1. Introduction
Tobacco use remains one of the leading preventable causes of morbidity and mortality worldwide [
1]. It significantly contributes to the global burden of non-communicable diseases, including cardiovascular diseases, chronic respiratory illnesses, and various forms of cancer [
2]. Despite increasing awareness and global public health efforts, tobacco consumption remains widespread in many low- and middle-income countries, particularly among men [
3].
Among tobacco-related cancers, kidney, prostate, and bladder cancers represent the most common malignancies of the genitourinary system [
4]. These cancers are associated with substantial mortality, years of life lost, and rising treatment costs, especially in countries with aging populations and limited cancer screening capacity [
5]. According to recent global cancer estimates, the incidence and mortality of these cancers have increased steadily over the past two decades, with wide disparities across regions and income levels [
6].
There is strong epidemiological evidence linking tobacco smoke exposure to increased risk of both kidney and bladder cancers [
7], with dose-dependent relationships observed in multiple meta-analyses. While the link between smoking and prostate cancer is less direct, studies have shown associations with more aggressive disease, higher mortality, and poorer treatment outcomes in smokers [
8].
The Global Burden of Disease (GBD) study framework provides a comprehensive approach to quantifying disease burden and its associated risk factors across countries and time periods. Within this framework, the estimated annual percentage change (EAPC) is commonly used to describe temporal trends in age-standardized rates and allows for comparison of changing burdens among different populations.
The BRICS countries—Brazil, Russia, India, China, and South Africa—collectively account for over 40% of the global population and face a growing burden of non-communicable diseases [
9]. Despite varying levels of tobacco control policy implementation, these countries share similar challenges, including high smoking prevalence (especially among men), demographic aging, limited access to early cancer detection, and underfunded cancer surveillance systems [
10]. Few studies have systematically quantified the burden of kidney, prostate, and bladder cancers attributable to tobacco smoke exposure across BRICS countries using the GBD 2021 framework [
11].
In this study, we aimed to estimate the deaths, disability-adjusted life years (DALYs), years of life lost (YLLs), and years lived with disability (YLDs) of kidney, prostate, and bladder cancers attributable to both active and secondhand tobacco smoke exposure in BRICS countries from 1990 to 2021, and to explore temporal trends, sex and age disparities, and national heterogeneity. These findings aim to inform regional policy responses and highlight priority areas for targeted tobacco control, early detection, and survivorship care within the BRICS context.
2. Methods
2.1. Data Sources
This study utilized data from the GBD 2021, which provides annual estimates for 371 diseases and 88 risk factors across 204 countries and territories from 1990 to 2021. All data were accessed via the Global Health Data Exchange (GHDx) results tool (
https://ghdx.healthdata.org/gbd-results-tool [accessed on 19 February 2025]). The GBD estimates adhere to the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) framework and are based on standardized methodologies for data collection, modeling, and risk attribution.
The GBD employs a comparative risk assessment framework using multiple data sources, including national surveys, cancer registries, vital registration systems, and household exposure assessments. These data are modeled using Bayesian meta-regression (DisMod-MR 2.1) to generate consistent estimates where empirical data are sparse.
2.2. Cancer Definitions
We assessed the burden of three genitourinary cancers based on the following International Classification of Diseases (ICD-10 and ICD-9) codes used in the GBD 2021:
Kidney cancer: C64–C65 (ICD-10), 189.0–189.1 (ICD-9);
Prostate cancer: C61 (ICD-10), 185 (ICD-9);
Bladder cancer: C67 (ICD-10), 188 (ICD-9).
The cancer burden metrics were estimated for each cancer site separately, including incidence, mortality, and DALYs, which are composed of YLLs and YLDs.
2.3. Exposure and Risk Attribution
Tobacco exposure included both active smoking and secondhand (passive) smoke exposure. Active smoking refers to current use of cigarettes, cigars, or other tobacco products, while secondhand smoke refers to inhalation of tobacco combustion products by nonsmokers in domestic or public environments.
Risk–outcome pairs were selected based on strong evidence of causality, following criteria from the World Cancer Research Fund (WCRF) and International Agency for Research on Cancer (IARC). Population exposure distributions were derived from nationally representative surveys and modeled using Bayesian meta-regression approaches in DisMod-MR 2.1.
The comparative risk assessment (CRA) framework was used to estimate the proportion of cancer burden attributable to tobacco smoke. This involved the computation of population attributable fractions (PAFs), which represent the proportion of disease burden that could be avoided if exposure to tobacco were reduced to the theoretical minimum risk exposure level (TMREL), defined as no smoking or exposure to secondhand smoke.
PAFs were calculated by combining the exposure distribution, relative risk (RR) functions from meta-analyses, and TMREL values. These fractions were applied to cancer-specific estimates of deaths, DALYs, YLLs, and YLDs to determine the burden attributable to tobacco smoke.
2.4. Indicators and Stratification
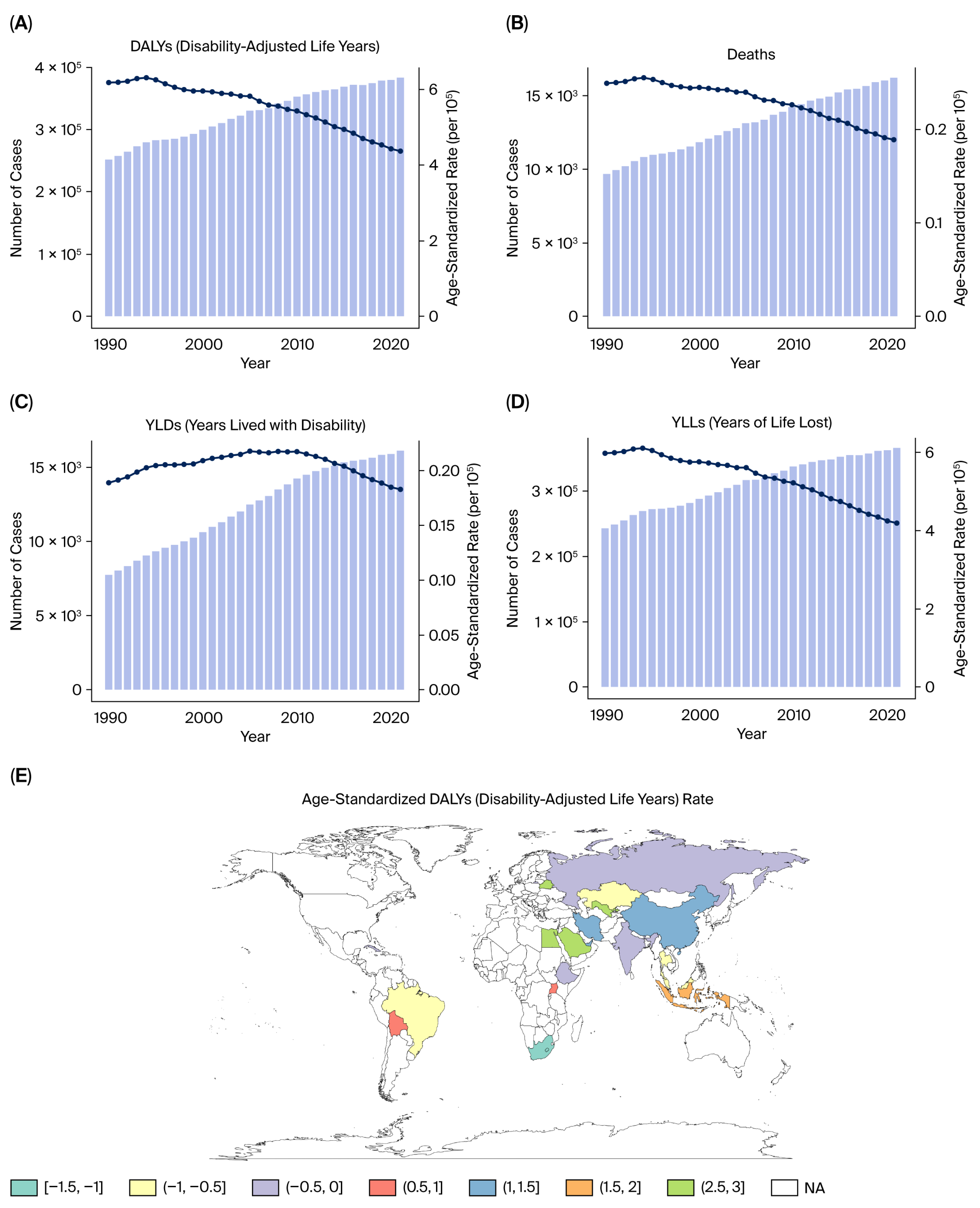
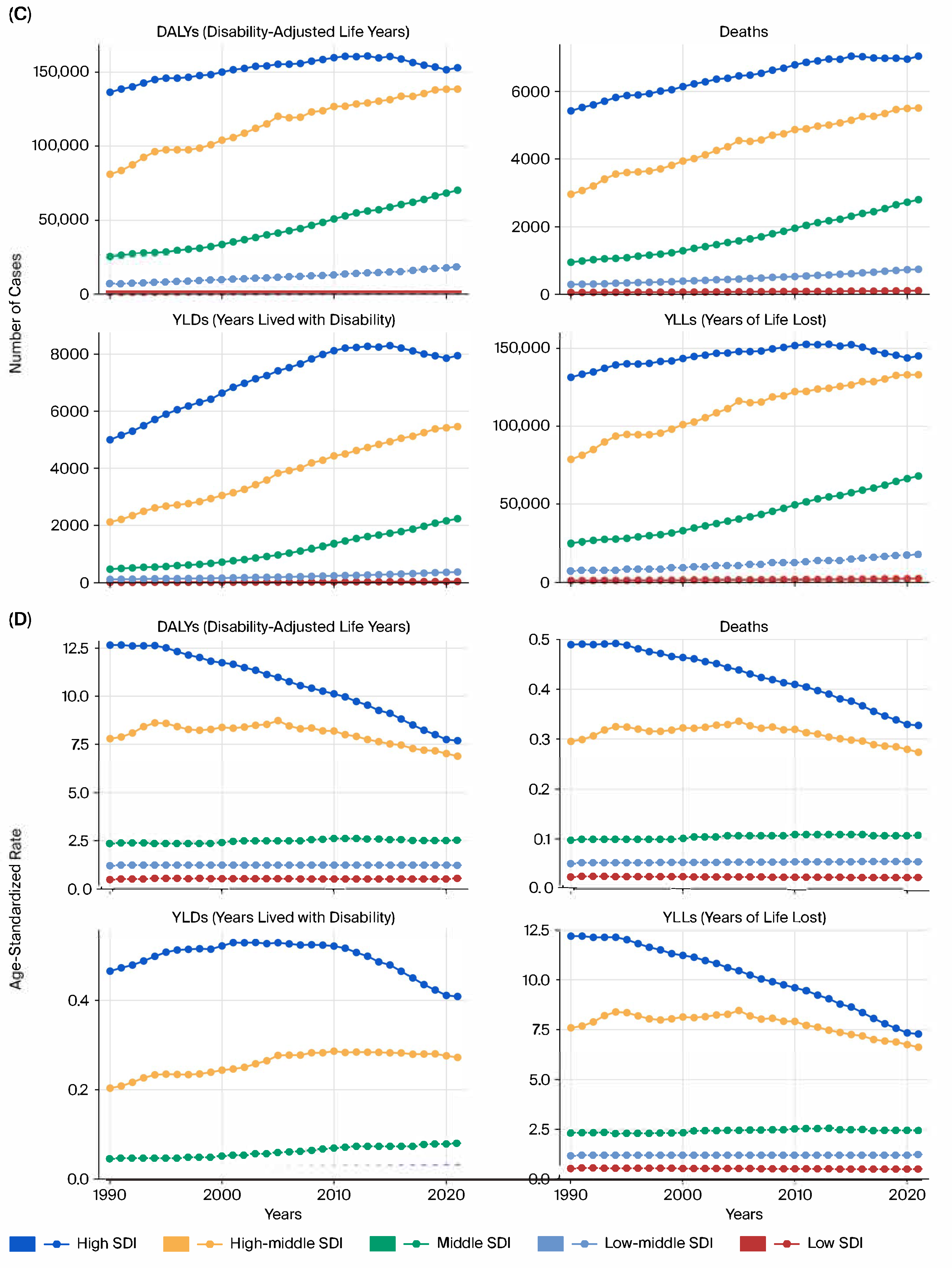
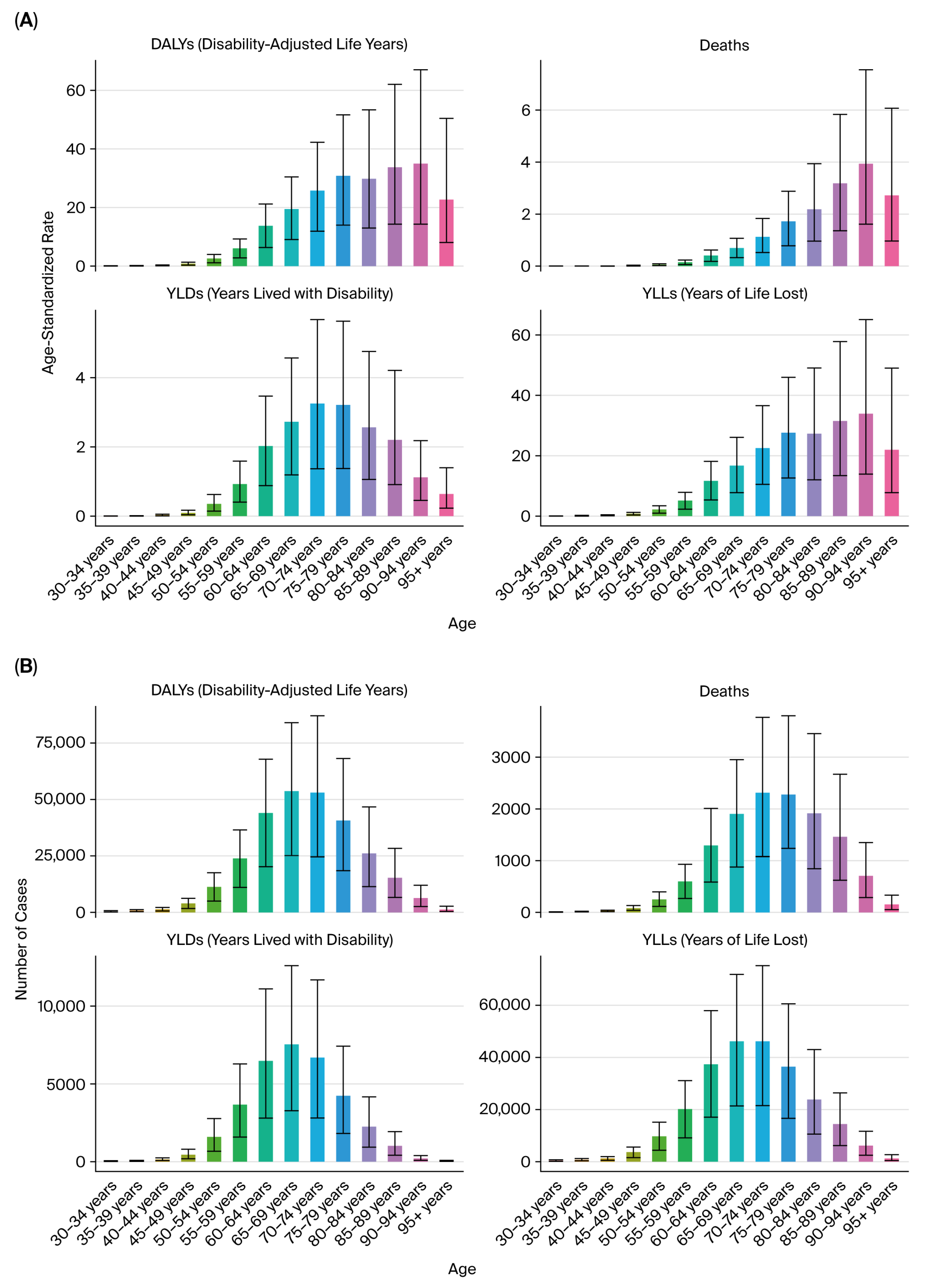
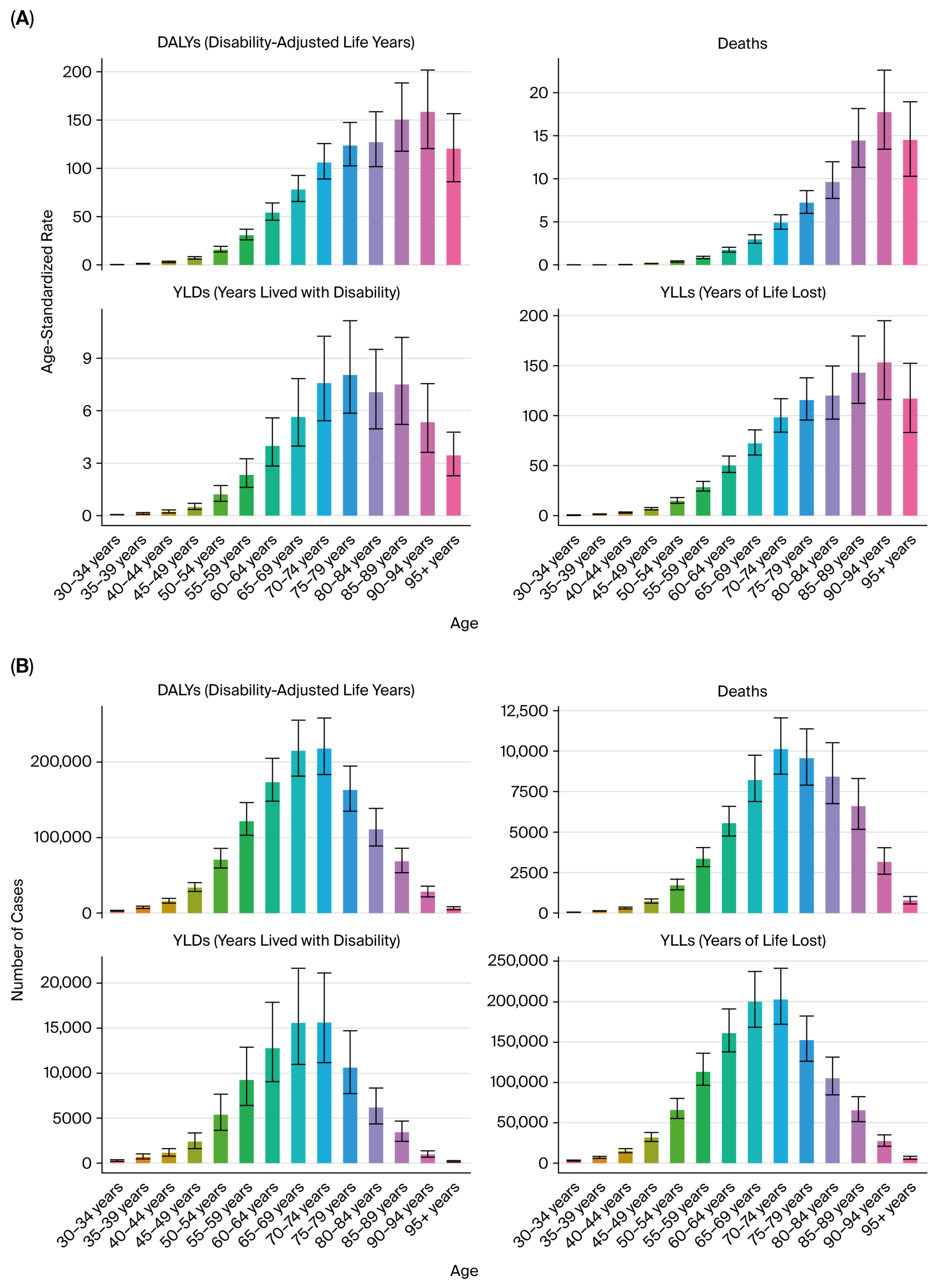
We reported the following key epidemiological indicators attributable to tobacco smoke exposure for each cancer type: deaths, DALYs, YLLs, and YLDs.
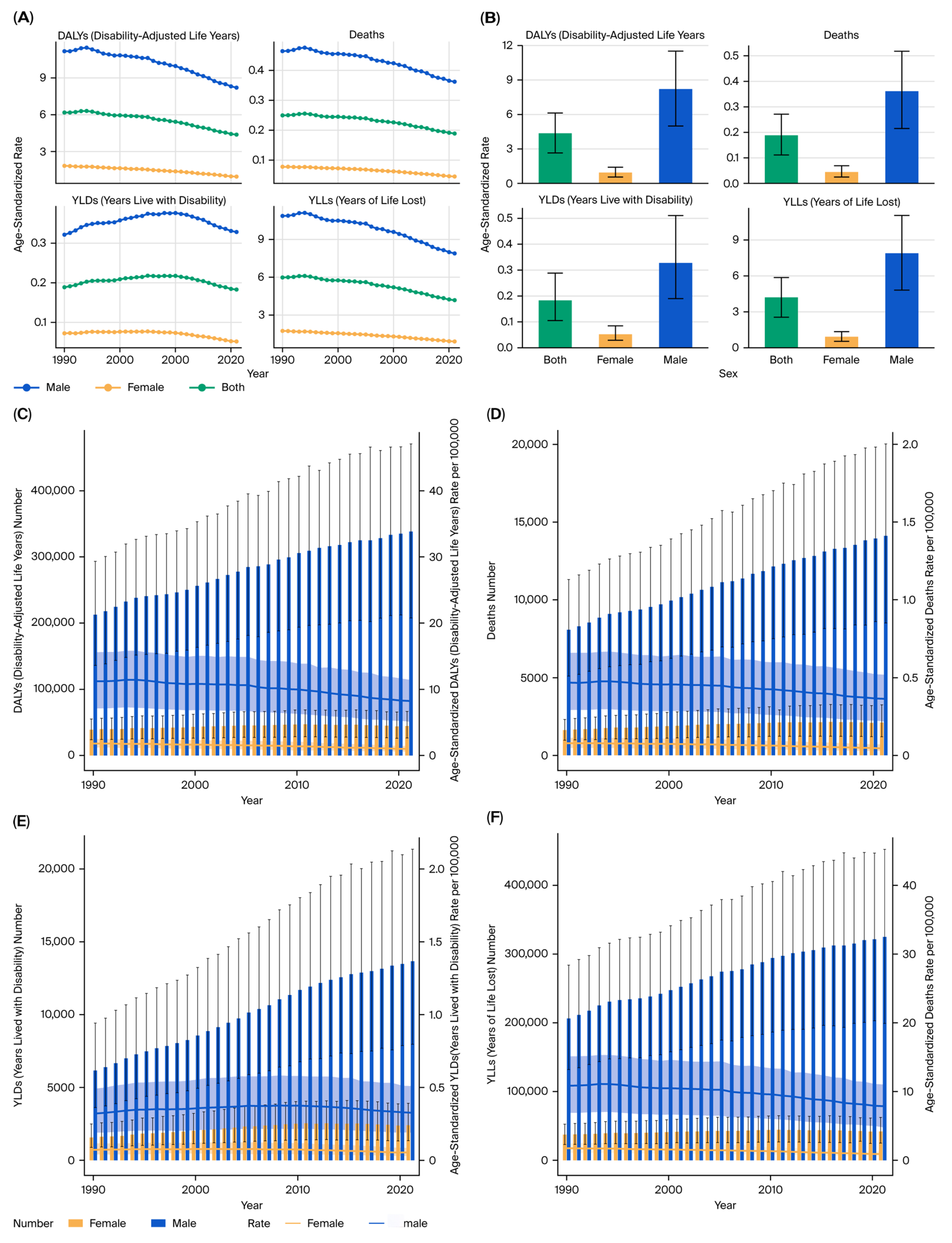
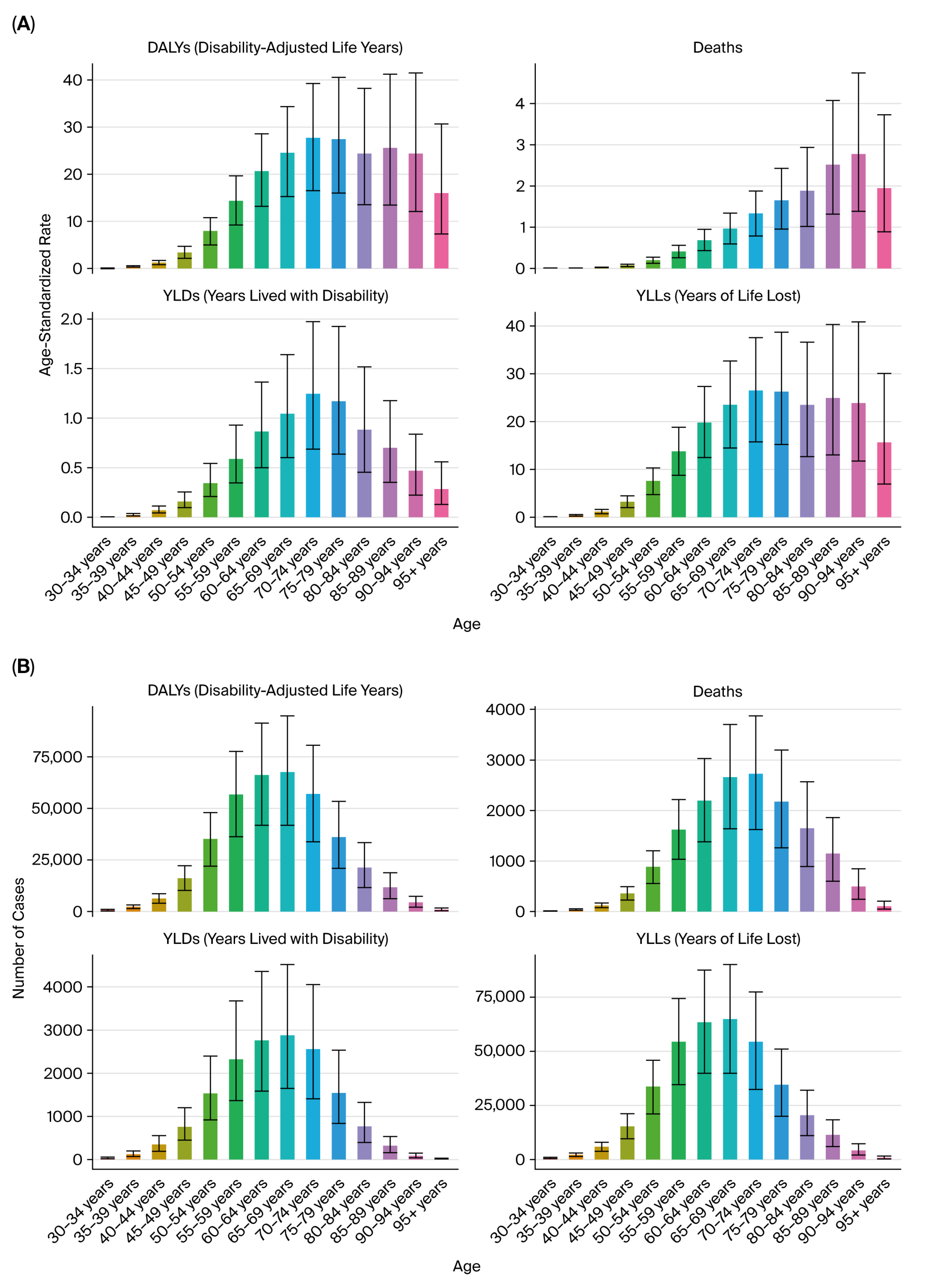
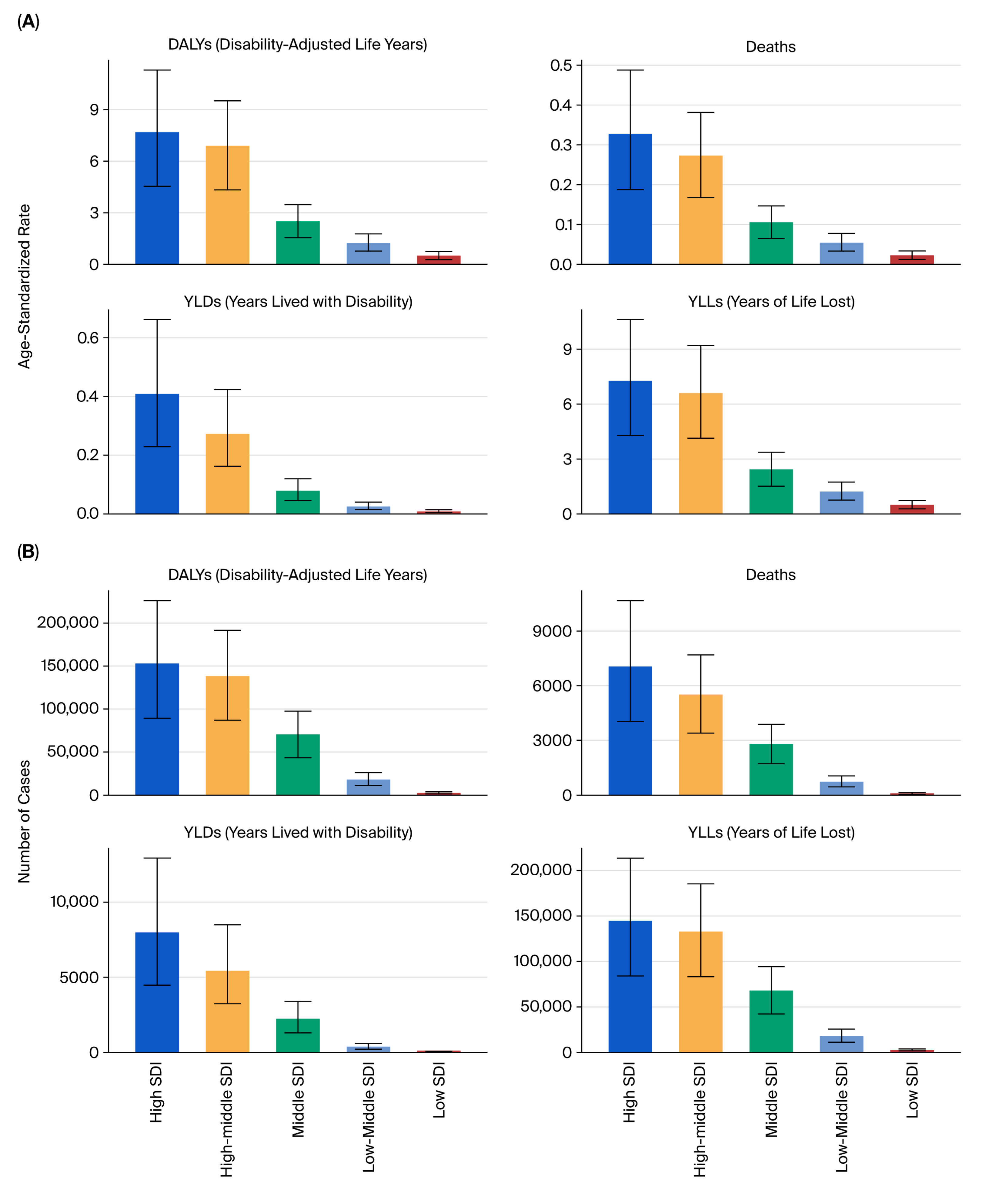
All estimates were age-standardized rates (ASRs) per 100,000 population using the GBD reference population, enabling cross-region and cross-year comparability. The results were stratified by sex, age group (in 5-year intervals from <20 to >95 years), and sociodemographic index (SDI) quintiles (low, low–middle, middle, high–middle, and high SDI).
All key estimates are presented with 95% uncertainty intervals (UIs), derived from 1000 posterior draws of the GBD model outputs.
2.5. Trend Analysis
We calculated the EAPC of ASRs using a log-linear regression model to evaluate long-term temporal changes in burden:
where β is the slope of the regression line fitted to the natural logarithm of the ASR over the calendar year. An EAPC > 0 with the lower bound of the 95% confidence interval (CI) > 0 indicates an increasing trend; an EAPC < 0 with the upper CI < 0 indicates a decreasing trend.
Confidence intervals (95% CIs) for all EAPC estimates were calculated from the regression model’s variance–covariance matrix, allowing interpretation of trend significance.
2.6. Uncertainty Estimation and Data Quality
All estimates were generated using 1000 posterior draws to calculate 95% uncertainty intervals (95% UIs), defined as the 2.5th and 97.5th percentiles of the posterior distribution.
The GBD applies multiple imputation and Bayesian-smoothing methods in countries with incomplete or poor-quality registry data (notably, India and South Africa) to reduce reporting bias. The remaining uncertainty due to underreporting was incorporated into the UIs.
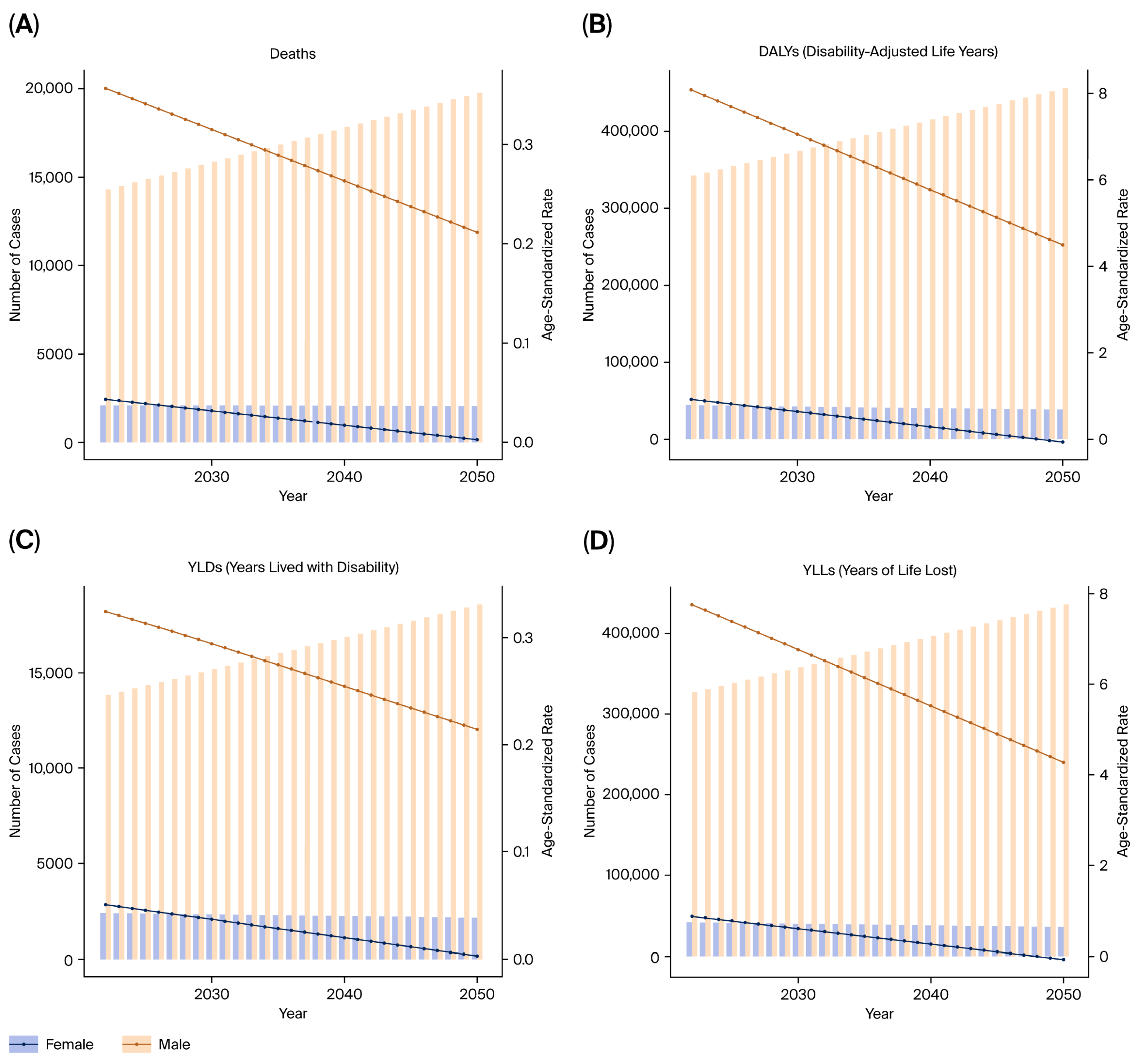
2.7. Forecasting
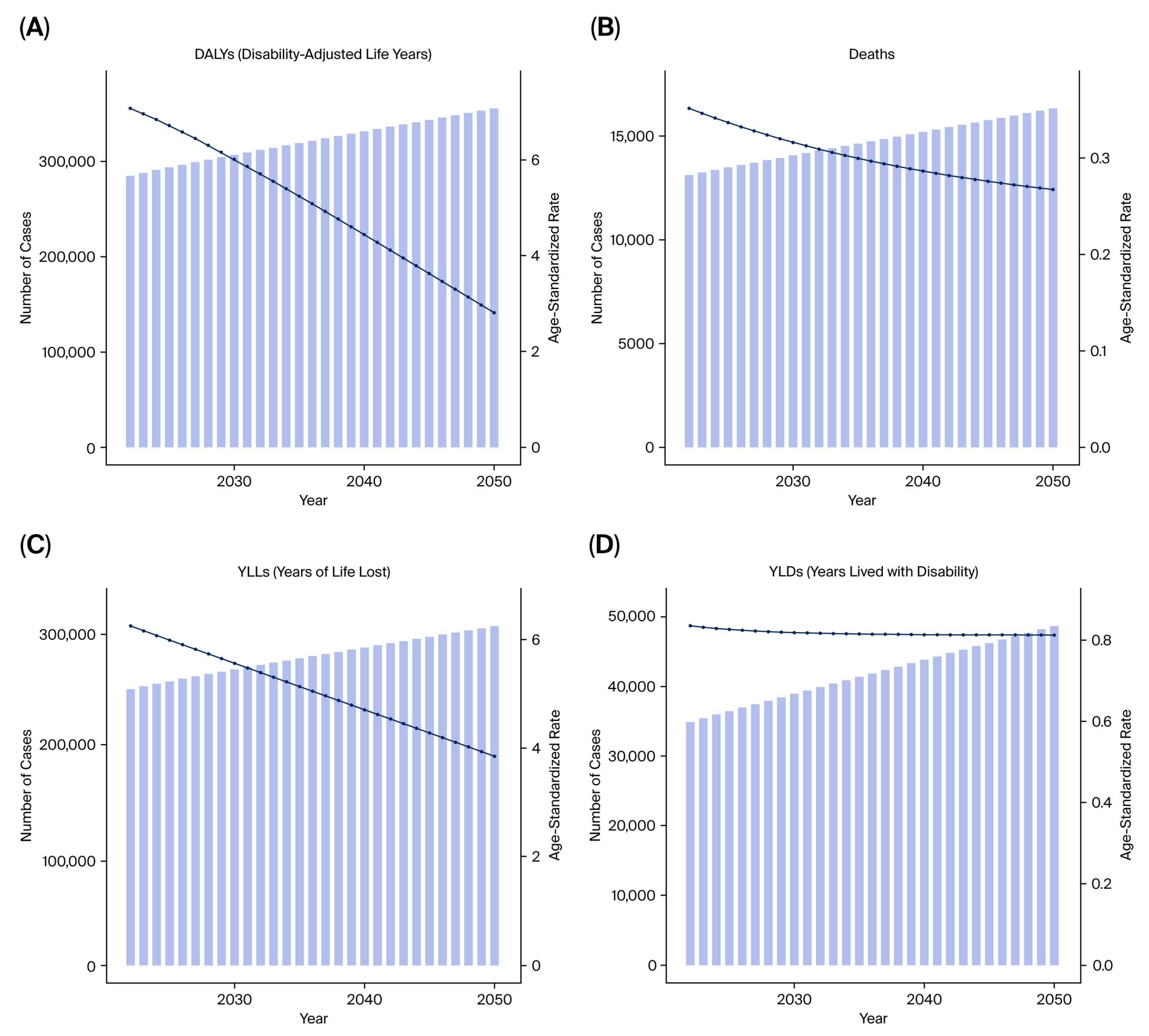
Future projections were modeled using the autoregressive integrated moving average (ARIMA) method, which predicts future values of DALYs, YLLs, and YLDs based on historical temporal patterns from 1990 to 2021. The model performance was evaluated by comparing predicted versus observed values via 10-fold cross-validation.
2.8. Software
All data processing and statistical analyses were performed using R (version 4.2.2; R Foundation for Statistical Computing, Vienna, Austria) and Python (version 3.10; Python Software Foundation, Wilmington, DE, USA). Data visualization was conducted using ggplot2 (version 3.4.4; part of the tidyverse collection, RStudio, PBC, Boston, MA, USA), matplotlib (version 3.7.5; The Matplotlib Development Team, USA), and seaborn (version 0.12.2; The Seaborn Development Team, USA).
6. Discussion
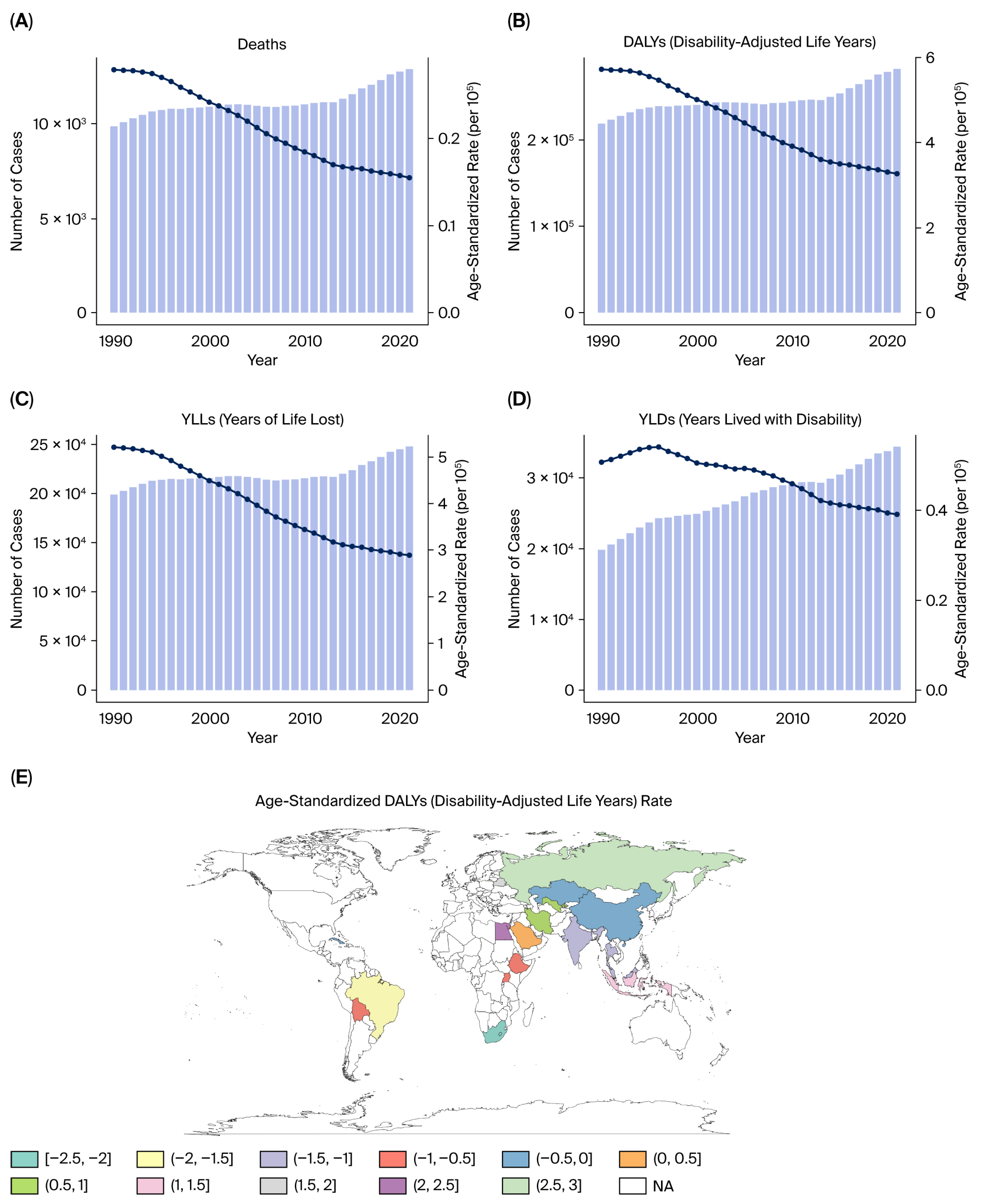
This study provides a comprehensive evaluation of the burden of kidney, prostate, and bladder cancers attributable to tobacco smoke exposure in the five BRICS countries from 1990 to 2021, based on data from the Global Burden of Disease 2021 framework. Across all three malignancies, tobacco exposure accounted for substantial proportions of deaths and DALYs, with persistently higher burdens in men and in high- or high–middle-SDI settings.
Despite improvements in public health infrastructure and the gradual decline in smoking prevalence in some regions, the cumulative burden of tobacco-related urological cancers remains high, reflecting both population aging and the delayed impact of smoking on cancer incidence.
Although certain countries have implemented national tobacco-control strategies, the overall tobacco-related cancer burden in BRICS remains considerable, emphasizing the need for integrated cancer-prevention and cessation efforts.
6.1. Comparative Patterns Across BRICS Countries
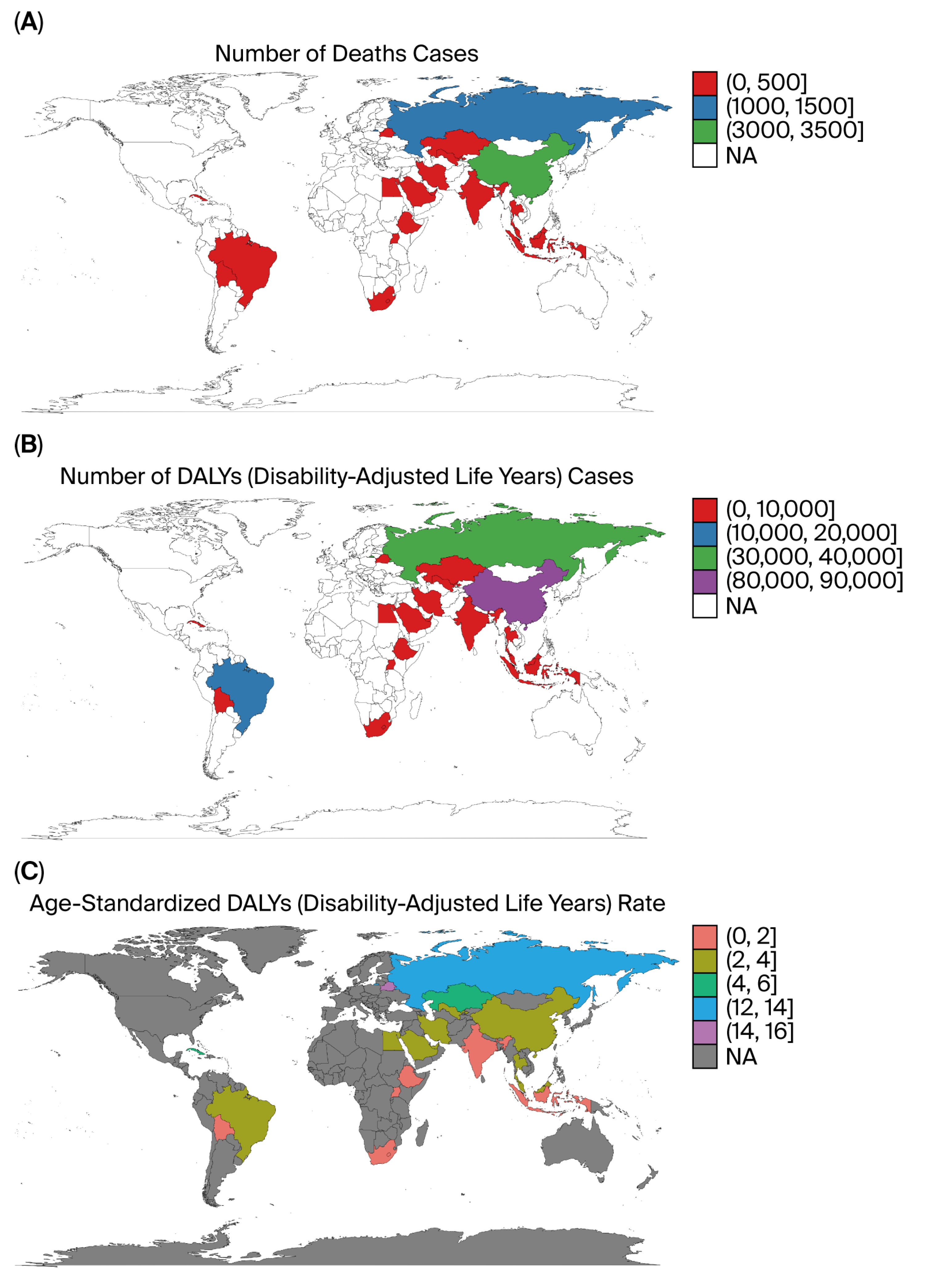
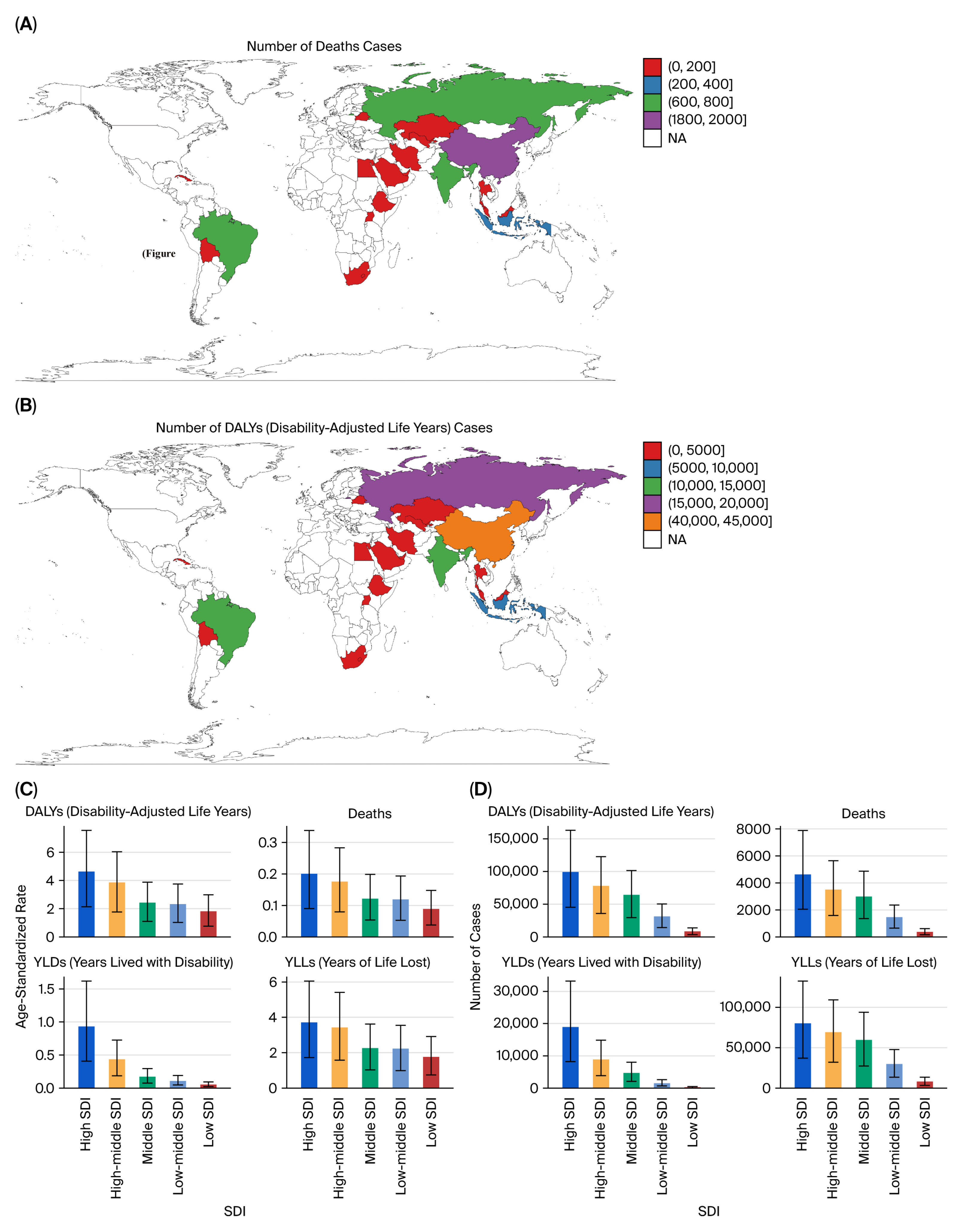
Marked heterogeneity was observed across the BRICS nations. China and Russia contributed the largest absolute number of deaths and DALYs, reflecting their large populations and high historical smoking prevalence, whereas South Africa exhibited the highest per capita rates, particularly for bladder cancer. India showed the lowest age-standardized rates but steady increases in absolute burden, likely due to demographic growth, urbanization, and delayed tobacco-control implementation. Brazil demonstrated gradual improvement after the introduction of stringent FCTC-aligned policies in the 2000s [
1,
2,
11,
12]. These inter-country differences mirror diverse epidemiological transitions, tobacco market penetration, and healthcare capacities within the BRICS bloc.
Sex-specific differences were pronounced: male-to-female DALY ratios exceeded 3:1 for kidney and bladder cancers [
4,
5,
6], consistent with higher smoking prevalence, occupational exposures, and androgen-driven susceptibility that may influence detoxification enzyme activity and DNA repair capacity [
13,
14,
15,
16,
17].
6.2. Biological and Exposure Mechanisms
Tobacco smoke remains one of the most pervasive and biologically potent carcinogenic exposures worldwide [
7,
8]. It contains numerous mutagens—including aromatic amines, nitrosamines, and polycyclic aromatic hydrocarbons—that induce DNA adducts, promote oxidative stress, and alter immune and vascular homeostasis.
For bladder cancer, these metabolites are filtered by the kidneys and concentrated in the urine, leading to direct urothelial contact and a mutation spectrum characterized by G → T transversions, consistent with tobacco-specific carcinogen signatures [
18,
19].
In the kidneys, chronic exposure to tobacco toxins induces tubular cell injury, endothelial dysfunction, and persistent inflammatory signaling, all of which can accelerate tumor initiation and progression [
20,
21].
Although the causal association with prostate cancer is less direct, several epidemiological studies have linked active smoking to higher Gleason grades, biochemical recurrence, and prostate-cancer-specific mortality [
22,
23,
24].
Secondhand smoke exposure contributes meaningfully to disease burden, particularly among women and children in households with a high smoking prevalence, yet remains underrepresented in current global surveillance frameworks.
6.3. Socioeconomic and Health System Implications
The social gradient of tobacco-attributable cancer burden underscores both behavioral and structural inequities across BRICS countries [
9,
11]. High- and high–middle-SDI countries such as China, Brazil, and Russia contributed the largest absolute number of DALYs and deaths, reflecting more complete cancer registry coverage, greater diagnostic capacity, and aging populations. In contrast, low-SDI countries such as India and South Africa exhibited faster growth in age-standardized DALY rates [
11], suggesting delayed enforcement of tobacco control measures, limited early detection, and under-resourced oncology infrastructure.
When compared with developed countries (i.e., high-SDI or high-income regions), the BRICS nations generally exhibit slower declines or stable trends in tobacco-attributable genitourinary cancers. Developed regions such as Western Europe and North America achieved earlier and more comprehensive implementation of tobacco control policies [
2,
9]—including taxation, advertising bans, and smoke-free regulations—resulting in sustained reductions in smoking prevalence and tobacco-related cancer mortality. In contrast, BRICS countries continue to face rising absolute burdens driven by population aging, delayed policy enforcement, and limited access to early-detection programs. This divergence highlights the importance of policy maturity and long-term investment in preventive infrastructure for achieving meaningful reductions in tobacco-related cancer burden.
Moreover, the relative risk coefficients (RRs) applied in the GBD model are largely derived from high-income cohorts; region-specific exposure–response functions in BRICS populations should be validated to improve precision. This limitation may partly explain the residual uncertainty and potential underestimation of the true regional burden.
6.4. Tobacco Control and Prevention Policies
Tobacco control remains the most effective and cost-efficient strategy for reducing the burden of urological malignancies. All BRICS countries are parties to the WHO Framework Convention on Tobacco Control (FCTC), yet the scale and rigor of implementation differ markedly [
12]. Brazil and Russia have achieved significant reductions in smoking prevalence via taxation, graphic warnings, and advertising restrictions, whereas China and India continue to face challenges in enforcement, particularly in rural areas and among low-income groups.
Integrating cessation counseling, pharmacotherapy, and routine screening for high-risk individuals into primary care could reduce incidence while improving early-stage diagnosis.
Additionally, policies addressing secondhand smoke exposure—such as smoke-free public spaces, family education, and workplace bans—should be expanded to protect non-smokers, especially women and children.
Despite existing commitments, the growing burden of tobacco-related cancers suggests that FCTC enforcement and cross-sector coordination remain insufficient.
6.5. Interpretation and Public Health Implications
The three malignancies analyzed in this study—kidney, prostate, and bladder cancers—illustrate distinct yet converging patterns of tobacco-attributable burden. Although the age-standardized rates of bladder cancer have declined in several BRICS countries, the absolute number of DALYs continues to rise due to population aging and improved survival. Kidney and prostate cancers exhibit stable or increasing trends, suggesting an epidemiological shift toward older adults and chronic disease coexistence.
These observations indicate that primary prevention via tobacco control remains essential even in the context of improved treatment access. Sustained taxation, plain packaging, and nationwide smoke-free laws could collectively prevent hundreds of thousands of DALYs annually across BRICS. From a health system perspective, integrating tobacco cessation services into universal health coverage and non-communicable disease programs would yield high cost-effectiveness and improve long-term survivorship outcomes [
2,
9,
11].
Moreover, the inclusion of secondhand smoke exposure metrics in routine cancer surveillance would allow more comprehensive risk monitoring, particularly for women and younger populations who are currently underrepresented in burden assessments.
6.6. Limitations
This analysis was subject to several methodological and data-related limitations inherent to the GBD approach.
First, relative risk coefficients used for estimating the burden of smoking-attributable cancers are predominantly derived from high-income countries and may not fully capture regional heterogeneity in exposure intensity, product type, or concurrent environmental risk factors.
Although the GBD framework applies 1000 posterior draws to quantify uncertainty, these estimates still rely on model assumptions that may underestimate the true variability in low- and middle-income settings.
Second, under-registration of cancer cases and incomplete death-certificate data, especially in India and South Africa, may contribute to residual underestimation despite the use of Bayesian meta-regression (DisMod-MR 2.1) and multiple imputation.
Third, the contribution of secondhand smoke could not be precisely quantified due to limited exposure data and potential misclassification.
Fourth, this study focused on aggregated national data and did not incorporate individual-level modifiers, such as occupation, alcohol consumption, genetic susceptibility, or healthcare accessibility.
Nevertheless, the application of standardized GBD methodology across countries and time provides valuable comparability for policy assessment and international benchmarking.
7. Conclusions
Tobacco-attributable kidney, prostate, and bladder cancers remain a substantial and persistent public health challenge in BRICS countries. Despite gradual declines in smoking prevalence in certain regions, the overall burden continues to increase due to demographic expansion, prolonged exposure histories, and incomplete enforcement of tobacco control policies. Strengthening FCTC implementation, expanding evidence-based cessation services, and promoting early detection programs targeting high-risk smokers are key strategies to mitigate future cancer burden. Policymakers should integrate tobacco-related cancer prevention into national NCD strategies, ensuring adequate funding and cross-sector collaboration. Future research should refine exposure–response relationships using region-specific cohorts and explore molecular mechanisms linking tobacco toxicity with renal and urothelial carcinogenesis, thereby supporting precision prevention and therapeutic innovation.
In summary, this study underscored the critical intersection between behavioral risk factors, aging populations, and health system responses by quantifying the long-term trends of three major urological cancers attributable to tobacco smoke in BRICS countries. Strengthened policy implementation and continuous surveillance will be essential to reverse these trends and achieve equitable cancer control across the BRICS region.