Timely and Personalized Interventions and Vigilant Care in Neurodegenerative Conditions: The FIT4TeleNEURO Pragmatic Trial
Abstract
1. Introduction
2. Materials and Methods
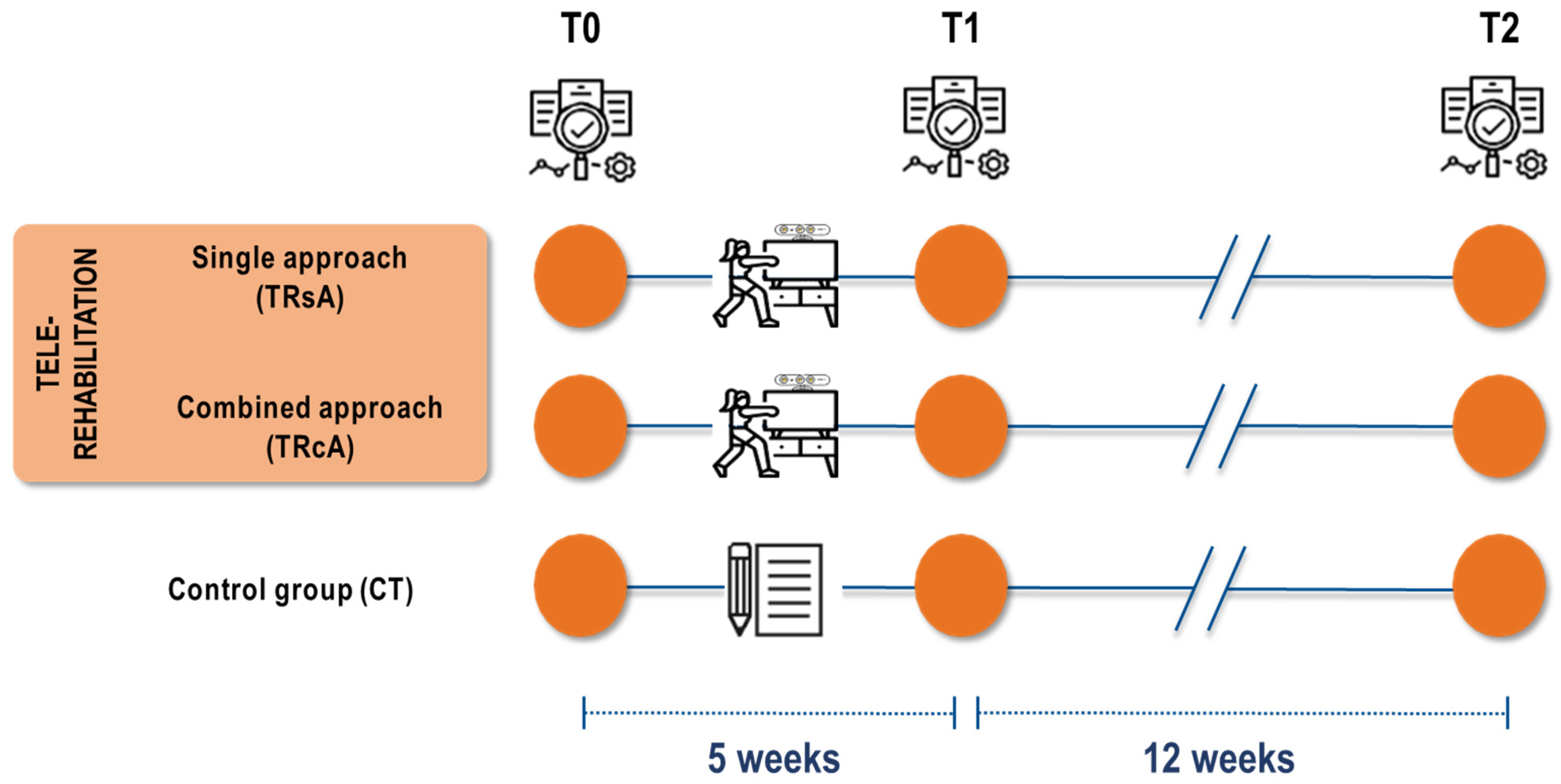
2.1. Trial Design and Setting
2.2. Sample Size
2.3. Study Population, Recruitment, and Randomization
2.4. Inclusion and Exclusion Criteria
- Age between 25 and 85 years.
- The preserved cognitive level at the Montreal Cognitive Assessment test (MoCA test > 15.5) [48].
- No rehabilitation program being implemented at the time of enrollment.
- Stable drug treatment (last three months) with L-Dopa or dopamine agonists (PD group) or disease modifying therapies (DMTs) (MS group).
- The presence of comorbidities that could hinder patients from safely participating in a home program or indicate clinical instability (e.g., severe orthopedic issues or significant cognitive impairments).
- Unsuitable environmental factors, such as inadequate space for rehabilitation activities or the absence of a stable internet connection.
- The presence of major psychiatric complications or personality disorders, assessed through a clinical interview.
- The presence of severe impairments in visual and/or auditory perception.
- Falls resulting in injuries or more than two falls in the six months prior to recruitment (for both PD and MS groups).
- Relapse ongoing/less than 3 months since the last relapse (MS group).
- The presence of “frequent” freezing as recorded at the administration of Section II (daily life activity) of the UPDRS (score ≥ 3) (PD group).
- EDSS-FS (cerebellar function) ≥ 3 (MS group).
2.5. Trial Interventions
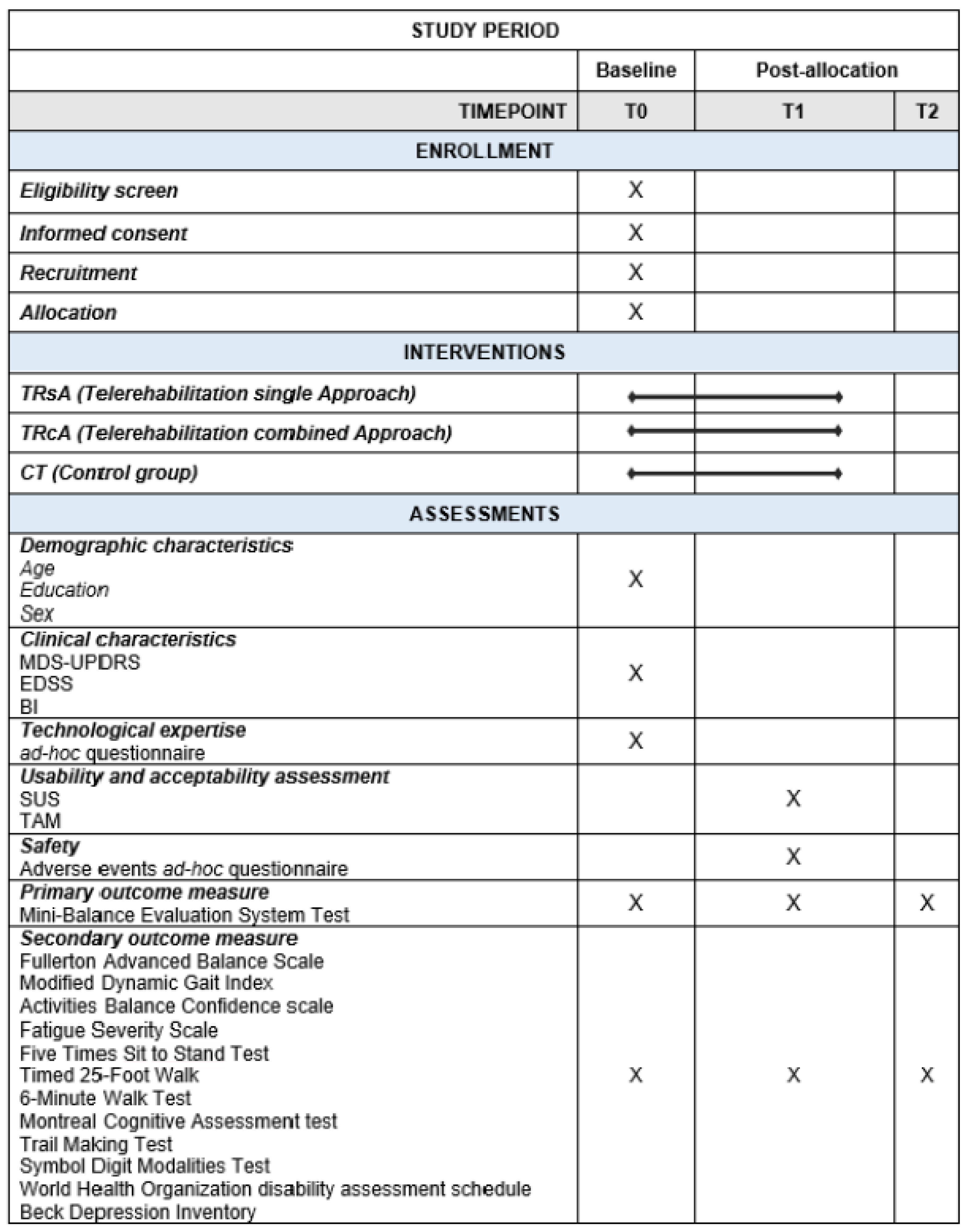
2.6. Outcome Measures
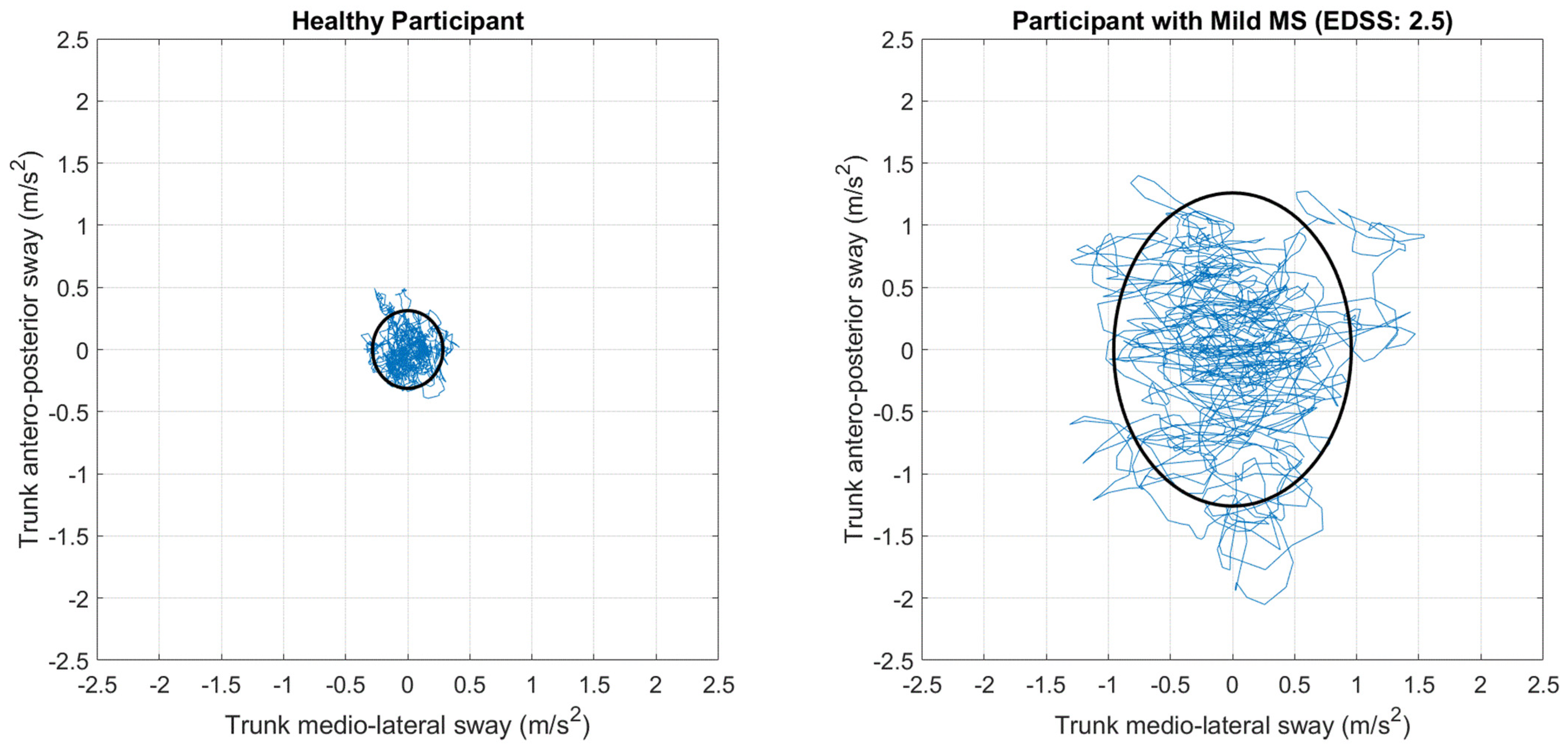
2.6.1. Primary Outcome Measure
2.6.2. Secondary Outcome Measures
2.6.3. Other Outcome Measures
2.7. Data Collection
2.8. Statistical Analysis
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Feigin, V.L.; Nichols, E.; Alam, T.; Bannick, M.S.; Beghi, E.; Blake, N.; Culpepper, W.J.; Dorsey, E.R.; Elbaz, A.; Ellenbogen, R.G.; et al. Global, regional, and national burden of neurological disorders, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Turner-Stokes, L.; Sykes, N.; Silber, E. Long-term neurological conditions: Management at the interface between neurology, rehabilitation and palliative care. Clin. Med. 2008, 8, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Korn, T. Pathophysiology of multiple sclerosis. J. Neurol. 2008, 255 (Suppl. S6), 2–6. [Google Scholar] [CrossRef] [PubMed]
- Zéphir, H. Progress in understanding the pathophysiology of multiple sclerosis. Rev. Neurol. 2018, 174, 358–363. [Google Scholar] [CrossRef]
- Ford, H. Clinical presentation and diagnosis of multiple sclerosis. Clin. Med. 2020, 20, 380–383. [Google Scholar] [CrossRef]
- McGinley, M.P.; Goldschmidt, C.H.; Rae-Grant, A.D. Diagnosis and Treatment of Multiple Sclerosis: A Review. JAMA 2021, 325, 765–779. [Google Scholar] [CrossRef]
- Halabchi, F.; Alizadeh, Z.; Sahraian, M.A.; Abolhasani, M. Exercise prescription for patients with multiple sclerosis; potential benefits and practical recommendations. BMC Neurol. 2017, 17, 185. [Google Scholar] [CrossRef]
- Lai, C.H.; Chen, H.C.; Liou, T.H.; Li, W.; Chen, S.C. Exercise Interventions for Individuals with Neurological Disorders: A Systematic Review of Systematic Reviews. Am. J. Phys. Med. Rehabil. 2019, 98, 921–930. [Google Scholar] [CrossRef]
- Hayes, S.; Galvin, R.; Kennedy, C.; Finlayson, M.; McGuigan, C.; Walsh, C.D.; Coote, S. Interventions for preventing falls in people with multiple sclerosis. Cochrane Database Syst. Rev. 2019, 11, Cd012475. [Google Scholar] [CrossRef]
- Shen, X.; Wong-Yu, I.S.; Mak, M.K. Effects of Exercise on Falls, Balance, and Gait Ability in Parkinson’s Disease: A Meta-analysis. Neurorehabilit. Neural Repair 2016, 30, 512–527. [Google Scholar] [CrossRef]
- Jankovic, J.; Tolosa, E. Parkinson’s Disease and Movement Disorders; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Tranchant, C.; Bhatia, K.P.; Marsden, C.D. Movement disorders in multiple sclerosis. Mov. Disord. 1995, 10, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, D.; Gervasoni, E.; Pupillo, E.; Bianchi, E.; Aprile, I.; Imbimbo, I.; Russo, R.; Cruciani, A.; Turolla, A.; Jonsdottir, J.; et al. Educational and Exercise Intervention to Prevent Falls and Improve Participation in Subjects with Neurological Conditions: The NEUROFALL Randomized Controlled Trial. Front. Neurol. 2019, 10, 865. [Google Scholar] [CrossRef] [PubMed]
- Beghi, E.; Gervasoni, E.; Pupillo, E.; Bianchi, E.; Montesano, A.; Aprile, I.; Agostini, M.; Rovaris, M.; Cattaneo, D. Prediction of Falls in Subjects Suffering From Parkinson Disease, Multiple Sclerosis, and Stroke. Arch. Phys. Med. Rehabil. 2018, 99, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Nilsagård, Y.; Gunn, H.; Freeman, J.; Hoang, P.; Lord, S.; Mazumder, R.; Cameron, M. Falls in people with MS--an individual data meta-analysis from studies from Australia, Sweden, United Kingdom and the United States. Mult. Scler. 2015, 21, 92–100. [Google Scholar] [CrossRef]
- Paul, S.S.; Canning, C.G.; Sherrington, C.; Lord, S.R.; Close, J.C.T.; Fung, V.S.C. Three simple clinical tests to accurately predict falls in people with Parkinson’s disease. Mov. Disord. 2013, 28, 655–662. [Google Scholar] [CrossRef]
- Cieza, A.; Causey, K.; Kamenov, K.; Hanson, S.W.; Chatterji, S.; Vos, T. Global estimates of the need for rehabilitation based on the Global Burden of Disease study 2019: A systematic analysis for the Global Burden of Disease Study 2019. Lancet 2021, 396, 2006–2017. [Google Scholar] [CrossRef]
- Topol, E. The Topol Review—Preparing the Healthcare Workforce to Deliver the Digital Future: An Independent Report on Behalf of the Secretary of State for Health and Social Care. 2019. Available online: https://topol.hee.nhs.uk/wp-content/uploads/HEE-Topol-Review-2019.pdf (accessed on 11 May 2021).
- Dorsey, E.R.; Topol, E.J. Telemedicine 2020 and the next decade. Lancet 2020, 395, 859. [Google Scholar] [CrossRef]
- Maggio, M.G.; Baglio, F.; Arcuri, F.; Borgnis, F.; Contrada, M.; Diaz, M.D.M.; Leochico, C.F.; Neira, N.J.; Laratta, S.; Suchan, B.; et al. Cognitive telerehabilitation: An expert consensus paper on current evidence and future perspective. Front. Neurol. 2024, 15, 1338873. [Google Scholar] [CrossRef] [PubMed]
- Özden, F.; Sari, Z.; Tuğay, N. Telerehabilitation; Springer: Berlin/Heidelberg, Germany, 2024. [Google Scholar]
- Pagliari, C.; Di Tella, S.; Jonsdottir, J.; Mendozzi, L.; Rovaris, M.; De Icco, R.; Milanesi, T.; Federico, S.; Agostini, M.; Goffredo, M.; et al. Effects of home-based virtual reality telerehabilitation system in people with multiple sclerosis: A randomized controlled trial. J. Telemed. Telecare 2024, 30, 344–355. [Google Scholar] [CrossRef]
- Pagliari, C.; Di Tella, S.; Bonanno, C.; Cacciante, L.; Cioeta, M.; De Icco, R.; Jonsdottir, J.; Federico, S.; Franceschini, M.; Goffredo, M.; et al. Enhancing the effect of rehabilitation on multiple sclerosis: A randomized clinical trial investigating the impact of remotely-supervised transcranial direct current stimulation and virtual reality telerehabilitation training. Mult. Scler. Relat. Disord. 2025, 94, 106256. [Google Scholar] [CrossRef]
- Shaw, M.T.; Best, P.; Frontario, A.; E Charvet, L. Telerehabilitation benefits patients with multiple sclerosis in an urban setting. J. Telemed. Telecare 2021, 27, 39–45. [Google Scholar] [CrossRef]
- Di Tella, S.; Pagliari, C.; Blasi, V.; Mendozzi, L.; Rovaris, M.; Baglio, F. Integrated telerehabilitation approach in multiple sclerosis: A systematic review and meta-analysis. J. Telemed. Telecare 2020, 26, 385–399. [Google Scholar] [CrossRef] [PubMed]
- Fjeldstad-Pardo, C.; Thiessen, A.; Pardo, G. Telerehabilitation in Multiple Sclerosis: Results of a Randomized Feasibility and Efficacy Pilot Study. Int. J. Telerehabilitation 2018, 10, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Amatya, B.; Kesselring, J.; Galea, M. Telerehabilitation for persons with multiple sclerosis. Cochrane Database Syst. Rev. 2015, 2015, CD010508. [Google Scholar] [CrossRef]
- Amatya, B.; Galea, M.; Kesselring, J.; Khan, F. Effectiveness of telerehabilitation interventions in persons with multiple sclerosis: A systematic review. Mult. Scler. Relat. Disord. 2015, 4, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Isernia, S.; Di Tella, S.; Rossetto, F.; Borgnis, F.; Realdon, O.; Cabinio, M.; Pagliari, C.; Torchio, A.; Castagna, A.; Blasi, V.; et al. Exploring cognitive reserve’s influence: Unveiling the dynamics of digital telerehabilitation in Parkinson’s Disease Resilience. npj Digit. Med. 2024, 7, 116. [Google Scholar] [CrossRef]
- Goffredo, M.; Baglio, F.; DE Icco, R.; Proietti, S.; Maggioni, G.; Turolla, A.; Pournajaf, S.; Jonsdottir, J.; Zeni, F.; Federico, S.; et al. Efficacy of non-immersive virtual reality-based telerehabilitation on postural stability in Parkinson’s disease: A multicenter randomized controlled trial. Eur. J. Phys. Rehabil. Med. 2023, 59, 689–696. [Google Scholar] [CrossRef]
- Del Pino, R.; de Echevarría, A.O.; Díez-Cirarda, M.; Ustarroz-Aguirre, I.; Caprino, M.; Liu, J.; Gand, K.; Schlieter, H.; Gabilondo, I.; Gómez-Esteban, J.C. Virtual coach and telerehabilitation for Parkinson’s disease patients: vCare system. J. Public Health 2023, 1–14. [Google Scholar]
- Eldemir, S.; Guclu-Gunduz, A.; Eldemir, K.; Saygili, F.; Yilmaz, R.; Akbostancı, M.C. The effect of task-oriented circuit training-based telerehabilitation on upper extremity motor functions in patients with Parkinson’s disease: A randomized controlled trial. Park. Relat. Disord. 2023, 109, 105334. [Google Scholar] [CrossRef]
- Bianchini, E.; Onelli, C.; Morabito, C.; Alborghetti, M.; Rinaldi, D.; Anibaldi, P.; Marcolongo, A.; Salvetti, M.; Pontieri, F.E. Feasibility, Safety, and Effectiveness of Telerehabilitation in Mild-to-Moderate Parkinson’s Disease. Front. Neurol. 2022, 13, 909197. [Google Scholar] [CrossRef]
- Truijen, S.; Abdullahi, A.; Bijsterbosch, D.; van Zoest, E.; Conijn, M.; Wang, Y.; Struyf, N.; Saeys, W. Effect of home-based virtual reality training and telerehabilitation on balance in individuals with Parkinson disease, multiple sclerosis, and stroke: A systematic review and meta-analysis. Neurol. Sci. 2022, 43, 2995–3006. [Google Scholar] [CrossRef] [PubMed]
- Vellata, C.; Belli, S.; Balsamo, F.; Giordano, A.; Colombo, R.; Maggioni, G. Effectiveness of Telerehabilitation on Motor Impairments, Non-motor Symptoms and Compliance in Patients with Parkinson’s Disease: A Systematic Review. Front. Neurol. 2021, 12, 627999. [Google Scholar] [CrossRef]
- Isernia, S.; Di Tella, S.; Pagliari, C.; Jonsdottir, J.; Castiglioni, C.; Gindri, P.; Salza, M.; Gramigna, C.; Palumbo, G.; Molteni, F.; et al. Effects of an Innovative Telerehabilitation Intervention for People with Parkinson’s Disease on Quality of Life, Motor, and Non-motor Abilities. Front. Neurol. 2020, 11, 846. [Google Scholar] [CrossRef] [PubMed]
- Seidler, K.J.; Duncan, R.P.; E McNeely, M.; E Hackney, M.; Earhart, G.M. Feasibility and preliminary efficacy of a telerehabilitation approach to group adapted tango instruction for people with Parkinson disease. J. Telemed. Telecare 2017, 23, 740–746. [Google Scholar] [CrossRef] [PubMed]
- Cotelli, M.; Manenti, R.; Brambilla, M.; Gobbi, E.; Ferrari, C.; Binetti, G.; Cappa, S.F. Cognitive telerehabilitation in mild cognitive impairment, Alzheimer’s disease and frontotemporal dementia: A systematic review. J. Telemed. Telecare 2019, 25, 67–79. [Google Scholar] [CrossRef]
- Rossetto, F.; Isernia, S.; Realdon, O.; Borgnis, F.; Blasi, V.; Pagliari, C.; Cabinio, M.; Alberoni, M.; Mantovani, F.; Clerici, M.; et al. A digital health home intervention for people within the Alzheimer’s disease continuum: Results from the Ability-TelerehABILITation pilot randomized controlled trial. Ann. Med. 2023, 55, 1080–1091. [Google Scholar] [CrossRef]
- Soke, F.; Guclu-Gunduz, A.; Kocer, B.; Fidan, I.; Keskinoglu, P. Task-oriented circuit training combined with aerobic training improves motor performance and balance in people with Parkinson′s Disease. Acta Neurol. Belg. 2021, 121, 535–543. [Google Scholar] [CrossRef]
- Sangelaji, B.; Nabavi, S.M.; Estebsari, F.; Banshi, M.R.; Rashidian, H.; Jamshidi, E.; Dastoorpour, M. Effect of Combination Exercise Therapy on walking distance, postural balance, fatigue and quality of life in multiple sclerosis patients: A clinical trial study. Iran. Red Crescent Med J. 2014, 16, e17173. [Google Scholar] [CrossRef]
- Learmonth, Y.; Paul, L.; Miller, L.; Mattison, P.; McFadyen, A. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: A randomized controlled pilot study. Clin. Rehabil. 2012, 26, 579–593. [Google Scholar] [CrossRef]
- Reis, A.A.; Upshur, R.; Moodley, K. Future-Proofing Research Ethics—Key Revisions of the Declaration of Helsinki 2024. JAMA 2025, 333, 20–21. [Google Scholar] [CrossRef]
- Postuma, R.B.; Berg, D.; Stern, M.; Poewe, W.; Olanow, C.W.; Oertel, W.; Obeso, J.; Marek, K.; Litvan, I.; Lang, A.E.; et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov. Disord. 2015, 30, 1591–1601. [Google Scholar] [CrossRef] [PubMed]
- Goetz, C.G.; Poewe, W.; Rascol, O.; Sampaio, C.; Stebbins, G.T.; Counsell, C.; Giladi, N.; Holloway, R.G.; Moore, C.G.; Wenning, G.K.; et al. Movement Disorder Society Task Force report on the Hoehn and Yahr staging scale: Status and recommendations. Mov. Disord. 2004, 19, 1020–1028. [Google Scholar] [CrossRef]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Cohen. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef]
- Santangelo, G.; Siciliano, M.; Pedone, R.; Vitale, C.; Falco, F.; Bisogno, R.; Siano, P.; Barone, P.; Grossi, D.; Santangelo, F.; et al. Normative data for the Montreal Cognitive Assessment in an Italian population sample. Neurol. Sci. 2015, 36, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Forman, C.R.; Nielsen, J.B.; Lorentzen, J. Neuroplasticity at Home: Improving Home-Based Motor Learning Through Technological Solutions. A Review. Front. Rehabil. Sci. 2021, 2, 789165. [Google Scholar] [CrossRef]
- Franchignoni, F.; Horak, F.; Godi, M.; Nardone, A.; Giordano, A. Using psychometric techniques to improve the Balance Evaluation Systems Test: The mini-BESTest. J. Rehabil. Med. 2010, 42, 323–331. [Google Scholar] [CrossRef]
- Carpinella, I.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Tacchino, A.; Brichetto, G.; Confalonieri, P.; Solaro, C.; Rovaris, M.; Ferrarin, M.; et al. Walking with Horizontal Head Turns Is Impaired in Persons with Early-Stage Multiple Sclerosis Showing Normal Locomotion. Front. Neurol. 2021, 12, 821640. [Google Scholar] [CrossRef]
- Schlenstedt, C.; Brombacher, S.; Hartwigsen, G.; Weisser, B.; Möller, B.; Deuschl, G. Comparing the fullerton advanced balance scale with the mini-bestest and berg balance scale to assess postural control in patients with parkinson disease. Arch. Phys. Med. Rehabil. 2015, 96, 218–225. [Google Scholar] [CrossRef]
- Mattos, F.G.M.; Gervasoni, E.; Anastasi, D.; Di Giovanni, R.; Tacchino, A.; Brichetto, G.; Carpinella, I.; Confalonieri, P.; Vercellino, M.; Solaro, C.; et al. Assessing balance in non-disabled subjects with multiple sclerosis: Validation of the Fullerton Advanced Balance Scale. Mult. Scler. Relat. Disord. 2020, 42, 102085. [Google Scholar] [CrossRef]
- Anastasi, D.; Carpinella, I.; Gervasoni, E.; Matsuda, P.N.; Bovi, G.; Ferrarin, M.; Cattaneo, D. Instrumented Version of the Modified Dynamic Gait Index in Patients with Neurologic Disorders. PM&R 2019, 11, 1312–1319. [Google Scholar]
- Cattaneo, D.; Regola, A.; Meotti, M. Validity of six balance disorders scales in persons with multiple sclerosis. Disabil. Rehabil. 2006, 28, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Franchignoni, F.; Giordano, A.; Ronconi, G.; Rabini, A.; Ferriero, G. Rasch validation of the Activities-specific Balance Confidence Scale and its short versions in patients with Parkinson’s disease. J. Rehabil. Med. 2014, 46, 532–539. [Google Scholar] [CrossRef]
- Siciliano, M.; Chiorri, C.; De Micco, R.; Russo, A.; Tedeschi, G.; Trojano, L.; Tessitore, A. Fatigue in Parkinson’s disease: Italian validation of the Parkinson Fatigue Scale and the Fatigue Severity Scale using a Rasch analysis approach. Park. Relat. Disord. 2019, 65, 105–110. [Google Scholar] [CrossRef]
- Ottonello, M.; Pellicciari, L.; Giordano, A.; Foti, C. Rasch analysis of the Fatigue Severity Scale in Italian subjects with multiple sclerosis. J. Rehabil. Med. 2016, 48, 597–603. [Google Scholar] [CrossRef]
- Stagsted, R.A.; Ramari, C.; Skjerbaek, A.G.; Thrue, C.; Dalgas, U.; Hvid, L.G. Lower extremity muscle power—A critical determinant of physical function in aging and multiple sclerosis. Exp. Gerontol. 2021, 150, 111347. [Google Scholar] [CrossRef] [PubMed]
- Duncan, R.P.; Leddy, A.L.; Earhart, G.M. Five Times Sit-to-Stand Test Performance in Parkinson’s Disease. Arch. Phys. Med. Rehabil. 2011, 92, 1431–1436. [Google Scholar] [CrossRef]
- Phan-Ba, R.; Pace, A.; Calay, P.; Grodent, P.; Douchamps, F.; Hyde, R.; Hotermans, C.; Delvaux, V.; Hansen, I.; Moonen, G.; et al. Comparison of the Timed 25-Foot and the 100-Meter Walk as Performance Measures in Multiple Sclerosis. Neurorehabilit. Neural Repair 2011, 25, 672–679. [Google Scholar] [CrossRef]
- Carpinella, I.; Bertoni, R.; Anastasi, D.; Cardini, R.; Lencioni, T.; Ferrarin, M.; Cattaneo, D.; Gervasoni, E. Walk Longer! Using Wearable Inertial Sensors to Uncover Which Gait Aspects Should Be Treated to Increase Walking Endurance in People with Multiple Sclerosis. Sensors 2024, 24, 7284. [Google Scholar] [CrossRef]
- Giovagnoli, A.R.; Del Pesce, M.; Mascheroni, S.; Simoncelli, M.; Laiacona, M.; Capitani, E. Trail making test: Normative values from 287 normal adult controls. Ital. J. Neurol. Sci. 1996, 17, 305–309. [Google Scholar] [CrossRef]
- Nocentini, U.; Giordano, A.; Di Vincenzo, S.; Panella, M.; Pasqualetti, P. The Symbol Digit Modalities Test—Oral version: Italian normative data. Funct. Neurol. 2006, 21, 93–96. [Google Scholar] [PubMed]
- Federici, S.; Bracalenti, M.; Meloni, F.; Luciano, J.V. World Health Organization disability assessment schedule 2.0: An international systematic review. Disabil. Rehabil. 2017, 39, 2347–2380. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Beck Depression Inventory; Springer: New York, NY, USA, 1996. [Google Scholar]
- Brooke, J. SUS: A ’Quick and Dirty’ Usability Scale. Usability Eval. Ind. 1996, 189, 4–7. [Google Scholar]
- Venkatesh, V.; Bala, H. Technology Acceptance Model 3 and a Research Agenda on Interventions. Decis. Sci. 2008, 39, 273–315. [Google Scholar] [CrossRef]
- Shah, S.; Vanclay, F.; Cooper, B. Improving the sensitivity of the Barthel Index for stroke rehabilitation. J. Clin. Epidemiol. 1989, 42, 703–709. [Google Scholar] [CrossRef]
- Montedori, A.; Bonacini, M.I.; Casazza, G.; Luchetta, M.L.; Duca, P.; Cozzolino, F.; Abraha, I. Modified versus standard intention-to-treat reporting: Are there differences in methodological quality, sponsorship, and findings in randomized trials? A cross-sectional study. Trials 2011, 12, 58. [Google Scholar] [CrossRef]
- Abraha, I.; Montedori, A. Modified intention to treat reporting in randomised controlled trials: Systematic review. BMJ 2010, 340, c2697. [Google Scholar] [CrossRef]
- Nizeyimana, E.; Joseph, C.; A Louw, Q. A scoping review of feasibility, cost-effectiveness, access to quality rehabilitation services and impact of telerehabilitation: A review protocol. Digit. Health 2022, 8, 20552076211066708. [Google Scholar] [CrossRef] [PubMed]
- Molina-Garcia, P.; Mora-Traverso, M.; Prieto-Moreno, R.; Díaz-Vásquez, A.; Antony, B.; Ariza-Vega, P. Effectiveness and cost-effectiveness of telerehabilitation for musculoskeletal disorders: A systematic review and meta-analysis. Ann. Phys. Rehabilitation Med. 2024, 67, 101791. [Google Scholar] [CrossRef]
- Kidholm, K.; Ekeland, A.G.; Jensen, L.K.; Rasmussen, J.; Pedersen, C.D.; Bowes, A.; Flottorp, S.A.; Bech, M. A Model for assessment of telemedicine applications: Mast. Int. J. Technol. Assess. Heal. Care 2012, 28, 44–51. [Google Scholar] [CrossRef]
- Callesen, J.; Cattaneo, D.; Brincks, J.; Jørgensen, M.-L.K.; Dalgas, U. How do resistance training and balance and motor control training affect gait performance and fatigue impact in people with multiple sclerosis? A randomized controlled multi-center study. Mult. Scler. J. 2020, 26, 1420–1432. [Google Scholar] [CrossRef] [PubMed]
- Matamala-Gomez, M.; Maisto, M.; Montana, J.I.; Mavrodiev, P.A.; Baglio, F.; Rossetto, F.; Mantovani, F.; Riva, G.; Realdon, O. The Role of Engagement in Teleneurorehabilitation: A Systematic Review. Front. Neurol. 2020, 11, 354. [Google Scholar] [CrossRef] [PubMed]

| Single Approach Treatment (TRsA) | Combined Approach Treatment (TRcA) | |
|---|---|---|
| Duration | 50 min | 50 min |
| Devices |  |  |
| Aim | To improve balance and coordination | To improve both balance and coordination, and strength and resistance |
| Example of exercises | The single approach includes exercises such as single-leg stance, standing while alternative reaching a target using the arms E.g. EXERCISE DESCRIPTION  | The combined approach includes exercises that simultaneously train both balance and strength such as flexion or abduction of lower limbs, and repetitive sit to stand movements from a chair E.g. EXERCISE DESCRIPTION  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baglio, F.; Rossetto, F.; Gervasoni, E.; Carpinella, I.; Smecca, G.; Aprile, I.; De Icco, R.; De Trane, S.; Pavese, C.; Lunetta, C.; et al. Timely and Personalized Interventions and Vigilant Care in Neurodegenerative Conditions: The FIT4TeleNEURO Pragmatic Trial. Healthcare 2025, 13, 682. https://doi.org/10.3390/healthcare13060682
Baglio F, Rossetto F, Gervasoni E, Carpinella I, Smecca G, Aprile I, De Icco R, De Trane S, Pavese C, Lunetta C, et al. Timely and Personalized Interventions and Vigilant Care in Neurodegenerative Conditions: The FIT4TeleNEURO Pragmatic Trial. Healthcare. 2025; 13(6):682. https://doi.org/10.3390/healthcare13060682
Chicago/Turabian StyleBaglio, Francesca, Federica Rossetto, Elisa Gervasoni, Ilaria Carpinella, Giulia Smecca, Irene Aprile, Roberto De Icco, Stefania De Trane, Chiara Pavese, Christian Lunetta, and et al. 2025. "Timely and Personalized Interventions and Vigilant Care in Neurodegenerative Conditions: The FIT4TeleNEURO Pragmatic Trial" Healthcare 13, no. 6: 682. https://doi.org/10.3390/healthcare13060682
APA StyleBaglio, F., Rossetto, F., Gervasoni, E., Carpinella, I., Smecca, G., Aprile, I., De Icco, R., De Trane, S., Pavese, C., Lunetta, C., Fundarò, C., Marcuccio, L., Zamboni, G., Molteni, F., Messa, C., & FIT4TeleNEURO Working Group. (2025). Timely and Personalized Interventions and Vigilant Care in Neurodegenerative Conditions: The FIT4TeleNEURO Pragmatic Trial. Healthcare, 13(6), 682. https://doi.org/10.3390/healthcare13060682







