Pilot Study on the Efficacy of a Novel Questionnaire for Assessing Psychological Health in Patients with Chronic Rhinosinusitis with Nasal Polyps Treated with Biologics
Abstract
1. Introduction
2. Materials and Methods
2.1. Research Design
2.2. Study Variables
2.3. Procedures
2.4. Data Analysis
2.5. Ethical Considerations
3. Results
3.1. Sample Characteristics
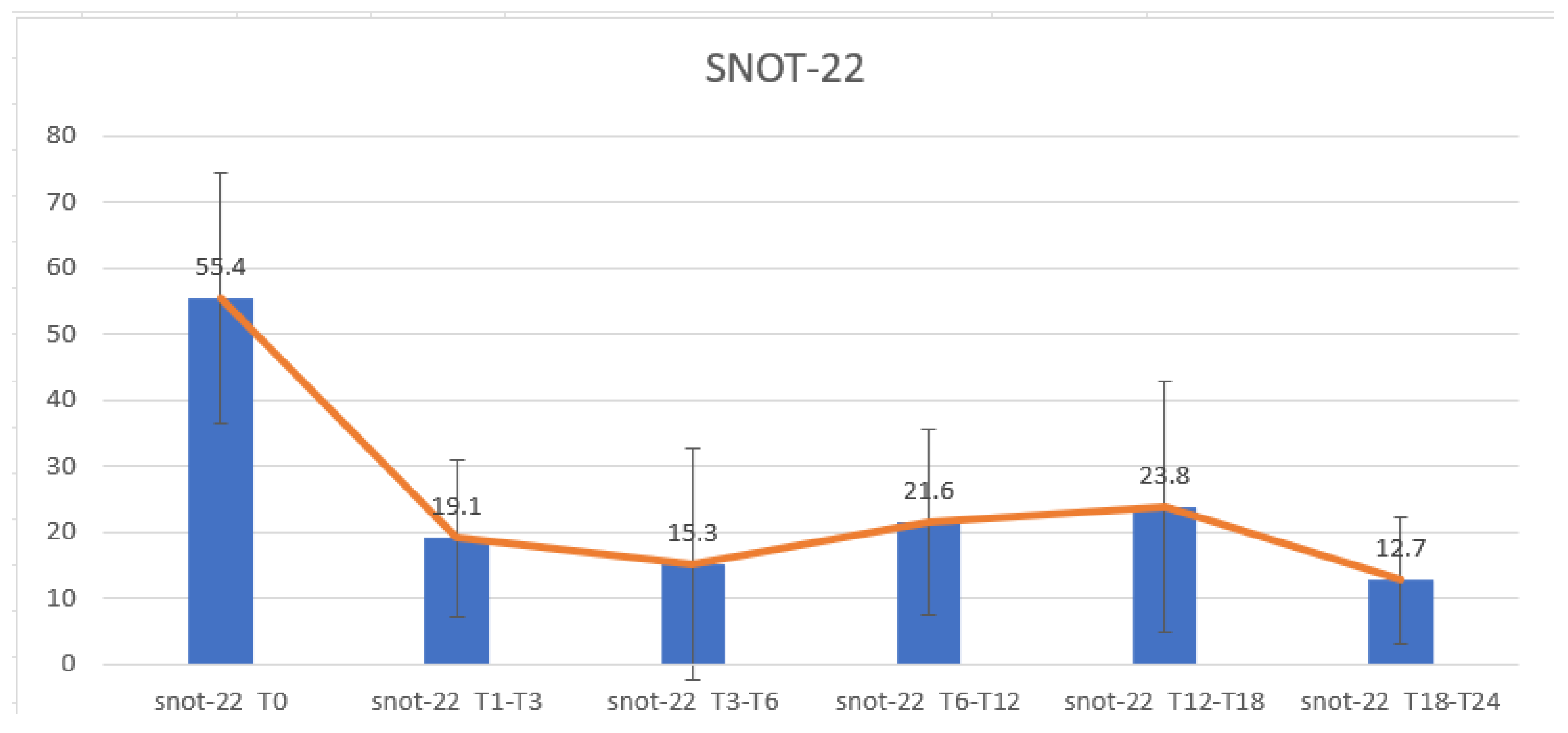
3.2. SNOT-22 Results Regarding the Efficacy of Dupilumab on Nasal Symptoms and QoL
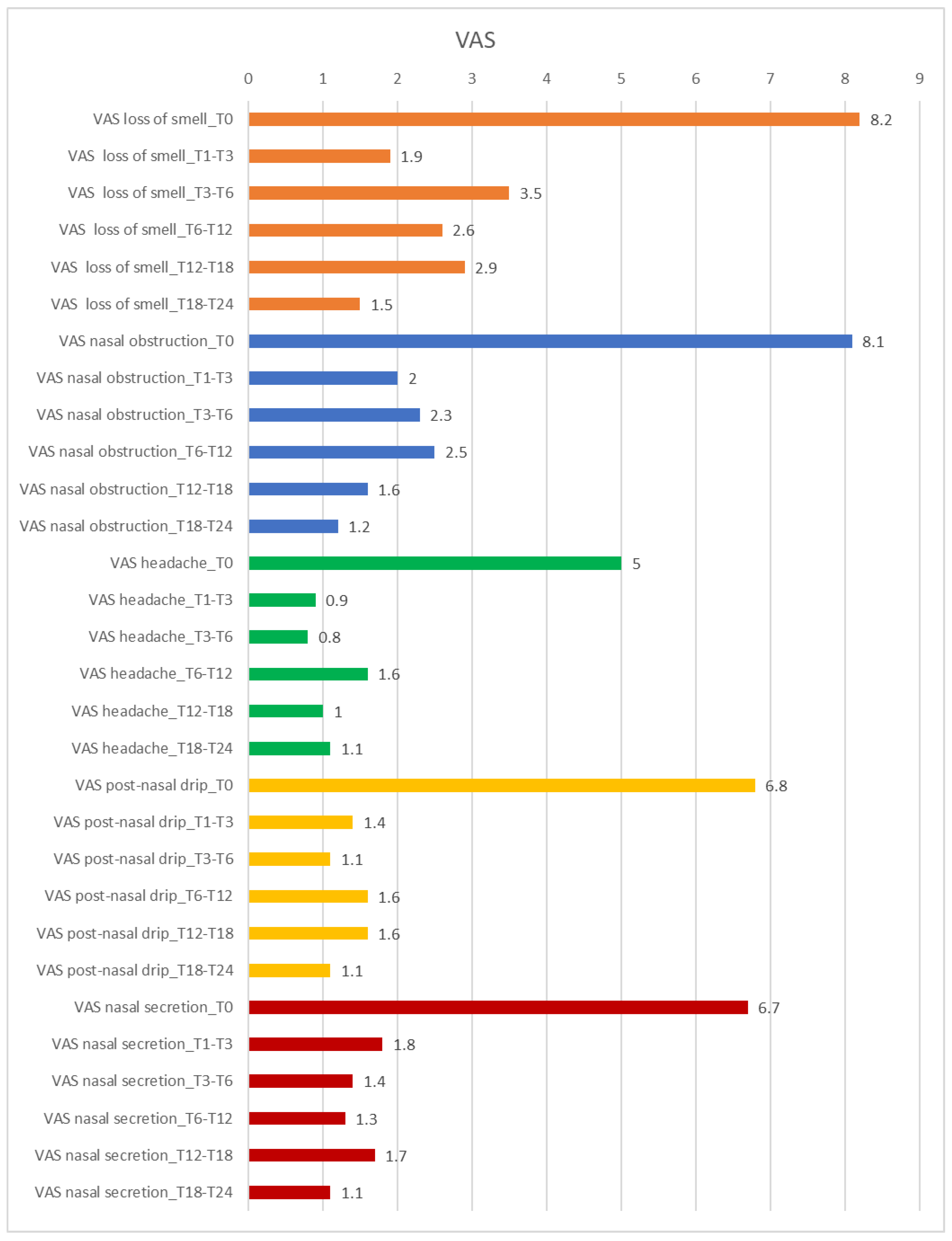
3.3. VAS Score Results Regarding the Efficacy of Dupilumab on Nasal Symptoms and QoL
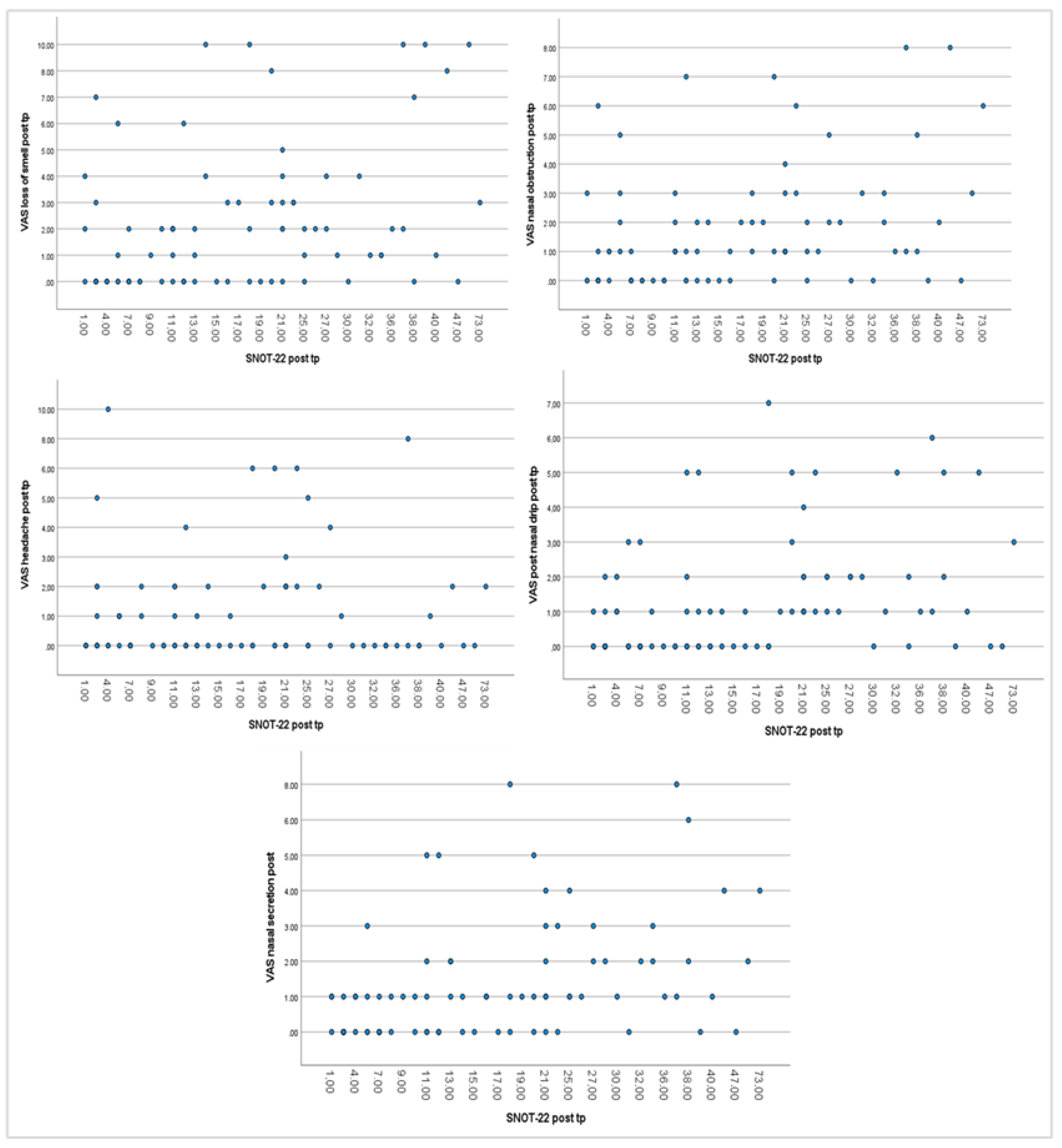
3.4. Correlation Analysis Between Parameters
3.5. Survey Results Regarding the Efficacy of Dupilumab on Psychological State
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CRSwNP | Chronic Rhinosinusitis with Nasal Polyps |
| QoL | Quality of Life |
| IL | Interleukin |
| IgE | Immunoglobulin E |
| NSAID-ERD | Nonsteroidal Anti-Inflammatory Drug-Exacerbated Respiratory Disease |
| INCS | Intranasal Corticosteroids |
| Mabs | Monoclonal antibodies |
| CRS | Chronic Rhinosinusitis |
| AIFA | Italian Agency of Drugs |
| NPS | Nasal Polyp Score |
| SNOT-22 | 22-item Sino-Nasal Outcome Test |
| VAS | Visual Analogue Scale |
| T | Time |
| M | Male |
| F | Female |
| HRQoL | Health-Related Quality of Life |
| CT | Computed Tomography |
| CRSsNP | Chronic Rhinosinusitis without Nasal Polyps |
| PROMs | Patient-Reported Outcome Measures |
References
- Stevens, W.W.; Schleimer, R.P.; Kern, R.C. Chronic Rhinosinusitis with Nasal Polyps. J. Allergy Clin. Immunol. Pract. 2016, 4, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Lund, V.J.; Hopkins, C.; Hellings, P.W.; Kern, R.; Reitsma, S.; Toppila-Salmi, S.; Bernal-Sprekelsen, M.; Mullol, J.; Alobid, I.; et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2020. Rhinology 2020, 58, 1–464. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Campion, N.J.; Villazala-Merino, S.; Liu, D.T.; Bartosik, T.; Landegger, L.D.; Ahmadi, N.; Mueller, C.A.; Vyskocil, E.; Stanek, V.; et al. Associations between the Quality of Life and Nasal Polyp Size in Patients Suffering from Chronic Rhinosinusitis without Nasal Polyps, with Nasal Polyps or Aspirin-Exacerbated Respiratory Disease. J. Clin. Med. 2020, 9, 925. [Google Scholar] [CrossRef]
- Post, M.W. Definitions of quality of life: What has happened and how to move on. Top. Spinal Cord. Inj. Rehabil. 2014, 20, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Alobid, I.; Bernal-Sprekelsen, M.; Mullol, J. Chronic rhinosinusitis and nasal polyps: The role of generic and specific questionnaires on assessing its impact on patient’s quality of life. Allergy 2008, 63, 1267–1279. [Google Scholar] [CrossRef]
- Rudmik, L.; Smith, T.L. Quality of life in patients with chronic rhinosinusitis. Curr. Allergy Asthma Rep. 2011, 11, 247–252. [Google Scholar] [CrossRef]
- Detmar, S.B.; Muller, M.J.; Schornagel, J.H.; Wever, L.D.; Aaronson, N.K. Health-related quality-of-life assessments and patient-physician communication: A randomized controlled trial. JAMA 2002, 288, 3027–3034. [Google Scholar] [CrossRef]
- Costa, D.S.J.; Mercieca-Bebber, R.; Rutherford, C.; Tait, M.A.; King, M.T. How is quality of life defined and assessed in published research? Qual. Life Res. 2021, 30, 2109–2121. [Google Scholar] [CrossRef]
- Kim, J.Y.; Ko, I.; Kim, M.S.; Yu, M.S.; Cho, B.J.; Kim, D.K. Association of Chronic Rhinosinusitis with Depression and Anxiety in a Nationwide Insurance Population. JAMA Otolaryngol. Head. Neck Surg. 2019, 145, 313–319. [Google Scholar] [CrossRef]
- Tomoum, M.O.; Klattcromwell, C.; DelSignore, A.; Ebert, C.; Senior, B.A. Depression and anxiety in chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2015, 5, 674–681. [Google Scholar] [CrossRef]
- Nanayakkara, J.P.; Igwe, C.; Roberts, D.; Hopkins, C. The impact of mental health on chronic rhinosinusitis symptom scores. Eur. Arch. Otorhinolaryngol. 2013, 270, 1361–1364. [Google Scholar] [CrossRef] [PubMed]
- Erskine, S.E.; Hopkins, C.; Clark, A.; Anari, S.; Robertson, A.; Sunkaraneni, S.; Wilson, J.A.; Beezhold, J.; Philpott, C.M. Chronic rhinosinusitis and mood disturbance. Rhinology 2017, 55, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Loperfido, A.; Cavaliere, C.; Begvarfaj, E.; Ciofalo, A.; D’Erme, G.; De Vincentiis, M.; Greco, A.; Millarelli, S.; Bellocchi, G.; Masieri, S. The Impact of Antibiotics and Steroids on the Nasal Microbiome in Patients with Chronic Rhinosinusitis: A Systematic Review According to PICO Criteria. J. Pers. Med. 2023, 13, 1583. [Google Scholar] [CrossRef] [PubMed]
- De Corso, E.; Pipolo, C.; Caminati, M.; Cantone, E.; Seccia, V.; Cecchi, L.; Nettis, E.; Garzaro, M.; Ottaviano, G.; Gelardi, M.; et al. Multidisciplinary Decision-Making-ITAlian Consensus After Two Years of Real Practice on the Management of Severe Uncontrolled CRSwNP by Biologics (ITACA Study). Curr. Allergy Asthma Rep. 2024, 24, 143–154. [Google Scholar] [CrossRef]
- Mullol, J.; Azar, A.; Buchheit, K.M.; Hopkins, C.; Bernstein, J.A. Chronic Rhinosinusitis with Nasal Polyps: Quality of Life in the Biologics Era. J. Allergy Clin. Immunol. Pract. 2022, 10, 1434–1453. [Google Scholar] [CrossRef]
- Nakayama, T.; Hirahara, K.; Onodera, A.; Endo, Y.; Hosokawa, H.; Shinoda, K.; Tumes, D.J.; Okamoto, Y. Th2 Cells in Health and Disease. Annu. Rev. Immunol. 2017, 35, 53–84. [Google Scholar] [CrossRef]
- Fionda, B.; Loperfido, A.; Bussu, F.; Lancellotta, V.; Casà, C.; Vavassori, A.; Vicenzi, L.; Re, A.; Deodato, F.; Morganti, A.G.; et al. The role of radiotherapy in Kimura’s disease: A multicenter systematic review of literature. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4205–4210. [Google Scholar]
- Dunican, E.M.; Fahy, J.V. The Role of Type 2 Inflammation in the Pathogenesis of Asthma Exacerbations. Ann. Am. Thorac. Soc. 2015, 12, 144–149. [Google Scholar] [CrossRef]
- Bachert, C.; Bhattacharyya, N.; Desrosiers, M.; Khan, A.H. Burden of Disease in Chronic Rhinosinusitis with Nasal Polyps. J. Asthma Allergy 2021, 14, 127–134. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Wagenmann, M.; Hosemann, W.; Lee, S.E.; Backer, V.; Mullol, J.; Gevaert, P.; Klimek, L.; Prokopakis, E.; et al. EUFOREA expert board meeting on uncontrolled severe chronic rhinosinusitis with nasal polyps (CRSwNP) and biologics: Definitions and management. J. Allergy Clin. Immunol. 2021, 147, 29–36. [Google Scholar] [CrossRef]
- Loperfido, A.; Ciofalo, A.; Cavaliere, C.; Begvarfaj, E.; Cascone, F.; Alfonzo, G.; Cadeddu, R.; Millarelli, S.; Bellocchi, G.; Greco, A.; et al. Dupilumab’s Impact on Blood Parameters in Nasal Polyposis: 18-Month Follow-Up in Real Life. J. Immunol. Res. 2023, 2023, 4027701. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, C.; Masieri, S.; Begvarfaj, E.; Loperfido, A.; Baroncelli, S.; Cascone, F.; Ciofalo, A. Long-Term Perspectives on Chronic Rhinosinusitis with Nasal Polyps: Evaluating Recurrence Rates after Functional Endoscopic Sinus Surgery in the Biologics Era-A 5-Year Follow-Up Study. J. Pers. Med. 2024, 14, 297. [Google Scholar] [CrossRef] [PubMed]
- Seys, S.F.; Schneider, S.; de Kinderen, J.; Reitsma, S.; Cavaliere, C.; Tomazic, P.V.; Morgenstern, C.; Mortuaire, G.; Wagenmann, M.; Bettio, G.; et al. Real-world effectiveness of dupilumab in a European cohort of chronic rhinosinusitis with nasal polyps (CHRINOSOR). J. Allergy Clin. Immunol. 2024, 155, 451–460. [Google Scholar] [CrossRef]
- Claeys, N.; Teeling, M.T.; Legrand, P.; Poppe, M.; Verschueren, P.; De Prins, L.; Cools, L.; Cypers, L.; Fokkens, W.J.; Hopkins, C.; et al. Corrigendum: Patients Unmet Needs in Chronic Rhinosinusitis with Nasal Polyps Care: A Patient Advisory Board Statement of EUFOREA. Front. Allergy 2021, 2, 789425. [Google Scholar] [CrossRef]
- Busse, W.W.; Pavord, I.D.; Siddiqui, S.; Khan, A.H.; Praestgaard, A.; Nash, S.; Jacob-Nara, J.A.; Rowe, P.J.; Deniz, Y. Dupilumab Improves Outcomes in Patients with Chronic Rhinosinusitis with Nasal Polyps and Coexisting Asthma Irrespective of Baseline Asthma Characteristics. J. Asthma Allergy 2023, 16, 411–419. [Google Scholar] [CrossRef]
- Cavaliere, C.; Loperfido, A.; Ciofalo, A.; Di Michele, L.; Begvarfaj, E.; Bellocchi, G.; Bugani, M.; de Vincentiis, M.; Greco, A.; Millarelli, S.; et al. Real-Life Evidence of Mepolizumab Treatment in Chronic Rhinosinusitis with Nasal Polyps: A Multicentric Study. J. Clin. Med. 2024, 13, 3575. [Google Scholar] [CrossRef]
- Cavaliere, C.; Frati, F.; Ridolo, E.; Greco, A.; de Vincentiis, M.; Masieri, S.; Makri, E.; Incorvaia, C. The spectrum of therapeutic activity of mepolizumab. Expert. Rev. Clin. Immunol. 2019, 15, 959–967. [Google Scholar] [CrossRef]
- Ciofalo, A.; Loperfido, A.; Baroncelli, S.; Masieri, S.; Bellocchi, G.; Caramia, R.; Cascone, F.; Filaferro, L.; Lo Re, F.; Cavaliere, C. Comparison between clinical and cytological findings in chronic rhinosinusitis with nasal polyps treated with Dupilumab. Eur. Arch. Otorhinolaryngol. 2024, 281, 6511–6521. [Google Scholar] [CrossRef]
- Bachert, C.; Han, J.K.; Desrosiers, M.; Hellings, P.W.; Amin, N.; Lee, S.E.; Mullol, J.; Greos, L.S.; Bosso, J.V.; Laidlaw, T.M.; et al. Efficacy and safety of dupilumab in patients with severe chronic rhinosinusitis with nasal polyps (LIBERTY NP SINUS-24 and LIBERTY NP SINUS-52): Results from two multicentre, randomised, double-blind, placebo-controlled, parallel-group phase 3 trials. Lancet 2019, 394, 1638–1650. [Google Scholar] [CrossRef]
- De Corso, E.; Bellocchi, G.; De Benedetto, M.; Lombardo, N.; Macchi, A.; Malvezzi, L.; Motta, G.; Pagella, F.; Vicini, C.; Passali, D. Biologics for severe uncontrolled chronic rhinosinusitis with nasal polyps: A change management approach. Consensus of the Joint Committee of Italian Society of Otorhinolaryngology on biologics in rhinology. Acta Otorhinolaryngol. Ital. 2022, 42, 1–16. [Google Scholar] [CrossRef]
- Plath, M.; Sand, M.; Cavaliere, C.; Plinkert, P.K.; Baumann, I.; Zaoui, K. Normative data for interpreting the SNOT-22. Acta Otorhinolaryngol. Ital. 2023, 43, 390–399. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.H.; Reaney, M.; Guillemin, I.; Nelson, L.; Qin, S.; Kamat, S.; Mannent, L.; Amin, N.; Whalley, D.; Hopkins, C. Development of Sinonasal Outcome Test (SNOT-22) Domains in Chronic Rhinosinusitis with Nasal Polyps. Laryngoscope 2022, 132, 933–941. [Google Scholar] [CrossRef] [PubMed]
- Gallo, S.; Russo, F.; Mozzanica, F.; Preti, A.; Bandi, F.; Costantino, C.; Gera, R.; Ottaviani, F.; Castelnuovo, P. Prognostic value of the Sinonasal Outcome Test 22 (SNOT-22) in chronic rhinosinusitis. Acta Otorhinolaryngol. Ital. 2020, 40, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Doulaptsi, M.; Prokopakis, E.; Seys, S.; Pugin, B.; Steelant, B.; Hellings, P. Visual analogue scale for sino-nasal symptoms severity correlates with sino-nasal outcome test 22: Paving the way for a simple outcome tool of CRS burden. Clin. Transl. Allergy 2018, 8, 32. [Google Scholar] [CrossRef]
- Schalek, P.; Otruba, L.; Hahn, A. Quality of life in patients with chronic rhinosinusitis: A validation of the Czech version of SNOT-22 questionnaire. Eur. Arch. Otorhinolaryngol. 2010, 267, 473–475. [Google Scholar] [CrossRef]
- Dietz de Loos, D.A.; Segboer, C.L.; Gevorgyan, A.; Fokkens, W.J. Disease-specific quality-of-life questionnaires in rhinitis and rhinosinusitis: Review and evaluation. Curr. Allergy Asthma Rep. 2013, 13, 162–170. [Google Scholar] [CrossRef]
- Banerji, A.; Piccirillo, J.F.; Thawley, S.E.; Levitt, R.G.; Schechtman, K.B.; Kramper, M.A.; Hamilos, D.L. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am. J. Rhinol. 2007, 21, 19–26. [Google Scholar] [CrossRef]
- Toros, S.Z.; Bölükbasi, S.; Naiboğlu, B.; Er, B.; Akkaynak, C.; Noshari, H.; Egeli, E. Comparative outcomes of endoscopic sinus surgery in patients with chronic sinusitis and nasal polyps. Eur. Arch. Otorhinolaryngol. 2007, 264, 1003–1008. [Google Scholar] [CrossRef]
- Orlandi, R.R.; Kingdom, T.T.; Hwang, P.H.; Smith, T.L.; Alt, J.A.; Baroody, F.M.; Batra, P.S.; Bernal-Sprekelsen, M.; Bhattacharyya, N.; Chandra, R.K.; et al. International Consensus Statement on Allergy and Rhinology: Rhinosinusitis. Int. Forum Allergy Rhinol. 2016, 6, S22–S209. [Google Scholar] [CrossRef]
- Canonica, G.W.; Malvezzi, L.; Blasi, F.; Paggiaro, P.; Mantero, M.; Senna, G.; Heffler, E.; Severe Asthma Network Italy (SANI). Chronic rhinosinusitis with nasal polyps impact in severe asthma patients: Evidences from the Severe Asthma Network Italy (SANI) registry. Respir. Med. 2020, 166, 105947. [Google Scholar] [CrossRef]
- Guilemany, J.M.; Mariño-Sánchez, F.S.; Angrill, J.; Alobid, I.; Centellas, S.; Pujols, L.; Berenguer, J.; Bernal-Sprekelsen, M.; Picado, C.; Mullol, J. The importance of smell in patients with bronchiectasis. Respir. Med. 2011, 105, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Handley, E.; Nicolson, C.H.; Hew, M.; Lee, A.L. Prevalence and Clinical Implications of Chronic Rhinosinusitis in People with Bronchiectasis: A Systematic Review. J. Allergy Clin. Immunol. Pract. 2019, 7, 2004–2012. [Google Scholar] [CrossRef] [PubMed]
- Hoehle, L.P.; Phillips, K.M.; Bergmark, R.W.; Caradonna, D.S.; Gray, S.T.; Sedaghat, A.R. Symptoms of chronic rhinosinusitis differentially impact general health-related quality of life. Rhinology 2016, 54, 316–322. [Google Scholar] [CrossRef] [PubMed]
- Fokkens, W.J.; Viskens, A.S.; Backer, V.; Conti, D.; De Corso, E.; Gevaert, P.; Scadding, G.K.; Wagemann, M.; Bernal-Sprekelsen, M.; Chaker, A.; et al. EPOS/EUFOREA update on indication and evaluation of Biologics in Chronic Rhinosinusitis with Nasal Polyps 2023. Rhinology 2023, 61, 194–202. [Google Scholar] [CrossRef]
- Millarelli, S.; Loperfido, A.; Mammarella, F.; Giorgione, C.; Celebrini, A.; Del Ninno, M.; Bellocchi, G. A Practical Clinical Protocol for Monitoring Patients with Severe Uncontrolled Chronic Rhinosinusitis with Nasal Polyposis Treated with Biologics. Allergy Rhinol. 2022, 13, 21526567221074335. [Google Scholar] [CrossRef]
- Fokkens, W.J.; De Corso, E.; Backer, V.; Bernal-Sprekelsen, M.; Bjermer, L.; von Buchwald, C.; Chaker, A.; Diamant, Z.; Gevaert, P.; Han, J.; et al. EPOS2020/EUFOREA expert opinion on defining disease states and therapeutic goals in CRSwNP. Rhinology 2024, 62, 287–298. [Google Scholar] [CrossRef]
- Bellocchi, G.; Loperfido, A.; Passali, F.M.; Millarelli, S.; Velletrani, G.; Perla, M.; Di Michele, L.; Di Girolamo, S. Biologics in severe uncontrolled chronic rhinosinusitis with nasal polyps: A bicentric experience. Acta Biomed. 2023, 94, 2023227. [Google Scholar]
- Fu, Q.L.; Ma, J.X.; Ou, C.Q.; Guo, C.; Shen, S.Q.; Xu, G.; Shi, J. Influence of self-reported chronic rhinosinusitis on health-related quality of life: A population-based survey. PLoS ONE 2015, 10, e0126881. [Google Scholar] [CrossRef]
- Luke, L.; Lee, L.; Gokani, S.A.; Boak, D.; Boardman, J.; Philpott, C. Understanding the Impact of Chronic Rhinosinusitis with Nasal Polyposis on Smell and Taste: An International Patient Experience Survey. J. Clin. Med. 2023, 12, 5367. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Turner, J.; Kelly, B. Emotional dimensions of chronic disease. West. J. Med. 2000, 172, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Hellings, P.W.; Peters, A.T.; Chaker, A.M.; Heffler, E.; Zhang, H.; Praestgaard, A.; Nash, S.; Khan, A.H.; Siddiqui, S.; Jacob-Nara, J.A.; et al. Rapid and sustained effects of dupilumab in severe chronic rhinosinusitis with nasal polyps. Int. Forum Allergy Rhinol. 2022, 12, 958–962. [Google Scholar] [CrossRef] [PubMed]
- Canonica, G.W.; Bourdin, A.; Peters, A.T.; Desrosiers, M.; Bachert, C.; Weidinger, S.; Simpson, E.L.; Daizadeh, N.; Chen, Z.; Kamat, S.; et al. Dupilumab Demonstrates Rapid Onset of Response Across Three Type 2 Inflammatory Diseases. J. Allergy Clin. Immunol. Pract. 2022, 10, 1515–1526. [Google Scholar] [CrossRef] [PubMed]
- Quintanilla-Dieck, L.; Litvack, J.R.; Mace, J.C.; Smith, T.L. Comparison of disease-specific quality-of-life instruments in the assessment of chronic rhinosinusitis. Int. Forum Allergy Rhinol. 2012, 2, 437–443. [Google Scholar] [CrossRef]

| Question | T0 | T1–T3 | T3–T6 | T6–T12 | T12–T18 | T18–T24 | |
|---|---|---|---|---|---|---|---|
| 1. I have full confidence in myself | Mean | 1.91 | 1.9 | 1.5 | 2.36 | 2.21 | 2.51 |
| SD | 1.1 | 1.0 | 1.3 | 1.0 | 1.1 | 0.9 | |
| 2. I often feel sad and discouraged | Mean | 1.01 | 0.7 | 1.13 | 0.27 | 0.29 | 0.23 |
| SD | 1.1 | 0.7 | 1.4 | 0.5 | 0.6 | 0.6 | |
| 3. Sometimes I feel in a condition of constant danger and I avoid going out because I feel safer at home | Mean | 0.41 | 0.1 | 0.63 | 0 | 0.14 | 0.05 |
| SD | 0.8 | 0.3 | 1.1 | 0.0 | 0.5 | 0.2 | |
| 4. Sometimes I think life is difficult and I can’t do anything about my future | Mean | 0.64 | 0.7 | 0.75 | 0 | 0.36 | 0.05 |
| SD | 1.1 | 1.3 | 1.4 | 0.0 | 0.6 | 0.2 | |
| 5. I think something bad might happen (to me or to some of my family members) | Mean | 0.56 | 1 | 0.5 | 0.09 | 0.29 | 0.23 |
| SD | 0.9 | 0.7 | 0.5 | 0.3 | 0.8 | 0.6 | |
| 6. Anxiety stops me from being active and taking initiative | Mean | 0.62 | 0.1 | 0.38 | 0.09 | 0.64 | 0.16 |
| SD | 0.9 | 0.3 | 0.7 | 0.3 | 0.8 | 0.4 | |
| 7. Sometimes I feel like I don’t care about my health anymore | Mean | 0.24 | 0 | 0.13 | 0 | 0 | 0.05 |
| SD | 0.6 | 0.0 | 0.4 | 0.0 | 0.0 | 0.2 | |
| 8. I have a hard time remembering important things to do/medicines to take | Mean | 0.34 | 0.3 | 0.5 | 0.27 | 0.21 | 0.16 |
| SD | 0.7 | 0.5 | 1.1 | 0.6 | 0.6 | 0.5 | |
| 9. I have lost interest in some activities previously considered interesting and/or enjoyable (hobbies, sports, interests) | Mean | 0.84 | 0.5 | 0.25 | 0.27 | 0.29 | 0.33 |
| SD | 1.0 | 0.7 | 0.5 | 0.6 | 0.5 | 0.6 | |
| 10. I worry about every little thing | Mean | 0.77 | 0.91 | 0.71 | 0.36 | 0.29 | 0.4 |
| SD | 1.0 | 0.9 | 1.1 | 0.5 | 0.5 | 0.8 | |
| 11. Sometimes I feel tense, nervous, irritable | Mean | 1.24 | 1.1 | 0.75 | 0.45 | 0.64 | 0.44 |
| SD | 1.0 | 1.1 | 0.9 | 0.7 | 0.7 | 0.7 | |
| 12. Sometimes I have a choking sensation and/or shortness of breath | Mean | 1.31 | 0.5 | 0.63 | 0.45 | 0.57 | 0.14 |
| SD | 1.1 | 0.9 | 0.7 | 0.7 | 0.6 | 0.4 | |
| 13. I have trouble falling asleep and/or wake up often during the night | Mean | 1.53 | 1.3 | 1 | 0.82 | 0.64 | 0.44 |
| SD | 1.1 | 1.3 | 1.3 | 1.0 | 0.9 | 0.7 | |
| 14. Sometimes I feel weak for no reason | Mean | 1.08 | 0.5 | 0.5 | 0.27 | 0.93 | 0.26 |
| SD | 1.0 | 0.7 | 0.8 | 0.6 | 1.1 | 0.5 | |
| 15. I often have palpitations | Mean | 0.67 | 0.9 | 0.13 | 0.18 | 0.57 | 0.23 |
| SD | 0.9 | 0.9 | 0.4 | 0.4 | 0.8 | 0.6 | |
| 16. I often feel tired, with no more energy | Mean | 1.22 | 1.2 | 0.63 | 0.55 | 0.71 | 0.3 |
| SD | 0.975 | 0.632 | 0.916 | 0.688 | 1.069 | 0.558 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masieri, S.; Cavaliere, C.; Loperfido, A.; Begvarfaj, E.; Ciofalo, A.; Primerano, F.M.; Velletrani, G.; Bugani, M.; Cirilli, P.; Passali, F.M.; et al. Pilot Study on the Efficacy of a Novel Questionnaire for Assessing Psychological Health in Patients with Chronic Rhinosinusitis with Nasal Polyps Treated with Biologics. Healthcare 2025, 13, 433. https://doi.org/10.3390/healthcare13040433
Masieri S, Cavaliere C, Loperfido A, Begvarfaj E, Ciofalo A, Primerano FM, Velletrani G, Bugani M, Cirilli P, Passali FM, et al. Pilot Study on the Efficacy of a Novel Questionnaire for Assessing Psychological Health in Patients with Chronic Rhinosinusitis with Nasal Polyps Treated with Biologics. Healthcare. 2025; 13(4):433. https://doi.org/10.3390/healthcare13040433
Chicago/Turabian StyleMasieri, Simonetta, Carlo Cavaliere, Antonella Loperfido, Elona Begvarfaj, Andrea Ciofalo, Francesco Maria Primerano, Gianluca Velletrani, Marcella Bugani, Pamela Cirilli, Francesco Maria Passali, and et al. 2025. "Pilot Study on the Efficacy of a Novel Questionnaire for Assessing Psychological Health in Patients with Chronic Rhinosinusitis with Nasal Polyps Treated with Biologics" Healthcare 13, no. 4: 433. https://doi.org/10.3390/healthcare13040433
APA StyleMasieri, S., Cavaliere, C., Loperfido, A., Begvarfaj, E., Ciofalo, A., Primerano, F. M., Velletrani, G., Bugani, M., Cirilli, P., Passali, F. M., Millarelli, S., Bellocchi, G., & Di Girolamo, S. (2025). Pilot Study on the Efficacy of a Novel Questionnaire for Assessing Psychological Health in Patients with Chronic Rhinosinusitis with Nasal Polyps Treated with Biologics. Healthcare, 13(4), 433. https://doi.org/10.3390/healthcare13040433







