Effects of Trehalose on Halitosis: A Randomized Cross-Over Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
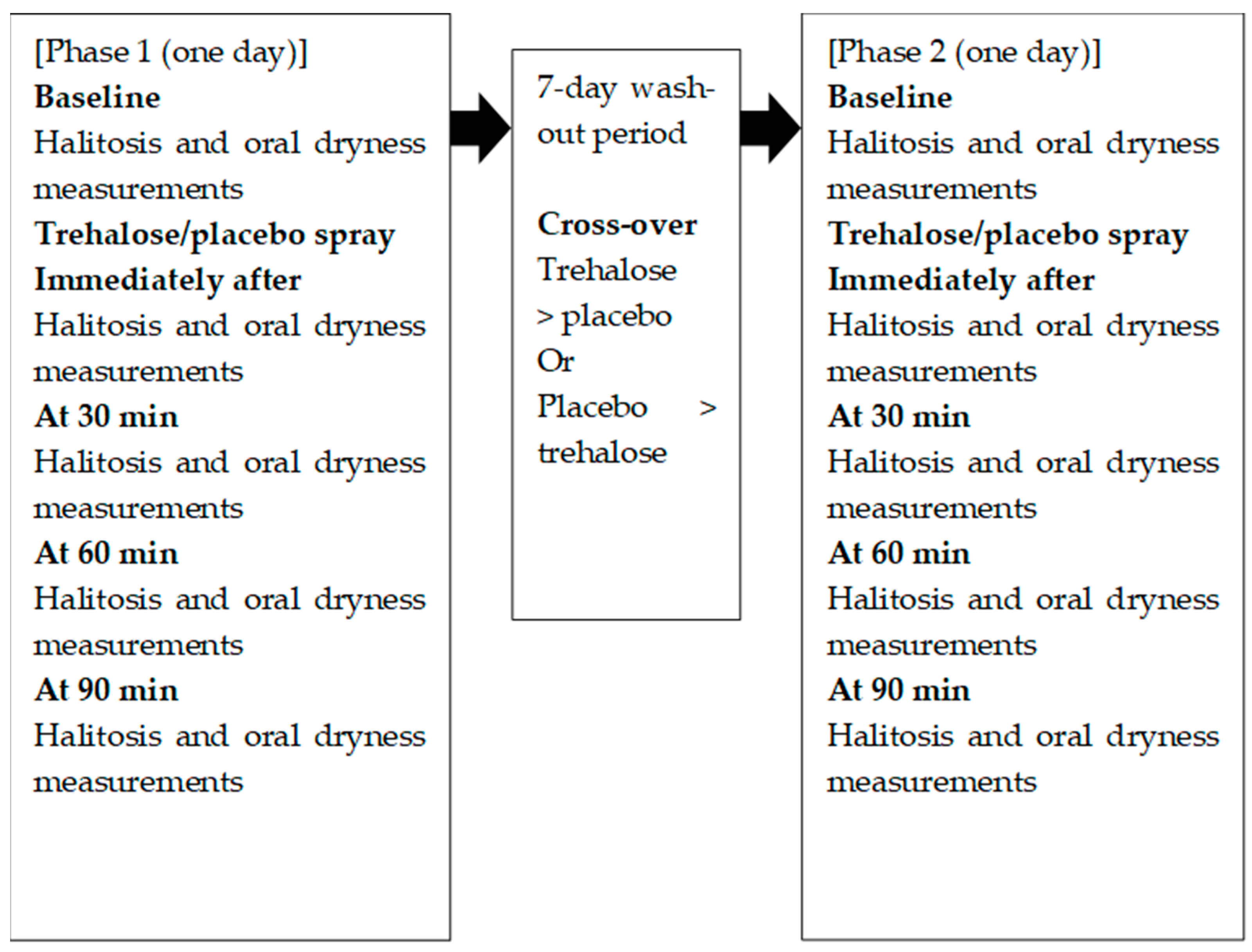
2.1. Trial Design
2.2. Participants
2.3. Intervention
2.4. Outcome Assessment
2.5. Adverse Events and Safety Monitoring
2.6. Sample Size Calculation
2.7. Randomization
2.8. Blinding
2.9. Statistical Analysis
3. Results
3.1. Flow Chart
3.2. Baseline Data
3.3. Outcomes
3.4. Harms
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| VSCs | volatile sulfur compounds |
| ITT | intention-to-treat |
References
- Memon, M.A.; Memon, H.A.; Faizan, E.; Muhammad, F.E.; Fahad, S.; Siddiqui, A.; Lee, K.Y.; Tahir, M.J.; Yousaf, Z. Aetiology and associations of halitosis: A systematic review. Oral. Dis. 2023, 29, 1432–1438. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.F.; Leite, F.R.M.; Ferreira, L.B.; Pola, N.M.; Scannapieco, F.A.; Demarco, F.F.; Nascimento, G.G. Estimated prevalence of halitosis: A systematic review and meta-regression analysis. Clin. Oral. Investig. 2018, 22, 47–55. [Google Scholar] [CrossRef]
- Wylleman, A.; Vuylsteke, F.; Dekeyser, C.; Teughels, W.; Quirynen, M.; Laleman, I. Alternative therapies in controlling oral malodour: A systematic review. J. Breath. Res. 2021, 15, 026009. [Google Scholar] [CrossRef]
- Yaegaki, K.; Coil, J.M. Examination, classification, and treatment of halitosis; clinical perspectives. J. Can. Dent. Assoc. 2000, 66, 257–261. [Google Scholar]
- Hampelska, K.; Jaworska, M.M.; Babalska, Z.L.; Karpinski, T.M. The role of oral microbiota in intra-oral halitosis. J. Clin. Med. 2020, 9, 2484. [Google Scholar] [CrossRef]
- Takeuchi, H.; Machigashira, M.; Takeuchi, N.; Nakamura, T.; Noguchi, K. The association of periodontopathic bacteria levels in saliva and tongue coating with oral malodor in periodontitis patients. Oral. Health Prev. Dent. 2017, 15, 285–291. [Google Scholar] [PubMed]
- Sikorska-Żuk, M.; Bochnia, M. Halitosis in children with adenoid hypertrophy. J. Breath. Res. 2018, 12, 026011. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.K.; Mou, F.Y.; Qian, X.X. Therapeutic efficacy of a probiotic preparation on idiopathic halitosis: A retrospective observational study. J. Breath. Res. 2024, 19, 016005. [Google Scholar] [CrossRef]
- Zhou, J.K.; Zheng, Y.; Wang, Y.P.; Ji, R. Prevalence and associated risk factors of Helicobacter pylori infection in community households in Lanzhou city. World J. Gastroenterol. 2024, 30, 5018–5031. [Google Scholar] [CrossRef]
- Delanghe, G.; Ghyselen, J.; van Steenberghe, D.; Feenstra, L. 1997 Multidisciplinary breath-odour clinic. Lancet 1997, 350, 187. [Google Scholar] [CrossRef]
- Dadamio, J.; Laleman, I.; Quirynen, M. The role of toothpastes in oral malodor management. Monogr. Oral. Sci. 2013, 23, 45–60. [Google Scholar] [PubMed]
- Dadamio, J.; Van Tournout, M.; Teughels, W.; Dekeyser, C.; Coucke, W.; Quirynen, M. Efficacy of different mouthrinse formulations in reducing oral malodour: A randomized clinical trial. J. Clin. Periodontol. 2013, 40, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Tangerman, A.; Winkel, E.G. Intra- and extra-oral halitosis: Finding of a new form of extra-oral blood-borne halitosis caused by dimethyl sulphide. J. Clin. Periodontol. 2007, 34, 748–755. [Google Scholar] [CrossRef] [PubMed]
- Quirynen, M.; Dadamio, J.; van den Velde, S.; de Smit, M.; Dekeyser, C.; van Tornout, M.; Vandekerckhove, B. Characteristics of 2000 patients who visited a halitosis clinic. J. Clin. Periodontol. 2009, 36, 970–975. [Google Scholar] [CrossRef]
- Scully, C.; Greenman, J. Halitology (breath odour: Aetiopathogenesis and management). Oral. Dis. 2012, 18, 333–345. [Google Scholar] [CrossRef]
- Mori, Y.; Yano, F.; Shimohata, N.; Suzuki, S.; Chung, U.I.; Takato, T. Trehalose inhibits oral dryness by protecting the cell membrane. Int. J. Oral. Maxillofac. Surg. 2010, 39, 916–921. [Google Scholar] [CrossRef]
- Hsu, S.; Dickinson, D. A new approach to managing oral manifestations of Sjogren’s syndrome and skin manifestations of lupus. J. Biochem. Mol. Biol. 2006, 39, 229–239. [Google Scholar] [CrossRef]
- Oh, D.J.; Lee, J.Y.; Kim, Y.K.; Kho, H.S. Effects of carboxymethylcellulose (CMC)-based artificial saliva in patients with xerostomia. Int. J. Oral. Maxillofac. Surg. 2008, 37, 1027–1031. [Google Scholar] [CrossRef]
- Yuan, J.; Tohara, H.; Mikushi, S.; Hoshino, T.; Yue, B.; Uematsu, H. The effect of “Oral Wet” for elderly people with xerostomia—The effect of oral rinse containing hialuronan. Kokubyo Gakkai Zasshi 2005, 72, 106–110. [Google Scholar] [CrossRef][Green Version]
- Franca, M.B.; Panek, A.D.; Eleutherio, E.C. Oxidative stress and its effects during dehydration. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2007, 146, 621–631. [Google Scholar] [CrossRef]
- Yoshizane, C.; Mizote, A.; Arai, C.; Arai, N.; Ogawa, R.; Endo, S.; Mitsuzumi, H.; Ushio, S. Daily consumption of one teaspoon of trehalose can help maintain glucose homeostasis: A double-blind, randomized controlled trial conducted in healthy volunteers. Nutr. J. 2020, 19, 68. [Google Scholar] [CrossRef] [PubMed]
- Ozek, D.; Kemer, O.E. Effect of the bioprotectant agent trehalose on corneal epithelial healing after corneal cross-linking for keratoconus. Arq. Bras. Oftalmol. 2018, 81, 505–509. [Google Scholar] [CrossRef]
- Plemons, J.M.; Al-Hashimi, I.; Marek, C.L. Managing xerostomia and salivary gland hypofunction: Executive summary of a report from the american Dental Association Council on Scientific Affairs. J. Am. Dent. Assoc. 2014, 145, 867–873. [Google Scholar] [CrossRef] [PubMed]
- Seemann, R.; Conceicao, M.D.; Filippi, A.; Greenman, J.; Lenton, P.; Nachnani, S.; Quirynen, M.; Roldan, S.; Schulze, H.; Sterer, N.; et al. Halitosis management by the general dental practitioner–results of an international consensus workshop. J. Breath. Res. 2014, 8, 017101. [Google Scholar] [CrossRef]
- Maruyama, T.; Ekuni, D.; Yokoi, A.; Nagasaki, J.; Sawada, N.; Morita, M. Effect of Antimicrobial Photodynamic Therapy on the Tongue Dorsum on Reducing Halitosis and the Duration of the Effect: A Randomized Clinical Trial. Healthcare 2024, 12, 980. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, Y.; Yoda, T.; Araki, R.; Sakai, T.; Toya, S.; Ito, K.; Funayama, S.; Enoki, Y.; Sato, T. Evaluation of oral wetness using an improved moisture-checking device for the diagnosis of dry mouth. Oral. Sci. Int. 2017, 14, 33–36. [Google Scholar] [CrossRef]
- Takeuchi, N.; Sawada, N.; Ekuni, D.; Morita, M. Association between oral condition and subjective psychological well-being among older adults attending a university hospital dental clinic: A cross-sectional study. PLoS ONE 2023, 18, e0295078. [Google Scholar] [CrossRef]
- Takeuchi, N.; Tamaki, N.; Esaki, M.; Honda, S.; Morita, M. Effect of long-term use of commercial mouthwash solution containing chlorine dioxide (ProFresh®) on oral malodor. J. Jpn. Acad. Malodor Syn. 2011, 2, 3–10. (In Japanese) [Google Scholar]
- Kuczyńska-Wiśnik, D.; Stojowska-Swędrzyńska, K.; Laskowska, E. Intracellular Protective Functions and Therapeutical Potential of Trehalose. Molecules 2024, 29, 2088. [Google Scholar] [CrossRef]
- Chen, A.; Tapia, H.; Goddard, J.M.; Gibney, P.A. Trehalose and its applications in the food industry. Compr. Rev. Food Sci. Food Saf. 2022, 21, 5004–5037. [Google Scholar] [CrossRef]
- Wolber, J.M.; Urbanek, B.L.; Meints, L.M.; Piligian, B.F.; Lopez-Casillas, I.C.; Zochowski, K.M.; Woodruff, P.J.; Swarts, B.M. The Trehalose-Specific Transporter LpqY-SugABC Is Required for Antimicrobial and Anti-Biofilm Activity of Trehalose Analogues in Mycobacterium smegmatis. Carbohydr. Res. 2017, 450, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.J.; Lee, S.K.; Song, N.; Nathan, T.O.; Swarts, B.M.; Eum, S.Y.; Ehrt, S.; Cho, S.N.; Eoh, H. Transient Drug-Tolerance and Permanent Drug-Resistance Rely on the Trehalose-Catalytic Shift in Mycobacterium tuberculosis. Nat. Commun. 2019, 10, 2928. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, S.; Karygianni, L.; Filippi, A.; Anderson, C.A.; Zürcher, A.; Hellwig, E.; Vach, K.; Macchiarelli, G.; Al-Ahmad, A. Combining culture and culture-independent methods reveals new microbial composition of halitosis patients' tongue biofilm. Microbiologyopen 2020, 9, e958. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; Updated August 2024; Cochrane: London, UK, 2024; Available online: www.training.cochrane.org/handbook (accessed on 27 December 2024).

| Variable | Total | Trehalose | Placebo |
|---|---|---|---|
| Age (years) | 23.6 ± 1.1 | - | - |
| Male/Female | 8 (88.9)/1 (11.1) | - | - |
| Systemic diseases | 0 (0.0) | - | - |
| Organoleptic score | 2.6 ± 0.8 | 2.7 ± 0.7 | 2.6 ± 0.8 |
| Organoleptic score > 1 | 9 (100.0) | ||
| Volatile sulfur compounds (ppb) | |||
| Hydrogen sulfide | 66.6 ± 72.5 | 56.2 ±89.9 | 77.0 ± 53.5 |
| Methyl mercaptan | 38.5 ± 37.6 | 46.8 ± 51.5 | 30.2 ± 14.1 |
| Dimethyl sulfide | 6.0 ± 9.7 | 4.8 ± 4.0 | 7.2 ± 13.4 |
| Oral moisture level | 30.0 ± 1.3 | 29.9 ± 1.6 | 30.1 ± 1.0 |
| Variable | Trehalose | Placebo | p * | Trehalose; Change from Baseline | Placebo; Change from Baseline | p * |
|---|---|---|---|---|---|---|
| Immediately after | ||||||
| Organoleptic score | 1.8 ± 0.8 | 1.8 ± 0.7 1 | 0.924 | −0.9 ± 0.8 | −0.8 ± 0.4 | 0.799 |
| Volatile sulfur compounds (ppb) | ||||||
| Hydrogen sulfide | 48.3 ± 43.3 | 32.3 ± 31.9 | 0.424 | −7.9 ± 71.2 | −44.7 ± 67.7 | 0.233 |
| Methyl mercaptan | 38.9 ± 38.9 | 29.1 ± 37.3 | 0.231 | −7.9 ± 32.1 | −1.1 ± 36.5 | 0.895 |
| Dimethyl sulfide | 8.1 ± 7.1 | 12.6 ± 28.2 | 0.248 | 3.3 ± 8.0 | 5.3 ± 18.0 | 0.756 |
| Oral moisture level | 30.3 ± 1.3 | 29.8 ± 0.8 | 0.215 | 0.4 ± 0.9 | −0.3 ± 0.7 | 0.047 |
| At 30 min | ||||||
| Organoleptic score | 1.9 ± 0.8 | 1.7 ± 0.7 | 0.534 | −0.8 ± 0.7 | −0.9 ± 0.9 | 0.591 |
| Volatile sulfur compounds (ppb) | ||||||
| Hydrogen sulfide | 37.9 ± 51.6 | 64.9 ± 69.9 | 0.397 | −18.3 ±57.1 | −12.1 ± 81.5 | 0.895 |
| Methyl mercaptan | 56.3 ± 65.1 | 25.6 ± 10.8 | 0.479 | 9.6 ± 58.7 | −4.7 ± 17.0 | 0.479 |
| Dimethyl sulfide | 3.1 ± 5.5 | 8.9 ± 14.3 | 0.609 | −1.7 ± 6.1 | 1.7 ± 5.1 | 0.168 |
| Oral moisture level | 29.5 ± 1.3 | 29.5 ± 1.3 | 0.895 | −0.4 ± 0.8 | −0.4 ± 0.9 | 0.894 |
| At 60 min | ||||||
| Organoleptic score | 2.0 ± 0.7 | 1.9 ± 0.8 | 0.737 | −0.7 ± 0.7 | −0.7 ± 1.0 | 0.962 |
| Volatile sulfur compounds (ppb) | ||||||
| Hydrogen sulfide | 49.2 ± 47.5 | 72.2 ± 49.3 | 0.232 | −7.0 ± 60.4 | −4.8 ± 77.2 | 0.860 |
| Methyl mercaptan | 54.6 ± 64.9 | 27.8 ± 14.5 | 0.691 | 7.8 ± 51.5 | −2.4 ± 18.6 | 0.757 |
| Dimethyl sulfide | 3.8 ± 4.8 | 4.9 ± 9.5 | 0.616 | −1.0 ± 6.1 | −2.3 ± 4.4 | 0.857 |
| Oral moisture level | 29.5 ± 1.8 | 29.4 ± 1.6 | 0.596 | −0.4 ± 1.5 | −1.0 ± 1.0 | 0.233 |
| At 90 min | ||||||
| Organoleptic score | 2.2 ± 0.8 | 1.9 ± 0.8 | 0.373 | −0.4 ± 0.8 | −0.7 ± 1.0 | 0.609 |
| Volatile sulfur compounds (ppb) | ||||||
| Hydrogen sulfide | 71.8 ± 45.8 | 101.6 ± 62.9 | 0.353 | 15.6 ± 89.7 | 24.6 ± 71.1 | 0.860 |
| Methyl mercaptan | 38.7 ± 43.6 | 35.4 ± 24.9 | 0.479 | −8.1 ± 28.0 | 5.2 ± 17.3 | 0.479 |
| Dimethyl sulfide | 8.4 ± 11.7 | 8.9 ± 11.5 | 0.824 | 3.7 ± 13.0 | 1.7 ± 4.1 | 0.535 |
| Oral moisture level | 29.0 ± 1.8 | 29.9 ± 2.0 | 0.353 | −0.9 ± 1.3 | −0.4 ± 1.1 | 0.426 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyai, H.; Tomofuji, T.; Mizuno, H.; Morita, M.; Nakahara, M.; Kataoka, K.; Sumita, I.; Uchida, Y.; Toyama, N.; Yokoi, A.; et al. Effects of Trehalose on Halitosis: A Randomized Cross-Over Clinical Trial. Healthcare 2025, 13, 619. https://doi.org/10.3390/healthcare13060619
Miyai H, Tomofuji T, Mizuno H, Morita M, Nakahara M, Kataoka K, Sumita I, Uchida Y, Toyama N, Yokoi A, et al. Effects of Trehalose on Halitosis: A Randomized Cross-Over Clinical Trial. Healthcare. 2025; 13(6):619. https://doi.org/10.3390/healthcare13060619
Chicago/Turabian StyleMiyai, Hisataka, Takaaki Tomofuji, Hirofumi Mizuno, Manabu Morita, Momoko Nakahara, Kota Kataoka, Ichiro Sumita, Yurika Uchida, Naoki Toyama, Aya Yokoi, and et al. 2025. "Effects of Trehalose on Halitosis: A Randomized Cross-Over Clinical Trial" Healthcare 13, no. 6: 619. https://doi.org/10.3390/healthcare13060619
APA StyleMiyai, H., Tomofuji, T., Mizuno, H., Morita, M., Nakahara, M., Kataoka, K., Sumita, I., Uchida, Y., Toyama, N., Yokoi, A., Yamanaka-Kohno, R., Takeuchi, N., Maruyama, T., & Ekuni, D. (2025). Effects of Trehalose on Halitosis: A Randomized Cross-Over Clinical Trial. Healthcare, 13(6), 619. https://doi.org/10.3390/healthcare13060619








