Efficacy of Oral Intake of Hydrogen-Rich Jelly Intake on Gingival Inflammation: A Double-Blind, Placebo-Controlled and Exploratory Randomized Clinical Trial
Abstract
1. Introduction
2. Materials and Methods
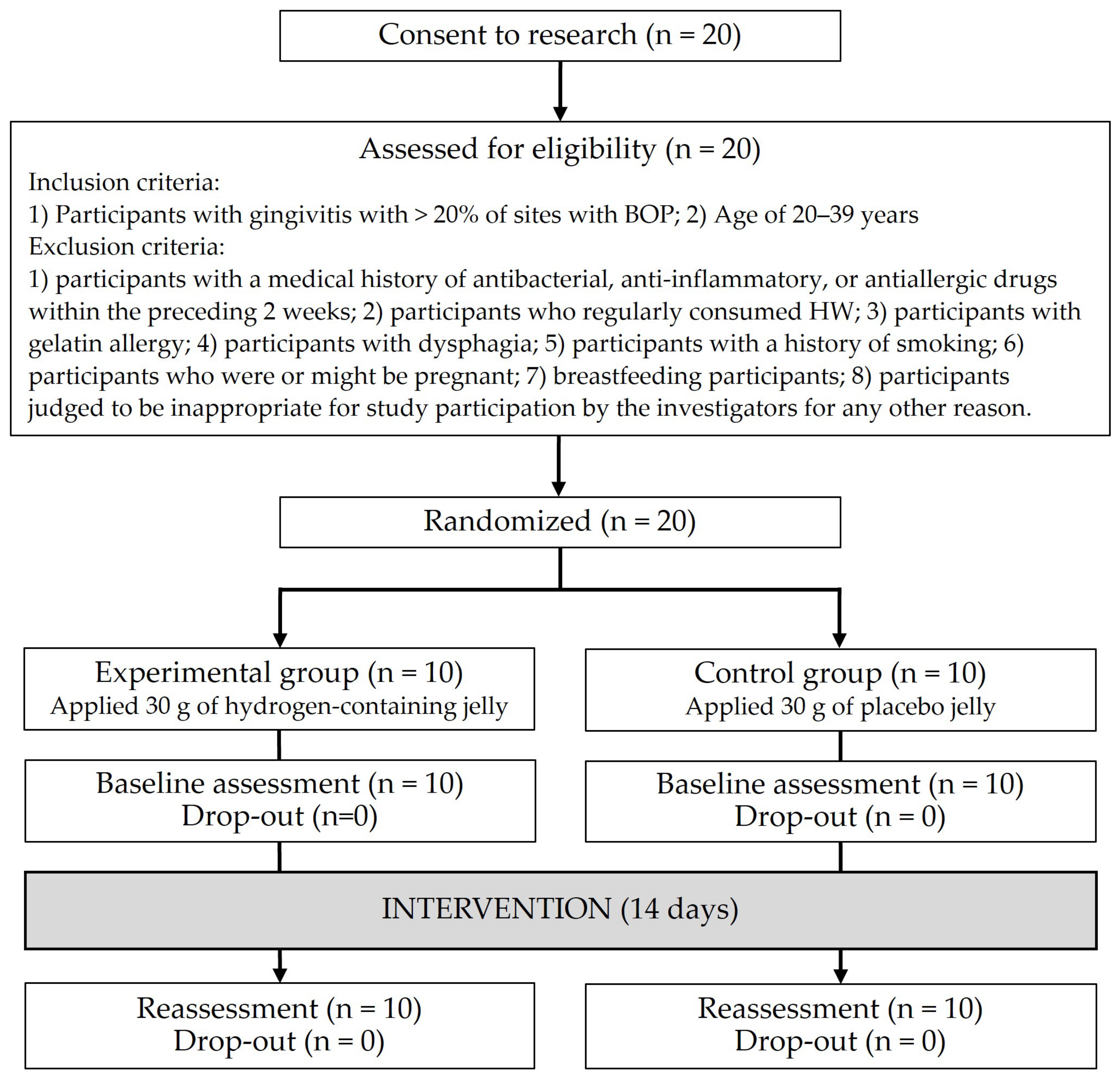
2.1. Trial Design
2.2. Blinding
2.3. Participants
2.4. Randomization
2.5. Sample Size Calculation
2.6. Intervention
2.7. Outcome Assessment
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, R.C. Periodontal disease. N. Eng. J. Med. 1990, 322, 373–382. [Google Scholar] [CrossRef] [PubMed]
- Madianos, P.N.; Bobetsis, Y.A.; Kinane, D.F. Generation of inflammatory stimuli: How bacteria set up inflammatory responses in the gingiva. J. Clin. Periodontol. 2005, 32, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Chapple, I.L.; Matthews, J.B. The role of reactive oxygen and antioxidant species in periodontal tissue destruction. Periodontol. 2000 2007, 43, 160–232. [Google Scholar] [CrossRef] [PubMed]
- Ekuni, D.; Tomofuji, T.; Tamaki, N.; Sanbe, T.; Azuma, T.; Yamanaka, R.; Yamamoto, T.; Watanabe, T. Mechanical stimulation of gingiva reduces plasma 8-OHdG level in rat periodontitis. Arch. Oral Biol. 2008, 53, 324–329. [Google Scholar] [CrossRef]
- Maruyama, T.; Tomofuji, T.; Endo, Y.; Irie, K.; Azuma, T.; Ekuni, D.; Tamaki, N.; Yamamoto, T.; Morita, M. Supplementation of green tea catechin in dentifrices suppresses gingival oxidative stress and periodontal inflammation. Arch. Oral Biol. 2011, 56, 48–53. [Google Scholar] [CrossRef]
- Tomofuji, T.; Ekuni, D.; Irie, K.; Azuma, T.; Endo, Y.; Tamaki, N.; Sanbe, T.; Murakami, J.; Yamamoto, T.; Morita, M. Preventive effects of a cocoa-enriched diet on gingival oxidative stress in experimental periodontitis. J. Periodontol. 2009, 80, 1799–1808. [Google Scholar] [CrossRef]
- Tamaki, N.; Tomofuji, T.; Ekuni, D.; Yamanaka, R.; Yamamoto, T.; Morita, M. Short-term effects of non-surgical periodontal treatment on plasma level of reactive oxygen metabolites in patients with chronic periodontitis. J. Periodontol. 2009, 80, 901–906. [Google Scholar] [CrossRef]
- Greenstein, G. The role of bleeding upon probing in the diagnosis of periodontal disease. A literature review. J. Periodontol. 1984, 55, 684–688. [Google Scholar] [CrossRef]
- Iwasaki, K.; Moynihan, P.; Manz, M.C.; Taylor, G.W.; Yoshihara, A.; Muramatsu, K.; Watanabe, R.; Miyazaki, H. Dietary antioxidants and periodontal disease in community-based older Japanese: A 2-year follow-up study. Public Health Nutr. 2013, 16, 330–338. [Google Scholar] [CrossRef]
- Dodington, D.W.; Fritz, P.C.; Sullivan, P.J.; Ward, W.E. Higher intakes of fruits and vegetables, β-Carotene, vitamin C, α-Tocopherol, EPA, and DHA are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. J. Nutr. 2015, 145, 2512–2519. [Google Scholar] [CrossRef]
- Ohsawa, I.; Ishikawa, M.; Takahashi, K.; Watanabe, M.; Nishimaki, K.; Yamagata, K.; Katsura, K.; Katayama, K.; Asoh, S.; Ohta, S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat. Med. 2007, 13, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Kasuyama, K.; Tomofuji, T.; Ekuni, D.; Tamaki, N.; Azuma, T.; Irie, K.; Endo, Y.; Morita, M. Hydrogen-rich water attenuates experimental periodontitis in a rat model. J. Clin. Periodontol. 2011, 38, 1085–1090. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Yamane, M.; Ekuni, D.; Kawabata, Y.; Kataoka, K.; Kasuyama, K.; Maruyama, T.; Tomofuji, T.; Morita, M. Drinking Hydrogen-Rich Water Has Additive Effects on Non-Surgical Periodontal Treatment of Improving Periodontitis: A Pilot Study. Antioxidants 2015, 4, 513–522. [Google Scholar] [CrossRef] [PubMed]
- Syndergaard, B.; Al-Sabbagh, M.; Kryscio, R.J.; Xi, J.; Ding, X.; Ebersole, J.L.; Miller, C.S. Salivary biomarkers associated with gingivitis and response to therapy. J. Periodontol. 2014, 85, e295–e303. [Google Scholar] [CrossRef]
- Maruyama, T.; Ekuni, D.; Higuchi, M.; Takayama, E.; Tokuno, S.; Morita, M. Relationship between Psychological Stress Determined by Voice Analysis and Periodontal Status: A Cohort Study. Int. J. Environ. Res. Public Health 2022, 19, 9489. [Google Scholar] [CrossRef]
- Nesse, W.; Abbas, F.; van der Ploeg, I.; Spiikervet, F.K.; Dijkstra, P.U.; Vissink, A. Periodontal inflamed surface area: Quantifying inflammatory burden. J. Clin. Periodontol. 2008, 35, 668–673. [Google Scholar] [CrossRef]
- O’Leary, T.J.; Drake, R.B.; Naylor, J.E. The plaque control record. J. Periodontol. 1972, 43, 38. [Google Scholar] [CrossRef]
- Tamaki, N.; Tomofuji, T.; Ekuni, D.; Yamanaka, R.; Morita, M. Periodontal treatment decreases plasma oxidized LDL level and oxidative stress. Clin. Oral Investig. 2011, 15, 953–958. [Google Scholar] [CrossRef]
- Vassalle, C.; Sciarrino, R.; Bianchi, S.; Battaglia, C.; Mercuri, A.; Maffei, S. Sex-related differences in association of oxidative stress status with coronary artery disease. Fertil. Steril. 2012, 97, 414–419. [Google Scholar] [CrossRef]
- National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Available online: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/Archive/CTCAE_4.0_2009-05-29_QuickReference_8.5x11.pdf (accessed on 27 December 2024).
- Grant, M.M.; Taylor, J.J.; Jaedicke, K.; Creese, A.; Gowland, C.; Burke, B.; Doudin, K.; Patel, U.; Milward, M.; Bissett, S.M.; et al. Discovery, validation, and diagnostic ability of multiple protein-based biomarkers in saliva and gingival crevicular fluid to distinguish between health and periodontal diseases. J. Clin. Periodontol. 2022, 49, 622–632. [Google Scholar] [CrossRef]
- Atanasova, T.; Stankova, T.; Bivolarska, A.; Vlaykova, T. Matrix metalloproteinases in oral health-special attention on MMP-8. Biomedicines 2023, 11, 1514. [Google Scholar] [CrossRef] [PubMed]
- Ikeuchi, T.; Moutsopoulos, N.M. Osteoimmunology in periodontitis; a paradigm for Th17/IL-17 inflammatory bone loss. Bone 2022, 163, 116500. [Google Scholar] [CrossRef] [PubMed]
- Bhatt, A.; Nayak, A.; Bhat, K.; Bogar, C.; Nayak, R.; Naik, S. Assessment of the effects of hydrogen water on human gingival fibroblast cell culture in patients with chronic periodontitis. J. Indian Soc. Periodontol. 2023, 27, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Paradowska-Stolarz, A.; Mikulewicz, M.; Laskowska, J.; Karolewicz, B.; Owczarek, A. The Importance of Chitosan Coatings in Dentistry. Mar. Drugs 2023, 21, 613. [Google Scholar] [CrossRef]
- Khabazian, A.; Mirhashemi, F.S.; Sadeghi, F. Investigating the effect of propolis-containing chewing gum in comparison with propolis-containing mouthwash on reducing gingival inflammation in patients with gingivitis. BMC Oral Health 2025, 25, 231. [Google Scholar] [CrossRef]
| Variable | Experimental Group (n = 10) | Control Group (n = 10) |
|---|---|---|
| Age (year) | 23 (21, 23) * | 23 (21.5, 23) |
| Male | 7 (70.0) † | 7 (70.0) |
| Percentage of sites with BOP (%) | 37.65 (30.85, 45.95) | 30.65 (26.125, 33.925) |
| Mean PPD (mm) | 1.978 (1.872, 2.125) | 1.942 (1.901, 2.005) |
| PISA (mm2) | 422.45 (381.4, 537.725) | 353.85 (294.7, 387.5) |
| PCR (%) | 66.95 (56.25, 79.95) | 62.05 (48.1, 78.0) |
| Serum ROM level (CARR U) | 291.0 (267.75, 333.0) | 295.0 (278.5, 394.25) |
| Serum OXY level (μmol HClO/mL) | 400.0 (316.5, 417.475) | 434.45 (330.4, 465.25) |
| Oxidative index | −0.248 (−1.053, 1.006) | 0.019 (−1.022, 0.618) |
| IL-1β level (pg/mL) | 34.40 (27.55, 42.22) | 29.63 (16.09, 52.13) |
| IL-6 level (pg/mL) | 1.36 (0.31, 12.33) | 0.70 (0.47, 1.90) |
| IL-10 level (pg/mL) | 3.39 (1.66, 6.50) | 1.68 (0.83, 3.03) |
| IL-17 level (pg/mL) | 2.21 (1.56, 3.43) | 1.05 (0.47, 2.69) |
| TNF-α level (pg/mL) | 110.64 (61.12, 133.22) | 44.66 (21.40, 126.12) |
| Variable | Experimental Group (n = 10) | Control Group (n = 10) | p Value |
|---|---|---|---|
| Percentage of sites with BOP (%) | 25.9 (16.7, 30.025) | 19.0 (15.625, 23.3) | 0.123 |
| Mean PPD (mm) | 1.857 (1.765, 2.026) | 1.849 (1.806, 1.886) | 0.739 |
| PISA (mm2) | 292.05 (174.775, 340.775) | 209.8 (173.85, 231.7) | 0.075 |
| PCR (%) | 63.45 (47.95, 73.425) | 58.05 (45.175, 75.925) | 0.739 |
| Serum ROM level (CARR U) | 303.5 (271.0, 325.55) | 321.0 (301.75, 369.5) | 0.123 |
| Serum OXY level (μmol HClO/mL) | 377.15 (304.7, 438.15) | 417.65 (336.35, 455.425) | 0.393 |
| Oxidative index | −0.188 (−0.910, 0.958) | 0.615 (−0.690, 1.196) | 0.393 |
| IL-1β level (pg/mL) | 25.53 (18.66, 31.32) | 16.98 (6.16, 41.32) | 0.529 |
| IL-6 level (pg/mL) | 1.59 (0.27, 2.51) | 0.33 (0.27, 0.52) | 0.075 |
| IL-10 level (pg/mL) | 1.59 (0.90, 4.69) | 0.93 (0.35, 1.78) | 0.243 |
| IL-17 level (pg/mL) | 1.50 (1.01, 2.69) | 0.71 (0.50, 1.20) | 0.113 |
| TNF-α level (pg/mL) | 50.38 (28.12, 111.32) | 27.79 (22.43, 56.09) | 0.356 |
| Variable | Experimental Group (n = 10) | Control Group (n = 10) | p Value |
|---|---|---|---|
| Percentage of sites with BOP (%) | −13.85 (−18.9, −10.55) | −10.95 (−14.2, −6.6) | 0.225 |
| Mean PPD (mm) | −0.109 (−0.148, −0.088) | −0.11 (−0.151, −0.080) | 0.116 |
| PISA (mm2) | −167.05 (−241.43, −120.35) | −148.25 (−170.4, −109.275) | 0.643 |
| PCR (%) | −6.8 (−12.73, 12.73) | −1.35 (−11.6, 8.0) | 0.868 |
| Serum ROM level (CARR U) | −2.00 (−15.75, 14.50) | 11.00 (−32.25, 41.5) | 0.735 |
| Serum OXY level (μmol HClO/mL) | −8.95 (−37.05, 41.075) | −4.750 (−45.33, 33.65) | 0.650 |
| Oxidative index | 0.170 (−0.629, 0.418) | 0.478 (−0.192, 0.905) | 0.570 |
| IL-1β level (pg/mL) | −7.81 (−20.25, 5.72) | −9.77 (−30.35, 1.75) | 0.155 |
| IL-6 level (pg/mL) | −1.78 (−11.10, 0.03) | −0.14 (−0.21, 0.01) | 0.092 |
| IL-10 level (pg/mL) | −1.11 (−3.21, −0.30) | −0.88 (−1.39, 0.46) | 0.673 |
| IL-17 level (pg/mL) | −0.79 (−1.20, −0.50) | −0.40 (−1.11, −0.05) | 0.085 |
| TNF-α level (pg/mL) | −37.05 (−83.79, −5.77) | −22.91 (−45.90, 7.92) | 0.441 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maruyama, T.; Takayama, E.; Tokuno, S.; Morita, M.; Ekuni, D. Efficacy of Oral Intake of Hydrogen-Rich Jelly Intake on Gingival Inflammation: A Double-Blind, Placebo-Controlled and Exploratory Randomized Clinical Trial. Healthcare 2025, 13, 577. https://doi.org/10.3390/healthcare13050577
Maruyama T, Takayama E, Tokuno S, Morita M, Ekuni D. Efficacy of Oral Intake of Hydrogen-Rich Jelly Intake on Gingival Inflammation: A Double-Blind, Placebo-Controlled and Exploratory Randomized Clinical Trial. Healthcare. 2025; 13(5):577. https://doi.org/10.3390/healthcare13050577
Chicago/Turabian StyleMaruyama, Takayuki, Eiji Takayama, Shinichi Tokuno, Manabu Morita, and Daisuke Ekuni. 2025. "Efficacy of Oral Intake of Hydrogen-Rich Jelly Intake on Gingival Inflammation: A Double-Blind, Placebo-Controlled and Exploratory Randomized Clinical Trial" Healthcare 13, no. 5: 577. https://doi.org/10.3390/healthcare13050577
APA StyleMaruyama, T., Takayama, E., Tokuno, S., Morita, M., & Ekuni, D. (2025). Efficacy of Oral Intake of Hydrogen-Rich Jelly Intake on Gingival Inflammation: A Double-Blind, Placebo-Controlled and Exploratory Randomized Clinical Trial. Healthcare, 13(5), 577. https://doi.org/10.3390/healthcare13050577







