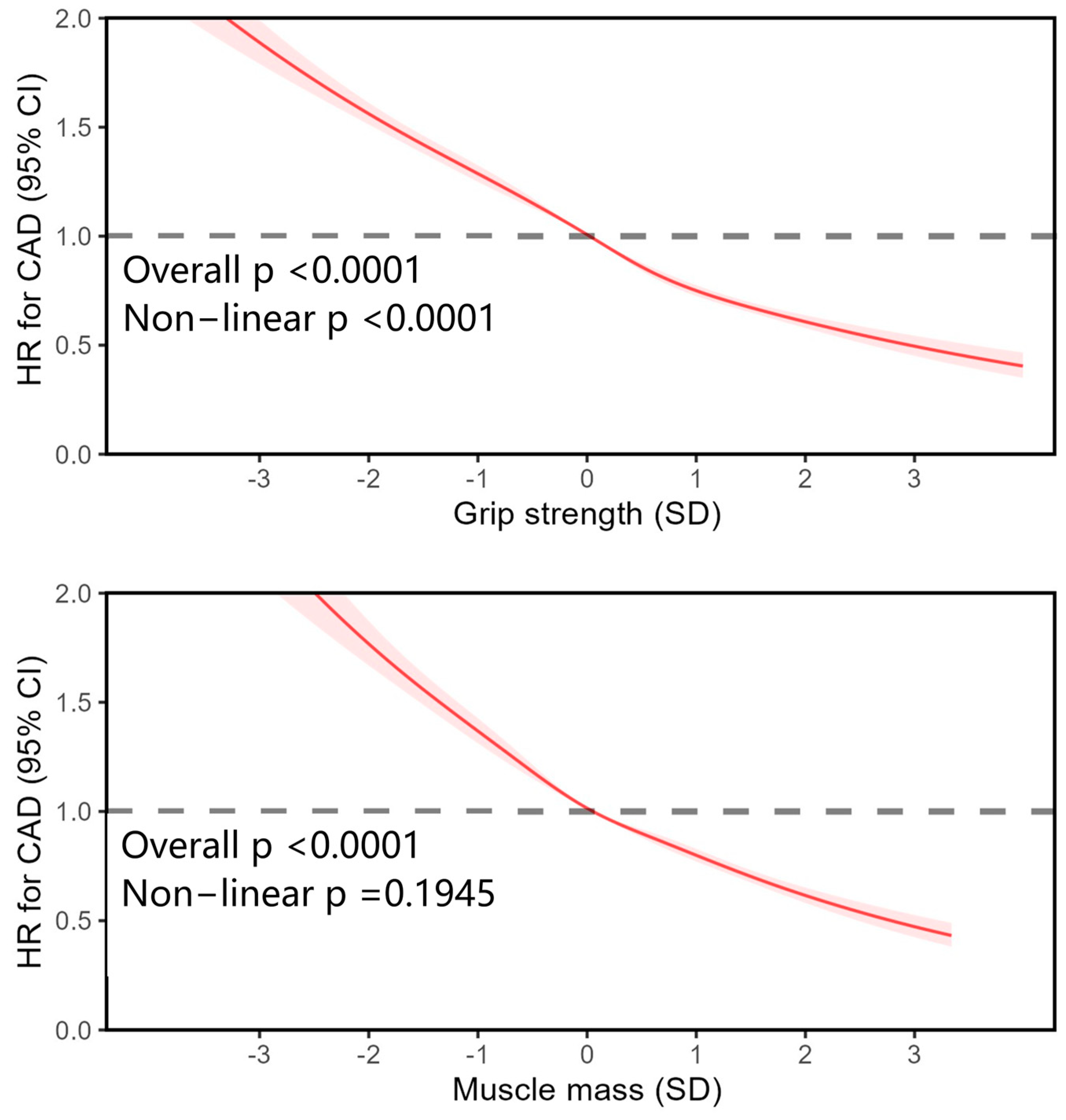
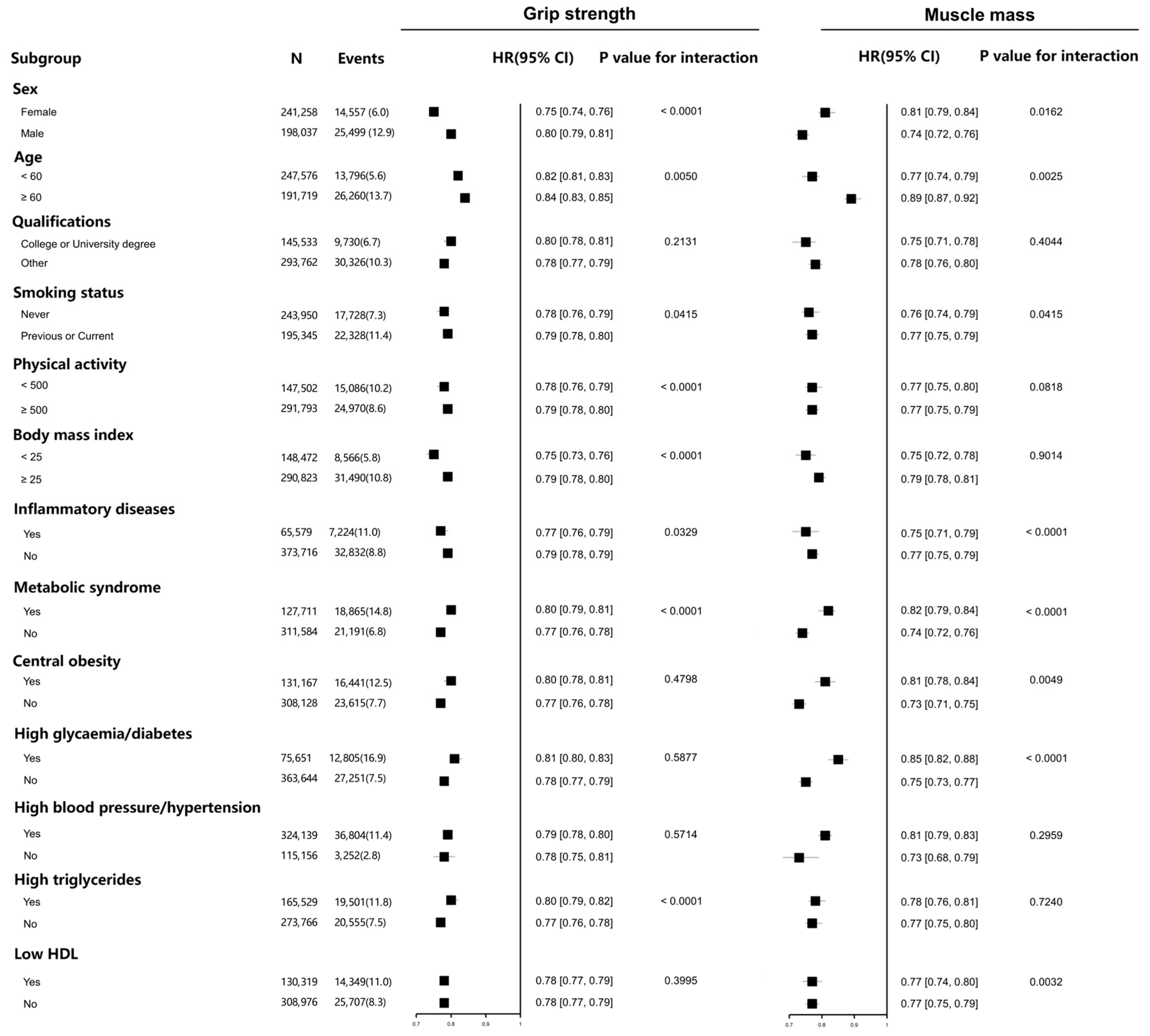
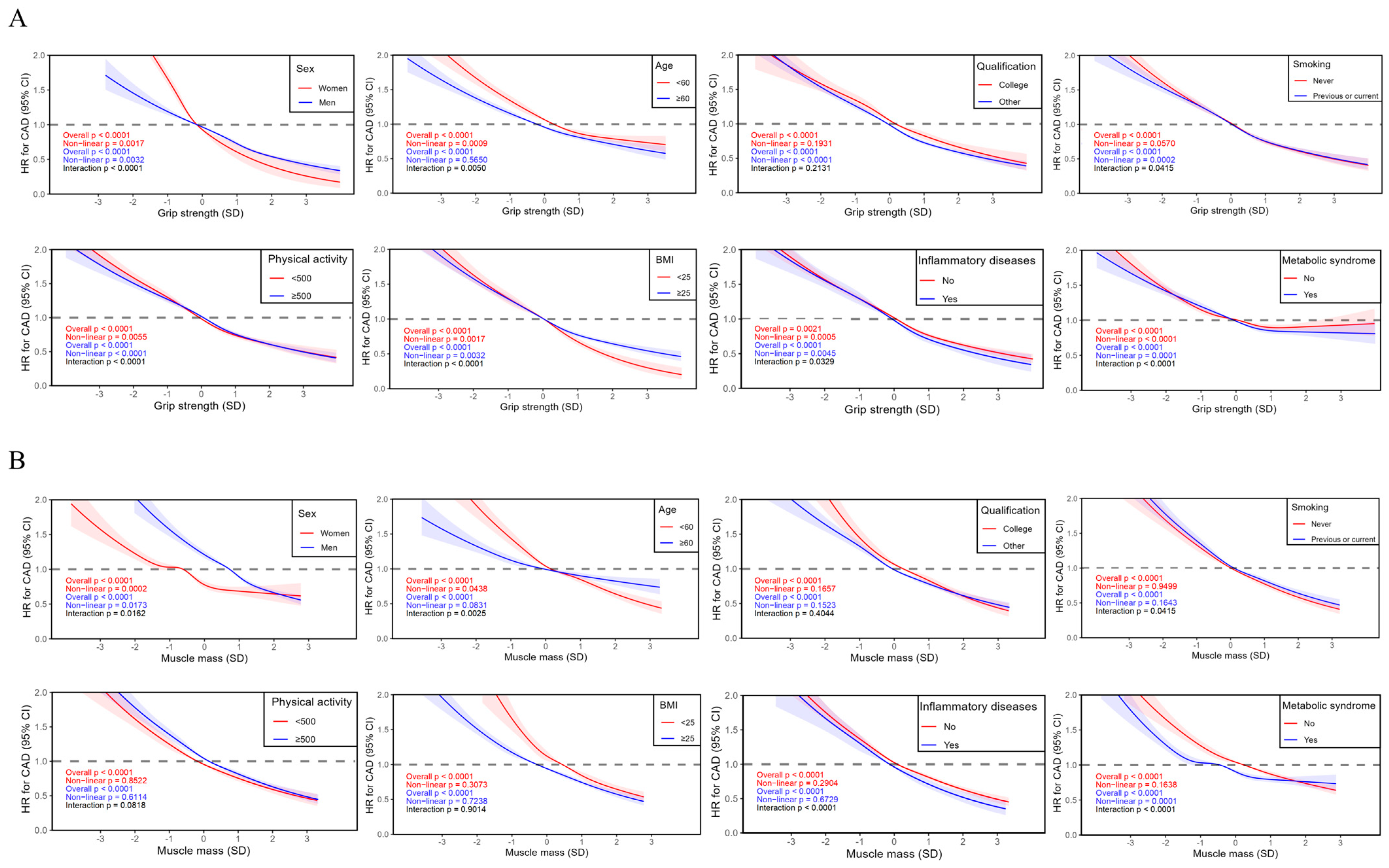
| Characteristic | Total | Quintiles of Grip Strength | Quintiles of Muscle Mass | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 (the Lowest) | 2 | 3 | 4 | 5 (the Highest) | 1 (the Lowest) | 2 | 3 | 4 | 5 (the Highest) | ||
| n | 439,295 | 87,858 | 87,859 | 87,859 | 87,859 | 87,860 | 87,858 | 87,859 | 87,859 | 87,859 | 87,860 |
| Sex (male), n (%) | 198,037 (45.1) | 39,577 (45.0) | 39,475 (44.9) | 38,146 (43.4) | 42,501 (48.4) | 38,338 (43.6) | 1552 (1.8) | 11,685 (13.3) | 39,927 (45.4) | 66,063 (75.2) | 78,810 (89.7) |
| Age, (mean (SD)) | 56.73 (8.10) | 59.75 (7.41) | 58.55 (7.66) | 57.12 (7.86) | 55.58 (7.92) | 52.64 (7.71) | 57.82 (7.68) | 57.46 (7.86) | 56.90 (8.08) | 56.39 (8.23) | 55.07 (8.35) |
| Deprivation, n (%) | |||||||||||
| Least deprived | 87,858 (20.0) | 15,113 (17.2) | 18,485 (21.0) | 17,360 (19.8) | 18,183 (20.7) | 18,717 (21.3) | 15,555 (17.7) | 17,654 (20.1) | 18,096 (20.6) | 18,356 (20.9) | 18,197 (20.7) |
| Second least deprived | 87,859 (20.0) | 15,989 (18.2) | 18,856 (21.5) | 17,200 (19.6) | 17,998 (20.5) | 17,816 (20.3) | 16,433 (18.7) | 17,911 (20.4) | 17,794 (20.3) | 17,909 (20.4) | 17,812 (20.3) |
| Medium deprived | 87,859 (20.0) | 17,009 (19.4) | 16,541 (18.8) | 19,414 (22.1) | 17,385 (19.8) | 17,510 (19.9) | 17,220 (19.6) | 18,145 (20.7) | 17,646 (20.1) | 17,766 (20.2) | 17,082 (19.4) |
| Second most deprived | 87,859 (20.0) | 18,153 (20.7) | 16,797 (19.1) | 17,925 (20.4) | 17,592 (20.0) | 17,392 (19.8) | 18,278 (20.8) | 17,548 (20.0) | 17,370 (19.8) | 17,270 (19.7) | 17,393 (19.8) |
| Most deprived | 87,860 (20.0) | 21,594 (24.6) | 17,180 (19.6) | 15,960 (18.2) | 16,701 (19.0) | 16,425 (18.7) | 20,372 (23.2) | 16,601 (18.9) | 16,953 (19.3) | 16,558 (18.8) | 17,376 (19.8) |
| Qualifications (College or University degree), n (%) | 293,762 (66.9) | 64,299 (73.2) | 60,701 (69.1) | 58,932 (67.1) | 56,281 (64.1) | 53,549 (60.9) | 66,014 (75.1) | 61,813 (70.4) | 59,099 (67.3) | 56,282 (64.1) | 50,554 (57.5) |
| Ethnicity, n (%) | |||||||||||
| White | 415,290 (94.5) | 80,828 (92.0) | 83,257 (94.8) | 83,783 (95.4) | 83,874 (95.5) | 83,548 (95.1) | 82,279 (93.6) | 83,503 (95.0) | 83,273 (94.8) | 83,029 (94.5) | 83,206 (94.7) |
| Mixed | 7793 (1.8) | 1907 (2.2) | 1486 (1.7) | 1401 (1.6) | 1492 (1.7) | 1507 (1.7) | 1678 (1.9) | 1470 (1.7) | 1567 (1.8) | 1487 (1.7) | 1591 (1.8) |
| South Asian | 7991 (1.8) | 3512 (4.0) | 1759 (2.0) | 1230 (1.4) | 919 (1.0) | 571 (0.6) | 1612 (1.8) | 1532 (1.7) | 1643 (1.9) | 1779 (2.0) | 1425 (1.6) |
| Black | 6852 (1.6) | 1231 (1.4) | 1066 (1.2) | 1162 (1.3) | 1349 (1.5) | 2044 (2.3) | 2237 (2.5) | 1165 (1.3) | 1091 (1.2) | 1206 (1.4) | 1153 (1.3) |
| Chinese | 1369 (0.3) | 380 (0.4) | 291 (0.3) | 283 (0.3) | 225 (0.3) | 190 (0.2) | 52 (0.1) | 189 (0.2) | 285 (0.3) | 358 (0.4) | 485 (0.6) |
| Smoking status, n (%) | |||||||||||
| Never | 243,950 (55.5) | 48,447 (55.1) | 48,722 (55.5) | 48,768 (55.5) | 48,533 (55.2) | 49,480 (56.3) | 50,964 (58.0) | 50,352 (57.3) | 46,809 (53.3) | 46,227 (52.6) | 49,598 (56.5) |
| Previous | 149,514 (34.0) | 30,198 (34.4) | 30,509 (34.7) | 30,236 (34.4) | 29,932 (34.1) | 28,639 (32.6) | 29,775 (33.9) | 29,607 (33.7) | 31,957 (36.4) | 31,803 (36.2) | 26,372 (30.0) |
| Current | 45,831 (10.4) | 9213 (10.5) | 8628 (9.8) | 8855 (10.1) | 9394 (10.7) | 9741 (11.1) | 7119 (8.1) | 7900 (9.0) | 9093 (10.3) | 9829 (11.2) | 11,890 (13.5) |
| Diet score, n (%) | |||||||||||
| Higher quintile | 9945 (2.3) | 1836 (2.1) | 2007 (2.3) | 2022 (2.3) | 2023 (2.3) | 2057 (2.3) | 2161 (2.5) | 2298 (2.6) | 1975 (2.2) | 1546 (1.8) | 1965 (2.2) |
| 4th quintile | 142,711 (32.5) | 27,624 (31.4) | 29,197 (33.2) | 29,272 (33.3) | 28,472 (32.4) | 28,146 (32.0) | 30,194 (34.4) | 31,760 (36.1) | 27,579 (31.4) | 26,141 (29.8) | 27,037 (30.8) |
| 3rd quintile | 212,420 (48.4) | 42,320 (48.2) | 42,007 (47.8) | 42,313 (48.2) | 42,857 (48.8) | 42,923 (48.9) | 43,147 (49.1) | 41,779 (47.6) | 42,633 (48.5) | 43,000 (48.9) | 41,861 (47.6) |
| 2nd quintile | 70,070 (16.0) | 14,998 (17.1) | 13,858 (15.8) | 13,474 (15.3) | 13,754 (15.7) | 13,986 (15.9) | 11,834 (13.5) | 11,473 (13.1) | 14,854 (16.9) | 16,143 (18.4) | 15,766 (17.9) |
| Lower quintile | 4149 (0.9) | 1080 (1.2) | 790 (0.9) | 778 (0.9) | 753 (0.9) | 748 (0.9) | 522 (0.6) | 549 (0.6) | 818 (0.9) | 1029 (1.2) | 1231 (1.4) |
| Physical activity, MET-min/week (%) | |||||||||||
| <500 | 147,502 (33.6) | 35,273 (40.1) | 30,642 (34.9) | 29,015 (33.0) | 27,026 (30.8) | 25,546 (29.1) | 38,747 (44.1) | 31,553 (35.9) | 28,845 (32.8) | 25,906 (29.5) | 22,451 (25.6) |
| ≥500 | 291,793 (66.4) | 52,585 (59.9) | 57,217 (65.1) | 58,844 (67.0) | 60,833 (69.2) | 62,314 (70.9) | 49,111 (55.9) | 56,306 (64.1) | 59,014 (67.2) | 61,953 (70.5) | 65,409 (74.4) |
| BMI, n (%) | |||||||||||
| <25 | 148,472 (33.8) | 28,231 (32.1) | 30,673 (34.9) | 30,854 (35.1) | 29,850 (34.0) | 28,864 (32.9) | 1907 (2.2) | 26,506 (30.2) | 39,458 (44.9) | 28,702 (32.7) | 51,899 (59.1) |
| ≥25 | 290,823 (66.2) | 59,627 (67.9) | 57,186 (65.1) | 57,005 (64.9) | 58,009 (66.0) | 58,996 (67.1) | 85,951 (97.8) | 61,353 (69.8) | 48,401 (55.1) | 59,157 (67.3) | 35,961 (40.9) |
| Inflammatory diseases (yes), n (%) | 65,579 (14.9) | 16,046 (18.3) | 12,906 (14.7) | 12,445 (14.2) | 12,122 (13.8) | 12,060 (13.7) | 17,043 (19.4) | 13,499 (15.4) | 12,287 (14.0) | 11,755 (13.4) | 10,995 (12.5) |
| Metabolic syndrome (yes), n (%) | 127,711 (29.1) | 30,621 (34.9) | 25,879 (29.5) | 24,513 (27.9) | 23,771 (27.1) | 22,927 (26.1) | 47,137 (53.7) | 24,224 (27.6) | 26,245 (29.9) | 20,731 (23.6) | 9374 (10.7) |
| Central obesity (yes), n (%) | 131,167 (29.9) | 30,361 (34.6) | 26,086 (29.7) | 25,157 (28.6) | 24,445 (27.8) | 25,118 (28.6) | 64,603 (73.5) | 25,272 (28.8) | 25,649 (29.2) | 13,720 (15.6) | 1923 (2.2) |
| High glycaemia/diabetes (yes), n (%) | 75,651 (17.2) | 19,809 (22.5) | 15,997 (18.2) | 14,665 (16.7) | 13,529 (15.4) | 11,651 (13.3) | 19,708 (22.4) | 15,129 (17.2) | 16,030 (18.2) | 14,340 (16.3) | 10,444 (11.9) |
| High blood pressure/hypertension (yes), n (%) | 324,139 (73.8) | 67,928 (77.3) | 66,157 (75.3) | 64,802 (73.8) | 63,806 (72.6) | 61,446 (69.9) | 70,697 (80.5) | 62,395 (71.0) | 63,377 (72.1) | 66,476 (75.7) | 61,194 (69.6) |
| High triglycerides (yes), n (%) | 165,529 (37.7) | 36,014 (41.0) | 33,707 (38.4) | 32,683 (37.2) | 32,599 (37.1) | 30,526 (34.7) | 38,602 (43.9) | 30,011 (34.2) | 32,522 (37.0) | 36,810 (41.9) | 27,584 (31.4) |
| Low HDL (yes), n (%) | 130,319 (29.7) | 28,100 (32.0) | 25,447 (29.0) | 25,301 (28.8) | 25,318 (28.8) | 26,153 (29.8) | 37,105 (42.2) | 27,688 (31.5) | 24,585 (28.0) | 22,690 (25.8) | 18,251 (20.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Yang, C.; Guo, T.; Guo, Y.; Xiong, J.; Chen, R.; Deng, S. Associations Between Physical Capability Markers and Risk of Coronary Artery Disease: A Prospective Study of 439,295 UK Biobank Participants. Healthcare 2025, 13, 1018. https://doi.org/10.3390/healthcare13091018
Liu D, Yang C, Guo T, Guo Y, Xiong J, Chen R, Deng S. Associations Between Physical Capability Markers and Risk of Coronary Artery Disease: A Prospective Study of 439,295 UK Biobank Participants. Healthcare. 2025; 13(9):1018. https://doi.org/10.3390/healthcare13091018
Chicago/Turabian StyleLiu, Duqiu, Chenxing Yang, Tianyu Guo, Yi Guo, Jinjie Xiong, Ru Chen, and Shan Deng. 2025. "Associations Between Physical Capability Markers and Risk of Coronary Artery Disease: A Prospective Study of 439,295 UK Biobank Participants" Healthcare 13, no. 9: 1018. https://doi.org/10.3390/healthcare13091018
APA StyleLiu, D., Yang, C., Guo, T., Guo, Y., Xiong, J., Chen, R., & Deng, S. (2025). Associations Between Physical Capability Markers and Risk of Coronary Artery Disease: A Prospective Study of 439,295 UK Biobank Participants. Healthcare, 13(9), 1018. https://doi.org/10.3390/healthcare13091018







