Effects of Resistance Training on Motor and Cognitive Function in Older Adults with Alzheimer’s Disease: A Systematic Review
Highlights
- Resistance exercise significantly improves muscle strength, motor performance, and functional capacity in older adults with Alzheimer’s disease.
- Resistance exercise programs lasting at least 12 weeks, performed three times per week at moderate intensities (50–70% of 1RM), appear to represent a safe and potentially neuroprotective intervention for individuals with Alzheimer’s disease.
- Incorporating resistance exercise into clinical care can help maintain independence and reduce caregiver burden in Alzheimer’s patients.
- Targeted physical training should be considered a key component of therapeutic strategies for managing Alzheimer’s-related physical decline.
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
- Medline: Alzheimer Disease OR “Alzheimer Dementia” OR senile dementia AND Resistance Training OR Muscle Strength OR strength training OR endurance training OR muscle strengthening exercise OR muscle exercise AND Cognitive function OR Motor Activity OR cognition OR cognitive function OR mental function OR motor function OR motor activity OR cognitive ability OR cognitive processes OR cognitive abilities.
- Scopus: (TITLE-ABS-KEY (“Alzheimer Disease” OR “Alzheimer Dementia” OR senile dementia AND TITLE-ABS-KEY (“Muscle Strength”) OR Resistance Training OR strength training OR endurance training OR muscle strengthening exercise OR muscle exercise ABS (“Cognitive function”) OR ALL (“Motor Activity”)) OR cognition OR cognitive function OR mental function OR motor function OR cognitive ability OR cognitive processes OR cognitive abilities.
- Web of Science: ((TI = (Alzheimer Disease)) OR “Alzheimer Dementia” OR senile dementia AND AB= (Muscle Strength)) OR Resistance Training OR strength training OR endurance training OR muscle strengthening exercise OR muscle exercise OR AB = (Cognitive function)) OR cognition OR cognitive function OR mental function OR motor function OR cognitive ability OR cognitive processes OR cognitive abilities.
- Lilacs: (Ti:(Alzheimer Disease)) OR “Alzheimer Dementia” OR senile dementia AND (ab: (Muscle Strength)) OR Resistance Training OR strength training OR endurance training OR muscle strengthening exercise OR muscle exercise (ab:(Resistance Training)) AND (mh: (clinical trial)) OR (tw: (double-blind)) OR (tw: (clinical experiment))) OR cognition OR cognitive function OR mental function OR motor function OR cognitive ability OR cognitive processes OR cognitive abilities.
- CENTRAL: Alzheimer Disease in Title Abstract Keyword OR “Alzheimer Dementia” OR senile dementia AND Motor Activity in Title Abstract Keyword OR Resistance Training OR strength training OR endurance training OR muscle strengthening exercise OR muscle exercise AND Cognitive function in Title Abstract Keyword) OR cognition OR cognitive function OR mental function OR motor function OR cognitive ability OR cognitive processes OR cognitive abilities.
2.4. Data Collection
2.5. Risk of Bias
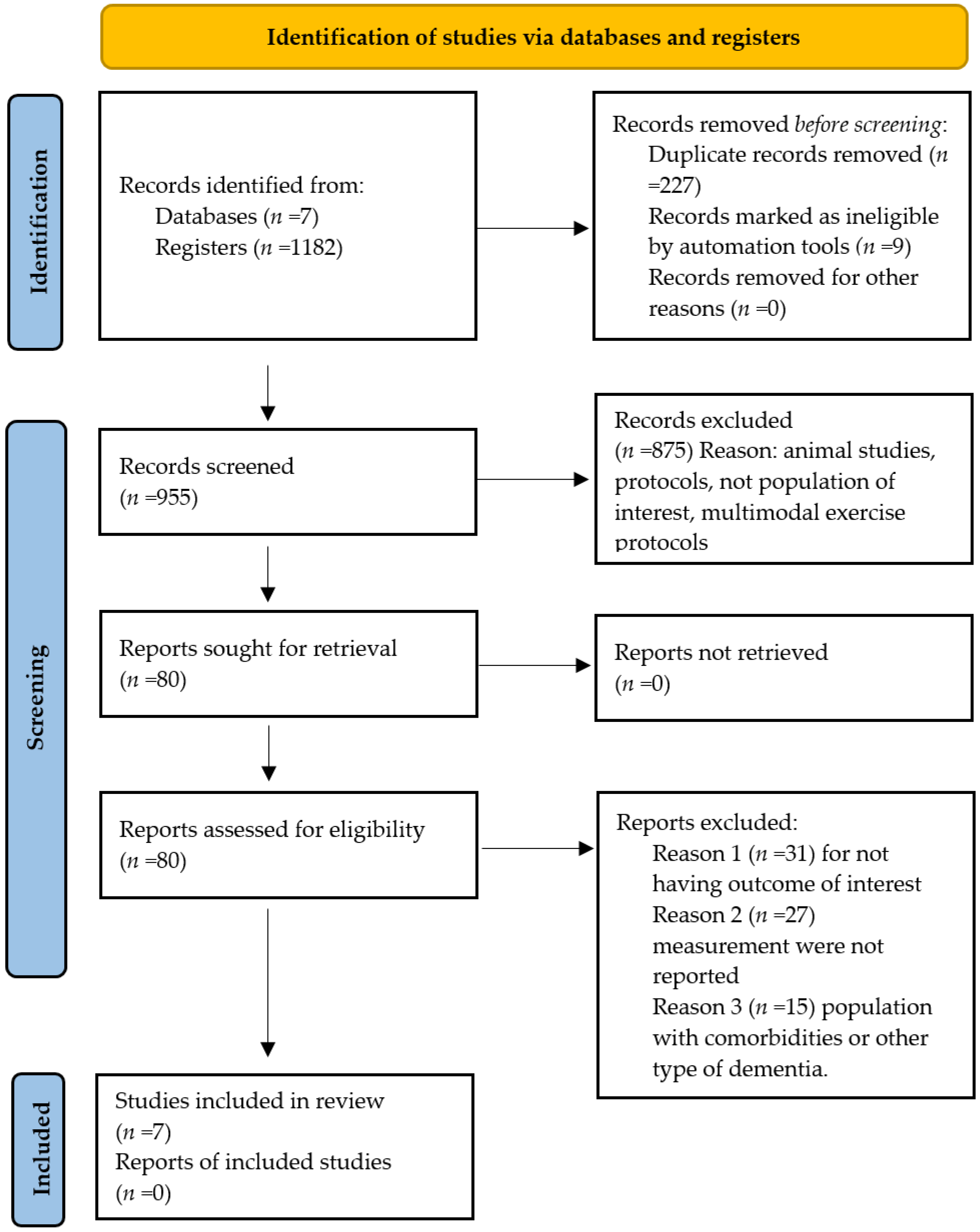
3. Results
3.1. Characteristics of Excluded Studies
3.2. Characteristics of Included Studies
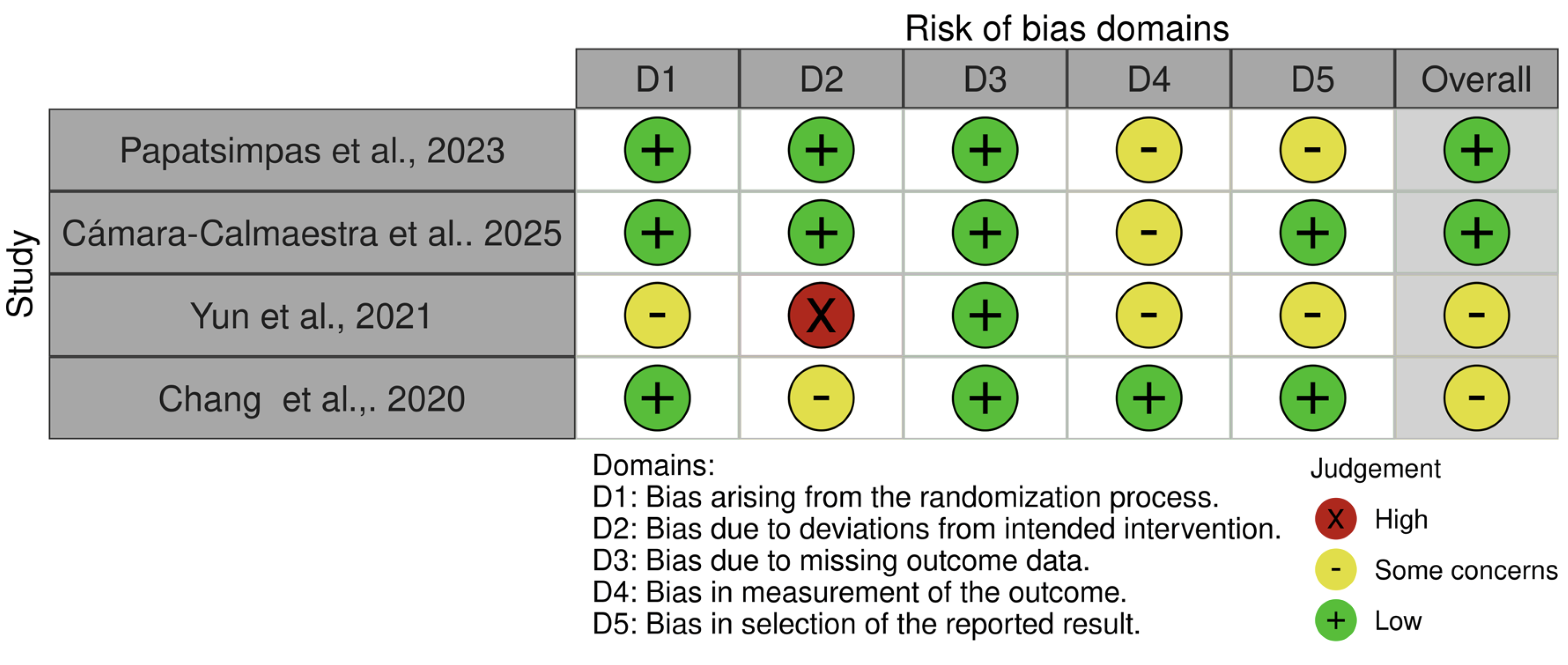
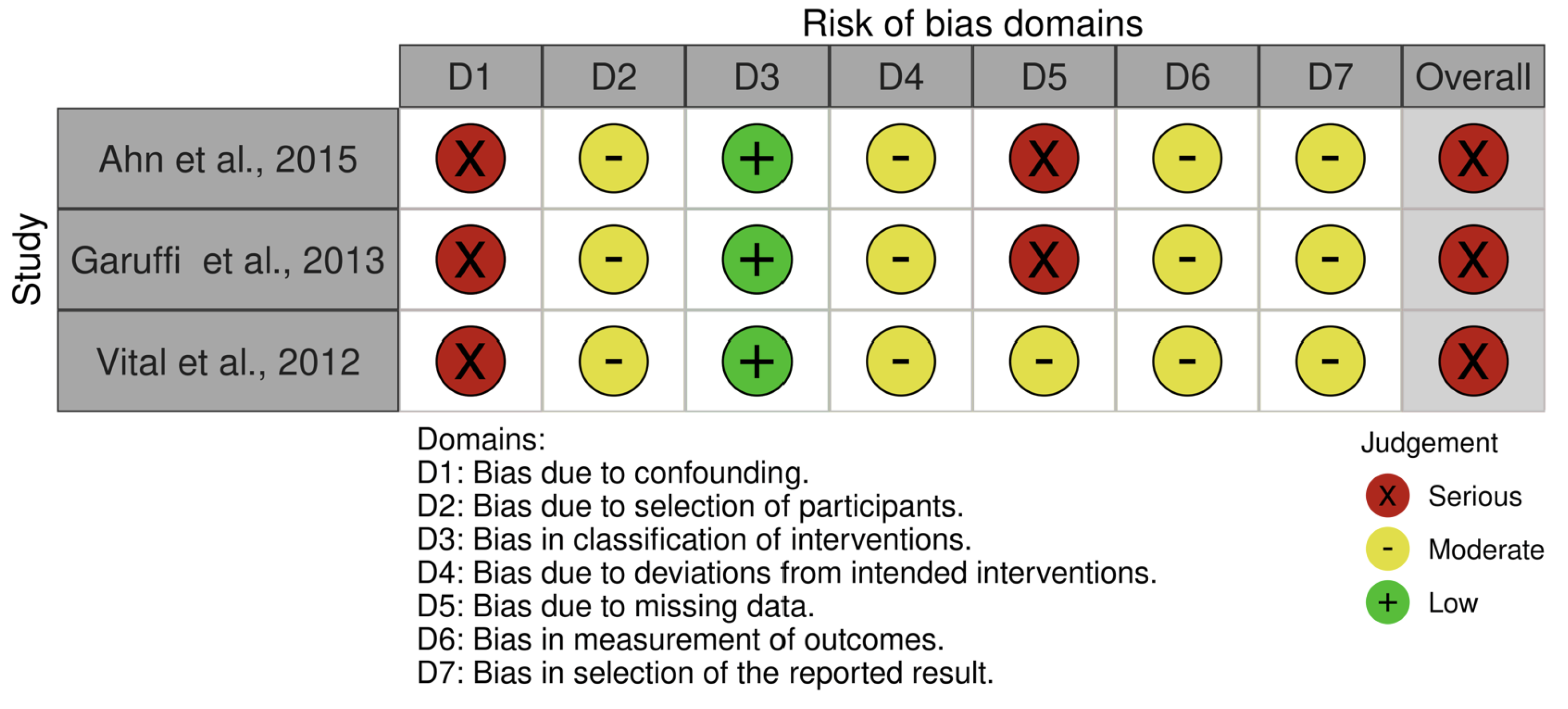
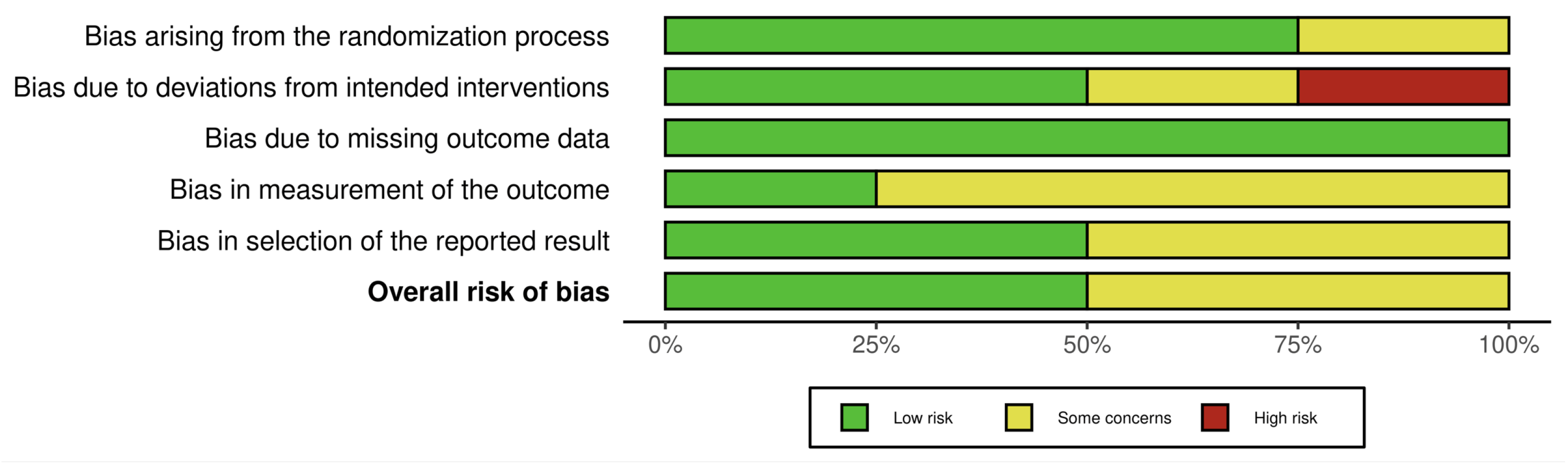
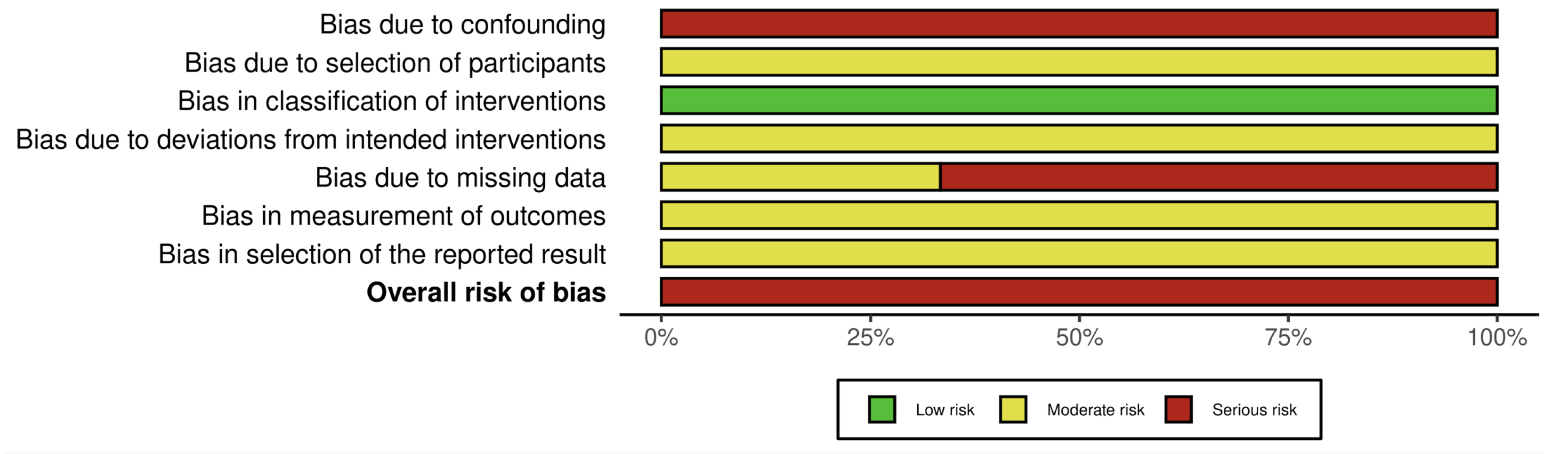
3.3. Risk of Bias Assessment
3.4. Cognitive Function Evaluation
3.5. Motor Function Evaluation
3.6. Synthesis Approach
4. Discussion
5. Limitations of the Study
6. Clinical Implications
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| BDNF | Brain-derived neurotrophic factors |
| MMSE | Mini-Mental State Examination |
| MoCA | Montreal Cognitive Assessment |
| MeSH | Medical Subject Headings |
| DeCS | Health Sciences Descriptors |
| TMT AB | Trail Making Test AB |
| DST FB | Forward and Backward |
| ACE-R | Addenbrooke’s Cognitive Examination Revised |
| SPPB | Short Physical Performance Battery |
| TUG | Timed Up and Go |
| MVC | Maximum Voluntary Isometric Contraction |
| IADL | Instrumental Activities of Daily Living Scale |
Appendix A
| Database | Search Strategy (with Fields and Operators) | Date of Search |
|---|---|---|
| MEDLINE (Ovid) | Fields: ti,ab,kw Strategy: 1. Alzheimer Disease. ti,ab,kw OR Alzheimer Dementia. ti,ab,kw OR senile dementia. ti,ab,kw 2. Resistance Training. ti,ab,kw OR Muscle Strength. ti,ab,kw OR strength training. ti,ab,kw OR endurance training. ti,ab,kw OR muscle strengthening exercise. ti,ab,kw OR muscle exercise. ti,ab,kw 3. Cognitive Function. ti,ab,kw OR Cognition. ti,ab,kw OR Cognitive Dysfunction. ti,ab,kw OR Motor Activity. ti,ab,kw OR Motor Function. ti,ab,kw 4. 1 AND 2 AND 3 | December 2024–April 2025 |
| SCOPUS | Fields: TITLE-ABS-KEY Strategy: (TITLE-ABS-KEY(“Alzheimer Disease” OR “Alzheimer Dementia” OR “senile dementia”)) AND (TITLE-ABS-KEY(“Resistance Training” OR “Muscle Strength” OR “strength training” OR “endurance training” OR “muscle strengthening exercise” OR “muscle exercise”)) AND (TITLE-ABS-KEY(“Cognitive Function” OR Cognition OR “Motor Activity” OR “Motor Function”)) | December 2024–April 2025 |
| Web of Science | Fields: TS = Topic (title, abstract, keywords) Strategy: TS = (“Alzheimer Disease” OR “Alzheimer Dementia” OR “senile dementia”) AND TS = (“Resistance Training” OR “Muscle Strength” OR “strength training” OR “endurance training” OR “muscle strengthening exercise” OR “muscle exercise”) AND TS = (“Cognitive Function” OR Cognition OR “Motor Activity” OR “Motor Function”) | December 2024–April 2025 |
| LILACS | Fields: ti = title; ab = abstract; mh = subject headings Strategy: (ti: (“Alzheimer Disease” OR “Alzheimer Dementia” OR “senile dementia”) AND ab: (“Resistance Training” OR “Muscle Strength” OR “strength training” OR “endurance training” OR “muscle strengthening exercise” OR “muscle exercise”) AND ab: (“Cognitive Function” OR Cognition OR “Motor Activity” OR “Motor Function”)) | December 2024–April 2025 |
| CENTRAL (Cochrane) | Fields: Title/Abstract/Keyword Strategy: (“Alzheimer Disease” OR “Alzheimer Dementia” OR “senile dementia”):ti,ab,kw AND (“Resistance Training” OR “Muscle Strength” OR “strength training” OR “endurance training” OR “muscle strengthening exercise” OR “muscle exercise”):ti,ab,kw AND (“Cognitive Function” OR Cognition OR “Motor Activity” OR “Motor Function”):ti,ab,kw | December 2024–April 2025 |
| Grey Literature (Google Scholar, OpenGrey) | Strategy: “Alzheimer Disease” OR “Alzheimer Dementia” OR “senile dementia” AND “Resistance Training” OR “strength training” OR “muscle strengthening exercise” AND “Cognitive Function” OR Cognition OR “Motor Activity” OR “Motor Function” | December 2024–April 2025 |
| ClinicalTrials.gov | Filters: • Condition/Disease: Alzheimer Disease • Intervention: Resistance Training OR Strength Training • Study type: Interventional Studies (Clinical Trials) • Status: Completed, Active not recruiting, Recruiting | December 2024–April 2025 |
| Study | Objective | n | Subgroups | Age (Years) | Sex (%F) | Measures | Main Findings |
|---|---|---|---|---|---|---|---|
| Papatsimpas et al., 2023 [23] | To investigate the effect of therapeutic exercise, through different types, on cognition and activities of daily living in patients with mild AD. | 171 | Multimodal exercise group n = 57 | 76.82 ± 5.73 | 63.2 | Trail Making Test AB (TMT AB), Digit Span Test Forward and Backward (DST FB), Cognitive Examination-Revised (ACE-R) | The resistance exercise group showed improvements in global cognitive function, including attention and orientation, memory, verbal fluency, language, and visuospatial ability. It also helped maintain independence in daily activities. |
| Resistance Group n = 57 | 76.07 ± 5.7 | 70.2 | |||||
| Control Group n = 57 | 78.75 ± 7.06 | 87.7 | |||||
| Cámara-Calmaestra et al., 2025 [24] | To assess the impact of resistance exercise on the risk of falls, fear of falling, muscle strength, neuropsychiatric symptoms, and ability to perform activities of daily living in people with Alzheimer’s disease | 60 | Intervention Group n = 30 | 81.7 ± 6.2 | 78 | Manual dynamometry, Short Physical Performance Battery (SPPB) | Resistance training was demonstrated to be an effective intervention for improving quality of life, significantly reducing fall risk, increasing handgrip strength, and lowering risks associated with Alzheimer’s disease. |
| Control Group n = 30 | 82.3 ± 7.6 | 74 | |||||
| Ahn et al., 2015 [26] | To examine the effects of a resistance exercise program aimed at improving muscle function in preventing and treating Alzheimer’s disease in older adults. | 23 | N = 23 | 74.21 ± 6.09 | NR | Chair stand test, single-leg balance test, Timed Up and Go (TUG) test, 2 min walk test, 8 m walk test, gait speed. | Cardiorespiratory function, gait speed, and static balance improved significantly, although no improvements were observed in dynamic balance. |
| Yun et al., 2021 [22] | To investigate the effects of a home-based multimodal exercise program on strength, mobility, fall risk, and functioning in older adults with mild to moderate AD. | 26 | Exercise Group n = 13 | 78.3 ± 5.3 | 100 | Maximum Voluntary Isometric Contraction (MVC), Mini-Mental State Examination (MMSE). | The exercise group also showed significant gains in lower-limb muscle strength, specifically in hip flexion and knee extension, among patients with moderate Alzheimer’s disease. |
| Control Group n = 13 | 78.2 ± 4.8 | ||||||
| Chang et al., 2020 [21] | To evaluate the effects of a resistance exercise program on reducing depressive symptoms in patients with mild Alzheimer’s disease and sarcopenia | 40 | Exercise Group n = 20 | 79.6 ± 5.4 | 100 | Mini-Mental State Examination (MMSE); Handgrip strength; Gait speed | Resistance exercises increase isometric muscle strength and can effectively manage depressive symptoms in elderly patients with Alzheimer’s disease and sarcopenia. |
| Control Group n = 20 | 79.1 ± 4.9 | ||||||
| Garuffi et al., 2013 [27] | To investigate the effects of resistance training on the performance of activities of daily living in patients with Alzheimer’s disease | 34 | Resistance Group n = 17 | 78.2 ± 7.3 | 82 | Mini-Mental State Examination (MMSE); 800 m walk; Functional evaluation: moving around the house, climbing stairs, getting up from the floor, manual skills, and putting on socks. | Positive results were also obtained in functional tasks such as climbing stairs, getting up from the floor, and putting on socks, reflecting improvements in lower-limb strength, dynamic balance, and flexibility in the treatment group. |
| Social Intervention Group n = 17 | 77.6 ± 6.5 | 76 | |||||
| Vital et al., 2012 [25] | To analyze the effects of weight training on cognitive functions in older adults with AD. | 34 | Resistance Group n = 17 | 78.2 ± 7.3 | NR | Mini-Mental State Examination (MMSE); Clock Drawing Test; Verbal Fluency Test | No significant differences were found regarding the effects of weight training on cognition in patients with AD. |
| Social Intervention Group n = 17 | 77.6 ± 6.5 |
| Study | Intervention Time | Frequency (Days/Week) | Duration per Session (Min) | Modality (Type) | Intensity | Volume (Sets/Reps/Total Min) | Muscle Groups | Progression Supervision/ Adherence |
|---|---|---|---|---|---|---|---|---|
| Papatsimpas et al., 2023 [23] | 12 weeks | 3 | 40–45 | Free-weight | 50–69% 1RM | 2 sets/12 rep/10 exercises | Major muscle groups | Supervision: Caregiver |
| Cámara-Calmaestra et al., 2021 [24] | 12 weeks | 3 | 30 | Free-weight | 80% RM | 3 sets/12 rep | Upper/lower limb | Not reported |
| Ahn et al., 2015 [26] | 5 months | 3 | 30–40 | Elastic bands | 60% HRmax RPE: 10–12 | (10 reps × 3 sets) | Upper/lower limb | Progression: Red or green band according to exercise capacity |
| Yun et al., 2021 [22] | 12 weeks | 3 | 30 | Bodyweight | Not reported | Not reported | Lower limb | Supervision: physiotherapist |
| Chang et al., 2020 [21] | 12 weeks | 3 | 40 | Elastic bands | Not reported | Not reported | Upper/lower limb/Trunk | Not reported |
| Garuffi et al., 2013 [27] | 16 weeks | 3 | 60 | Free-weight | 85% 1RM | 3 sets/20 rep | Major muscle groups | Not reported |
| Vital et al., 2012 [25] | 16 weeks | 3 | NR | Free-weight | 85% 1RM | 2-3 sets/20 rep | Major muscle groups | Progression: load adjustment if last series exceeded 22 rep7; Adherence: 70% |
| Study | Cognitive Function | Motor Function (Strength, Gait, Balance) | Physical Performance (SPPB, TUG, Walk Tests) | ADL/Functional Independence |
|---|---|---|---|---|
| Papatsimpas et al., 2023 [23] | + (ACE-R, DST, TMT improved) | 0 (no motor measures) | 0 (no physical performance tests) | + (independence maintained) |
| Cámara-Calmaestra et al., 2025 [24] | 0 (not assessed) | + (handgrip ↑, SPPB ↑, fall risk ↓) | + (SPPB improved) | + (daily activities improved) |
| Ahn et al., 2015 [26] | 0 (not assessed) | + (static balance ↑, strength ↑) | + (TUG, walk tests improved) | 0 (not evaluated) |
| Yun et al., 2021 [22] | + (MMSE improved) | + (MVC knee, hip flexion ↑) | + (no SPPB/TUG, but gait & mobility improved) | 0 (not evaluated) |
| Chang et al., 2020 [21] | + (MMSE improved) | + (MVC ↑, handgrip ↑) | + (gait speed ↑) | 0 (not evaluated) |
| Garuffi et al., 2013 [27] | 0 (not assessed) | + (strength ↑, dynamic balance ↑) | + (800 m walk ↑, functional tasks ↑) | + (getting up, stairs, floor transfers improved) |
| Vital et al., 2012 [25] | 0 (no significant effects) | N/A (no motor assessment) | N/A | 0 (not evaluated) |
| Outcome | Studies (n) | Participants (n) | Summary of Effect (Direction) | Certainty of Evidence | Key Reasons for Downgrading |
|---|---|---|---|---|---|
| Motor function | 6 | 354 | Consistent improvements in strength, gait, and mobility across studies. | ⊕⊕⊕◯ Moderate | Risk of bias (−1): several trials with “some concerns” and non-RCT designs. |
| Cognitive function | 3 | 231 | Small improvements observed in ACE-R, TMT, and verbal fluency; MMSE showed no consistent change. Evidence insufficient due to limited follow-up measures. | ⊕◯◯◯ Very low | Risk of bias (−1): non-RCTs with serious concerns. Inconsistency (−1): divergent findings across cognitive tests. Imprecision (−1): small samples and wide variability. |
| Risk fall | 1 | 60 | One study reported significant improvements in fall-risk indicators after resistance training. | ⊕◯◯◯ Very low | Imprecision (−1): small sample. Limited evidence base (−1): single study only. Potential risk of bias/inconsistency (−1): cannot be fully assessed with one study. |
| Activities of Daily Living / Functional Independence | 2 | 205 | Improvements seen in performance of instrumental activities of daily living; effect sizes small–moderate. | ⊕◯◯◯ Very low | Risk of bias (−1): one non-RCT; lack of blinding. Imprecision (−1): small samples and uncertainty. Inconsistency (−1): different ADL tools and effect magnitude. |
| Adverse events | 0 | - | Not reported in included studies | ⊕◯◯◯ Very low | Indirectness (−1): outcome not measured. Imprecision (−1): absence of data. Suspected reporting bias (−1): typical under-reporting in exercise trials. |
| Study | Intervention Time | Group | Exercise Protocol | Initial | Final | Follow/Difference | p Value |
|---|---|---|---|---|---|---|---|
| Papatsimpas et al., 2023 [23] | 12 weeks | Multimodal Exercise Group | Trail Making Test A | 132.5 ± 61.04 | 110.1 ± 59.48 | N/A | <0.001 |
| Trail Making Test B | 235.7 ± 66.46 | 147.2 ± 70.8 | |||||
| ACE-R | 74.04 ± 7.42 | 79.25 ± 6.46 | |||||
| Digit Span Test | 8.16 ± 1.6 | 9.63 ± 1.75 | |||||
| IADL | 5.93 ± 1.76 | 6.04 ± 1.71 | |||||
| Resistance Exercise Group | Trail Making Test A | 165.56 ± 71.1 | 147.2 ± 70.8 | ||||
| Trail Making Test B | 264.07 ± 55.1 | 248.5 ± 58.7 | |||||
| ACE-R | 70.51 ± 8.83 | 75.7 ± 8.61 | |||||
| Digit Span Test | 8.28 ± 2.01 | 9.54 ± 1.80 | |||||
| IADL | 5.61 ± 1.85 | 5.86 ± 1.73 | |||||
| Control Group | Trail Making Test A | 175.30 ± 59.7 | 219.6 ± 59.73 | ||||
| Trail Making Test B | 280.35 ± 39.3 | 291.8 ± 27.9 | |||||
| ACE-R | 70.53 ± 6.80 | 64.28 ± 6.51 | |||||
| Digit Span Test | 7.54 ± 1.65 | 5.58 ± 1.87 | |||||
| IADL | 5.56 ± 1.67 | 4.14 ± 1.82 | |||||
| Cámara-Calmaestra et al., 2025 [24] | 12 weeks | Intervention Group | SPPB | 5.8 ± 2.3 | 7.3 ± 2.6 | Follow 3 months: 6.8 ± 2.6 | <0.001 |
| Hand Grip (kg) | 12.2 ± 5.8 | 15.3 ± 6.0 | Follow 3 months: 14.7 ± 6.4 | <0.001 | |||
| IADL | 2.8 ± 1.5 | 2.9 ± 1.5 | Follow 3 months: 2.8 ± 1.5 | 0.059 | |||
| Control Group | SPPB | 5.8 ± 2.4 | 5.8 ± 2.5 | Follow 3 months: 5.7 ± 2.4 | <0.001 | ||
| Hand Grip (Kg) | 11.9 ± 7.0 | 12.0 ± 6.9 | Follow 3 months: 11.9 ± 7.0 | <0.001 | |||
| IADL | 2.3 ± 1.4 | 2.2 ± 1.3 | Follow 3 months: 2.1 ± 1.4 | 0.059 | |||
| Ahn et al., 2015 [26] | 5 months | Intervention Group | MMSE | 10;19 | NR | N/A | |
| Chair leg squat (reps) | 5.22 ± 5.07 | 11.89 ± 5.04 | <0.001 | ||||
| Left one-leg stance (s) | 0.87 ± 0.74 | 4.18 ± 2.73 | <0.001 | ||||
| Right one-leg stance (s) | 1.53 ± 1.46 | 4.48 ± 2.63 | <0.001 | ||||
| TUG test (reps) | 19.85 ± 10.19 | 18.22 ± 12.01 | NR | ||||
| Walking 2 min (steps) | 52.94 ± 40.64 | 169.71 ± 55.91 | <0.001 | ||||
| Walking 8 m (s) | 16.41 ± 6.90 | 13.18 ± 5.33 | <0.001 | ||||
| Walking round 8 m (s) | 31.35 ± 12.84 | 27.71 ± 10.09 | <0.001 | ||||
| Gait speed (cm/s) | 55.63 ± 18.30 | 68.97 ± 22.57 | <0.001 | ||||
| Yun et al., 2021 [22] | 12 weeks | Exercise group | MMSE | 18.8 ± 1.0 | NR | ||
| MVC Hip flexors (N/kg) | 0.98 ± 0.19 | 1.26 ± 0.24 | Diff: 0.28 ± 0.15 | 0.002 | |||
| MVC Knee Extensor (N/kg) | 1.26 ± 0.24 | 1.45 ± 0.19 | Diff: 0.18 ± 0.07 | 0.002 | |||
| Control Group | MMSE | 18.98 ± 1.0 | NR | ||||
| MVC Hip flexors (N/kg) | 1.03 ± 0.22 | 1.02 ± 0.21 | Diff: −0.01 ± 0.02 | 0.122 | |||
| MVC Knee Extensor (N/kg) | 1.30 ± 0.17 | 1.27 ± 0.16 | Diff: −0.02 ± 0.04 | 0.124 | |||
| Chang et al., 2020 [21] | 12 weeks | Exercise Group | MMSE | 21.1 ± 1.3 | NR | ||
| MVC Shoulder abductors (N/kg) | 0.92 ± 0.28 | 1.07 ± 0.22 | Diff: 0.15 ± 0.07 | 0.003 | |||
| MVC Elbow Flexor (N/kg) | 1.13 ± 0.25 | 1.30 ± 0.25 | Diff: 0.17 ± 0.06 | <0.001 | |||
| MVC Hip flexor (N/kg) | 1.04 ± 0.24 | 1.35 ± 0.26 | Diff: 0.31 ± 0.15 | <0.001 | |||
| MVC Knee extensor (N/kg) | 1.32 ± 0.23 | 1.50 ± 0.22 | Diff: 0.18 ± 0.08 | <0.001 | |||
| Gait Speed (m/s) | 0.47 ± 015 | 0.52 ± 0.15 | Diff: 0.05 ± 0.02 | <0.001 | |||
| Hand Grip (kg) | 12.3 ± 4.9 | 12.9 ±4.6 | Diff: 0.6 ± 0.8 | 0.017 | |||
| Control Group | MMSE | 21.3 ± 1.4 | NR | ||||
| MVC Shoulder abductors (N/kg) | 0.93 ± 0.14 | 0.91 ± 0.25 | Diff: −0.02 | 0.003 | |||
| MVC Elbow Flexor (N/kg) | 1.13 ± 0.23 | 1.12 ± 0.24 | Diff: −0.02 | <0.001 | |||
| MVC Hip flexor (N/kg) | 1.07 ± 0.24 | 1.06 ± 0.22 | Diff: −0.02 | <0.001 | |||
| MVC Knee extensor (N/kg) | 1.35 ± 0.20 | 1.33 ± 0.19 | Diff: −0.02 | <0.001 | |||
| Gait Speed (m/s) | 13.3 ± 3.8 | 13.3 ± 3.7 | Diff. 0.0 ± 0.6 | 0.017 | |||
| Hand Grip (kg) | 0.48 ± 0.14 | 0.48 ± 0.13 | Diff: 0.00 ± 0.01 | <0.001 | |||
| Garuffi et al., 2013 [27] | 16 weeks | Resistance Group | MMSE | 18.4 ± 4.3 | NR | ||
| Climbing stairs (s) | 16.28 ± 9.74 | 14.16 ± 6.89 | Diff: −2.12 | 0.0 | |||
| Moving around the house (s) | 57.30 ± 16.7 | 52.12 ± 13.03 | Diff: −5.18 | 0.03 | |||
| Manual skills (s) | 23.43 ± 11.77 | 20.12 ± 10.04 | Diff: −3.31 | NR | |||
| Putting on socks (s) | 11.15 ± 7.19 | 9.04 ± 4.24 | Diff: −211 | 0.04 | |||
| Getting up from a chair (s) | 26.21 ± 26.66 | 22.37 ± 24.29 | Diff: −3.84 | NR | |||
| Walking 800 m (s) | 720.82 ± 118.81 | 738.58 ± 119.30 | Diff 17.76 | NR | |||
| Social Intervention Group | MMSE | 17.7 ± 5.3 | NR | ||||
| Climbing stairs (s) | 14.22 ± 7.20 | 15.79 ± 8.64 | Diff: 1.57 | 0.0 | |||
| Moving around the house (s) | 63.03 ± 34.99 | 55.15 ± 23.61 | Diff: −7.88 | 0.03 | |||
| Manual skills (s) | 21.74 ± 16.11 | 24.41 ± 22.27 | Diff. 2.67 | NR | |||
| Putting on socks (s) | 8.75 ± 5.41 | 10.10 ± 7.04 | Diff: 1.35 | 0.04 | |||
| Getting up from a chair (s) | 13.04 ± 11.12 | 24.99 ± 22.21 | Diff: 11.95 | NR | |||
| Walking 800 m (s) | 742.06 ± 159.91 | 756.13 ± 112.48 | Diff: 14.07 | NR | |||
| Vital et al., 2012 [25] | 16 weeks | Resistance Group | MMSE | 18.4 ± 4.3 | NR | N/A | NR |
| Clock drawing test | 6 (0–9) | 7 (0–9) | 0.4 | ||||
| Verbal fluency test | 5 (3–13) | 5 (0–12) | 0.8 | ||||
| Social Intervention Group | MMSE | 17.7 ± 5.3 | NR | NR | |||
| Clock drawing test | 4 (0–9) | 5 (1–10) | 0.9 | ||||
| Verbal fluency test | 6 (1–11) | 5 (0–13) | 0.6 |
References
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; van der Flier, W.M.; Jessen, F.; Hoozemanns, J.; Thal, D.R.; Boche, D.; Brosseron, F.; Teunissen, C.; Zetterberg, H.; Jacobs, A.H.; et al. Neuroinflammation in Alzheimer Disease. Nat. Rev. Immunol. 2025, 25, 321–352. [Google Scholar] [CrossRef] [PubMed]
- Andrade-Guerrero, J.; Martínez-Orozco, H.; Villegas-Rojas, M.M.; Santiago-Balmaseda, A.; Delgado-Minjares, K.M.; Pérez-Segura, I.; Baéz-Cortés, M.T.; Del Toro-Colin, M.A.; Guerra-Crespo, M.; Arias-Carrión, O.; et al. Alzheimer’s Disease: Understanding Motor Impairments. Brain Sci. 2024, 14, 1054. [Google Scholar] [CrossRef] [PubMed]
- Mandolesi, L.; Polverino, A.; Montuori, S.; Foti, F.; Ferraioli, G.; Sorrentino, P.; Sorrentino, G. Effects of Physical Exercise on Cognitive Functioning and Wellbeing: Biological and Psychological Benefits. Front. Psychol. 2018, 9, 509. [Google Scholar] [CrossRef]
- Liang, J.; Wang, C.; Zhang, H.; Huang, J.; Xie, J.; Chen, N. Exercise-Induced Benefits for Alzheimer’s Disease by Stimulating Mitophagy and Improving Mitochondrial Function. Front. Aging Neurosci. 2021, 13, 755665. [Google Scholar] [CrossRef]
- López-Ortiz, S.; Pinto-Fraga, J.; Valenzuela, P.L.; Martín-Hernández, J.; Seisdedos, M.M.; García-López, O.; Toschi, N.; Di Giuliano, F.; Garaci, F.; Mercuri, N.B.; et al. Physical Exercise and Alzheimer’s Disease: Effects on Pathophysiological Molecular Pathways of the Disease. Int. J. Mol. Sci. 2021, 22, 2897. [Google Scholar] [CrossRef]
- Lopez, P.; Radaelli, R.; Taaffe, D.R.; Newton, R.U.; Galvão, D.A.; Trajano, G.S.; Teodoro, J.L.; Kraemer, W.J.; Häkkinen, K.; Pinto, R.S. Resistance Training Load Effects on Muscle Hypertrophy and Strength Gain: Systematic Review and Network Meta-analysis. Med. Sci. Sports Exerc. 2021, 53, 1206–1216. [Google Scholar] [CrossRef]
- Al-Mhanna, S.B.; A Franklin, B.; Jakicic, J.M.; Stamatakis, E.; Pescatello, L.S.; Riebe, D.; Thompson, W.R.; Skinner, J.; Colberg, S.R.; Alkhamees, N.H.; et al. Impact of Resistance Training on Cardiometabolic Health-Related Indices in Patients with Type 2 Diabetes and Overweight/Obesity: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Br. J. Sports Med. 2025, 59, 733. [Google Scholar] [CrossRef]
- Gordon, B.R.; McDowell, C.P.; Hallgren, M.; Meyer, J.D.; Lyons, M.; Herring, M.P. Association of Efficacy of Resistance Exercise Training with Depressive Symptoms: Meta-analysis and Meta-regression Analysis of Randomized Clinical Trials. JAMA Psychiatry 2018, 75, 566–576. [Google Scholar] [CrossRef]
- Herring, M.P.; Meyer, J.D. Resistance Exercise for Anxiety and Depression: Efficacy and Plausible Mechanisms. Trends Mol. Med. 2024, 30, 204–206. [Google Scholar] [CrossRef]
- Xue, J.; Han, X.; Zheng, Y.; Zhang, Q.; Kong, L. Effectiveness of Resistance Training in Modulating Inflammatory Biomarkers among Asian Patients with Sarcopenia: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Front. Immunol. 2024, 15, 1385902. [Google Scholar] [CrossRef]
- Calle, M.C.; Fernandez, M.L. Effects of resistance training on the inflammatory response. Nutr. Res. Pract. 2010, 4, 259–269. [Google Scholar] [CrossRef]
- Vaughan, S.; Wallis, M.; Polit, D.; Steele, M.; Shum, D.; Morris, N. The effects of multimodal exercise on cognitive and physical functioning and brain-derived neurotrophic factor in older women: A randomised controlled trial. Age Ageing 2014, 43, 623–629. [Google Scholar] [CrossRef]
- Liang, S.; Liu, H.; Wang, X.; Lin, H.; Zheng, L.; Zhang, Y.; Peng, L.; Huang, S.; Chen, L. Aerobic exercise improves clearance of amyloid-β via the glymphatic system in a mouse model of Alzheimer’s Disease. Brain Res. Bull. 2025, 222, 111263. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, S.; Li, S. The effect of physical exercise intervention on the ability of daily living in patients with Alzheimer’s dementia: A meta-analysis. Front. Aging Neurosci. 2024, 16, 1391611. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, T. Preventive strategies for cognitive decline and dementia: Benefits of aerobic physical activity, especially open-skill exercise. Brain Sci. 2023, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Sanaeifar, F.; Pourranjbar, S.; Pourranjbar, M.; Ramezani, S.; Mehr, S.R.; Wadan, A.-H.S.; Khazeifard, F. Beneficial effects of physical exercise on cognitive-behavioral impairments and brain-derived neurotrophic factor alteration in the limbic system induced by neurodegeneration. Exp. Gerontol. 2024, 195, 112539. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Version 6.4 (Updated August 2023); Cochrane: Oxford, UK, 2023. [Google Scholar]
- American College of Sports Medicine. Progression Models in Resistance Training for Healthy Adults. Med. Sci. Sports Exerc. 2009, 41, 687–708. [Google Scholar] [CrossRef]
- McGuinness, L.A.; Higgins, J.P.T. Risk-of-Bias Visualization (robvis): An R Package and Shiny Web App for Visualizing Risk-of-Bias Assessments. Res. Synth. Methods 2020, 12, 55–61. [Google Scholar] [CrossRef]
- Chang, M.C.; Lee, A.Y.; Kwak, S.; Kwak, S.G. Effect of Resistance Exercise on Depression in Mild Alzheimer Disease Patients with Sarcopenia. Am. J. Geriatr. Psychiatry 2020, 28, 587–589. [Google Scholar] [CrossRef]
- Yun, J.H.; Kim, D.H.; Chang, M.C. A simple bedside exercise method to enhance lower limb muscle strength in moderate Alzheimer’s disease patients with sarcopenia. Healthcare 2021, 9, 680. [Google Scholar] [CrossRef]
- Papatsimpas, V.; Vrouva, S.; Papathanasiou, G.; Papadopoulou, M.; Bouzineki, C.; Kanellopoulou, S.; Moutafi, D.; Bakalidou, D. Does therapeutic exercise support improvement in cognitive function and instrumental activities of daily living in patients with mild Alzheimer’s disease? A randomized controlled trial. Brain Sci. 2023, 13, 1112. [Google Scholar] [CrossRef] [PubMed]
- Cámara-Calmaestra, R.; Martínez-Amat, A.; Aibar-Almazán, A.; Hita-Contreras, F.; de Miguel-Hernando, N.; Rodríguez-Almagro, D.; Jiménez-García, J.D.; Achalandabaso-Ochoa, A. Resistance exercise to reduce risk of falls in people with Alzheimer’s disease: A randomised clinical trial. Physiotherapy 2025, 126, 101440. [Google Scholar] [CrossRef] [PubMed]
- Vital, T.M.; Hernández, S.S.S.; Pedroso, R.V.; Teixeira, C.V.L.; Garuffi, M.; Stein, A.M.; Costa, J.L.R.; Stella, F. Effects of weight training on cognitive functions in elderly with Alzheimer’s disease. Dement. Neuropsychol. 2012, 6, 253–259. [Google Scholar] [CrossRef]
- Ahn, N.; Kim, K. Effects of an elastic band resistance exercise program on lower extremity muscle strength and gait ability in patients with Alzheimer’s disease. J. Phys. Ther. Sci. 2015, 27, 1953–1955. [Google Scholar] [CrossRef]
- Garuffi, M.; Costa, J.L.R.; Hernández, S.S.S.; Vital, T.M.; Stein, A.M.; dos Santos, J.G.; Stella, F. Effects of resistance training on the performance of activities of daily living in patients with Alzheimer’s disease. Geriatr. Gerontol. Int. 2013, 13, 322–328. [Google Scholar] [CrossRef]
- Smart, T.F.F.; Doleman, B.; Hatt, J.; Paul, M.; Toft, S.; Lund, J.N.; E Phillips, B. The role of resistance exercise training for improving cardiorespiratory fitness in healthy older adults: A systematic review and meta-analysis. Age Ageing 2022, 51, afac143. [Google Scholar] [CrossRef]
- Ferté, J.B.; Boyer, F.C.; Taiar, R.; Pineau, C.; Barbe, C.; Rapin, A. Impact of resistance training on the 6-minute walk test in individuals with chronic obstructive pulmonary disease: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2022, 65, 101582. [Google Scholar] [CrossRef]
- Azevedo, C.V.; Hashiguchi, D.; Campos, H.C.; Figueiredo, E.V.; Otaviano, S.F.S.D.; Penitente, A.R.; Arida, R.M.; Longo, B.M. The Effects of Resistance Exercise on Cognitive Function, Amyloidogenesis, and Neuroinflammation in Alzheimer’s Disease. Front. Neurosci. 2023, 17, 1131214. [Google Scholar] [CrossRef]
- Santos, P.R.P.D.; Cavalcante, B.R.; Vieira, A.K.D.S.; Guimarães, M.D.; Leandro Da Silva, A.M.; Armstrong, A.D.C.; Carvalho, R.G.D.S.; Carvalho, F.O.D.; Souza, M.F.D. Improving Cognitive and Physical Function through 12-Weeks of Resistance Training in Older Adults: Randomized Controlled Trial. J. Sports Sci. 2020, 38, 1936–1942. [Google Scholar] [CrossRef]
- Özbeyli, D.; Sarı, G.; Özkan, N.; Karademir, B.; Yüksel, M.; Çilingir Kaya, Ö.T.; Kasımay Çakır, Ö. Protective Effects of Different Exercise Modalities in an Alzheimer’s Disease-like Model. Behav. Brain Res. 2017, 328, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Vilela, T.C.; Muller, A.P.; Damiani, A.P.; Macan, T.P.; da Silva, S.; Canteiro, P.B.; de Sena Casagrande, A.; Pedroso, G.D.S.; Nesi, R.T.; de Andrade, V.M.; et al. Strength and Aerobic Exercises Improve Spatial Memory in Aging Rats through Stimulating Distinct Neuroplasticity Mechanisms. Mol. Neurobiol. 2017, 54, 7928–7937. [Google Scholar] [CrossRef] [PubMed]
- Rahmati, M.; Keshvari, M.; Xie, W.; Yang, G.; Jin, H.; Li, H.; Chehelcheraghi, F.; Li, Y. Resistance Training and Urtica Dioica Increase Neurotrophin Levels and Improve Cognitive Function by Increasing Age in the Hippocampus of Rats. Biomed. Pharmacother. 2022, 153, 113306. [Google Scholar] [CrossRef] [PubMed]
- Castaño, L.A.A.; Castillo de Lima, V.; Barbieri, J.F.; de Lucena, E.G.P.; Gáspari, A.F.; Arai, H.; Teixeira, C.V.L.; Coelho-Júnior, H.J.; Uchida, M.C. Resistance Training Combined with Cognitive Training Increases Brain Derived Neurotrophic Factor and Improves Cognitive Function in Healthy Older Adults. Front. Psychol. 2022, 13, 870561. [Google Scholar] [CrossRef]
- Coelho-Junior, H.; Marzetti, E.; Calvani, R.; Picca, A.; Arai, H.; Uchida, M. Resistance Training Improves Cognitive Function in Older Adults with Different Cognitive Status: A Systematic Review and Meta-Analysis. Aging Ment. Health 2022, 26, 213–224. [Google Scholar] [CrossRef]
- Castillo Quezada, H.; Martínez-Salazar, C.; Fuentealba-Urra, S.; Hernández-Mosqueira, C.; Araneda Garcés, N.; Rodríguez-Rodríguez, F.; Concha-Cisternas, Y.; Molina-Sotomayor, E. Effects of Two Physical Training Programs on the Cognitive Status of a Group of Older Adults in Chile. Int. J. Environ. Res. Public Health 2021, 18, 4186. [Google Scholar] [CrossRef]
- Adlard, P.A.; Perreau, V.M.; Pop, V.; Cotman, C.W. Voluntary Exercise Decreases Amyloid Load in a Transgenic Model of Alzheimer’s Disease. J. Neurosci. 2005, 25, 4217–4221. [Google Scholar] [CrossRef]
- de Andrade Santos, F.O.; Passos, A.A.; Arida, R.M.; Teixeira-Machado, L. Effectiveness of Resistance Exercise on Cognitive Function in Animal Models of Alzheimer Disease: A Systematic Review and Meta-Analysis. J. Prev. Alzheimers Dis. 2024, 11, 998–1012. [Google Scholar] [CrossRef]
- Boyle, P.A.; Buchman, A.S.; Wilson, R.S.; Leurgans, S.E.; Bennett, D.A. Association of muscle strength with the risk of Alzheimer disease and the rate of cognitive decline in community-dwelling older persons. Arch. Neurol. 2009, 66, 1339–1344. [Google Scholar] [CrossRef]
- Zhao, H.; Cheng, R.; Song, G.; Teng, J.; Shen, S.; Fu, X.; Yan, Y.; Liu, C. The effect of resistance training on the rehabilitation of elderly patients with sarcopenia: A meta-analysis. Int. J. Environ. Res. Public Health 2022, 19, 15491. [Google Scholar] [CrossRef]
- Rolland, Y.; Pillard, F.; Klapouszczak, A.; Reynish, E.; Thomas, D.; Andrieu, S.; Rivière, D.; Vellas, B. Exercise program for nursing home residents with Alzheimer’s disease: A 1-year randomized, controlled trial. J. Am. Geriatr. Soc. 2007, 55, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Landrigan, J.F.; Bell, T.; Crowe, M.; Clay, O.J.; Mirman, D. Lifting cognition: A meta-analysis of effects of resistance exercise on cognition. Psychol. Res. 2020, 84, 1167–1183. [Google Scholar] [CrossRef] [PubMed]
- Szuhany, K.L.; Bugatti, M.; Otto, M.W. A meta-analytic review of the effects of exercise on brain-derived neurotrophic factor. J. Psychiatr. Res. 2015, 60, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Sepúlveda-Lara, A.; Sepúlveda, P.; Marzuca-Nassr, G.N. Resistance Exercise Training as a New Trend in Alzheimer’s Disease Research: From Molecular Mechanisms to Prevention. Int. J. Mol. Sci. 2024, 25, 7084. [Google Scholar] [CrossRef]
- Rodríguez-Gutiérrez, E.; Torres-Costoso, A.; Pascual-Morena, C.; Pozuelo-Carrascosa, D.P.; Garrido-Miguel, M.; Martínez-Vizcaíno, V. Effects of Resistance Exercise on Neuroprotective Factors in Middle and Late Life: A Systematic Review and Meta-Analysis. Aging Dis. 2023, 14, 1264–1275. [Google Scholar] [CrossRef]
- Mansoor, M.; Ibrahim, A.; Hamide, A.; Tran, T.; Candreva, E.; Baltaji, J. Exercise-Induced Neuroplasticity: Adaptive Mechanisms and Preventive Potential in Neurodegenerative Disorders. Physiologia 2025, 5, 13. [Google Scholar] [CrossRef]
- Zhang, S.; Zhen, K.; Su, Q.; Chen, Y.; Lv, Y.; Yu, L. The effect of aerobic exercise on cognitive function in people with Alzheimer’s disease: A systematic review and meta-analysis of randomized controlled trials. Int. J. Environ. Res. Public Health 2022, 19, 15700. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, Z.; Peng, C. Effects of aerobic exercise on cognitive function and quality of life in patients with Alzheimer’s disease: A systematic review and meta-analysis. BMJ Open 2025, 15, e090623. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Serna-Orozco, M.F.; Pitto-Bedoya, S.; Salazar-Goyes, J.S.; Figueroa-Zúñiga, S.; Martínez-Muñoz, L.M.; Jaramillo-Losada, J. Effects of Resistance Training on Motor and Cognitive Function in Older Adults with Alzheimer’s Disease: A Systematic Review. Healthcare 2025, 13, 3079. https://doi.org/10.3390/healthcare13233079
Serna-Orozco MF, Pitto-Bedoya S, Salazar-Goyes JS, Figueroa-Zúñiga S, Martínez-Muñoz LM, Jaramillo-Losada J. Effects of Resistance Training on Motor and Cognitive Function in Older Adults with Alzheimer’s Disease: A Systematic Review. Healthcare. 2025; 13(23):3079. https://doi.org/10.3390/healthcare13233079
Chicago/Turabian StyleSerna-Orozco, Maria Fernanda, Stefania Pitto-Bedoya, Jhoan Sebastián Salazar-Goyes, Sebastián Figueroa-Zúñiga, Luisa María Martínez-Muñoz, and Jennifer Jaramillo-Losada. 2025. "Effects of Resistance Training on Motor and Cognitive Function in Older Adults with Alzheimer’s Disease: A Systematic Review" Healthcare 13, no. 23: 3079. https://doi.org/10.3390/healthcare13233079
APA StyleSerna-Orozco, M. F., Pitto-Bedoya, S., Salazar-Goyes, J. S., Figueroa-Zúñiga, S., Martínez-Muñoz, L. M., & Jaramillo-Losada, J. (2025). Effects of Resistance Training on Motor and Cognitive Function in Older Adults with Alzheimer’s Disease: A Systematic Review. Healthcare, 13(23), 3079. https://doi.org/10.3390/healthcare13233079