Targeted Physical Function Exercises for Frailty and Falls Management in Pre-Frail Community-Dwelling Older Adults: A Randomized Controlled Trial
Abstract
1. Introduction
2. Methods
2.1. Trial Design
2.2. Participants
2.3. Sample
2.4. Procedure
2.5. Intervention
2.6. Outcomes
2.7. Primary Outcome Measures
2.8. Secondary Outcome Measures
2.9. Ethical Consideration
2.10. Statistical Analysis
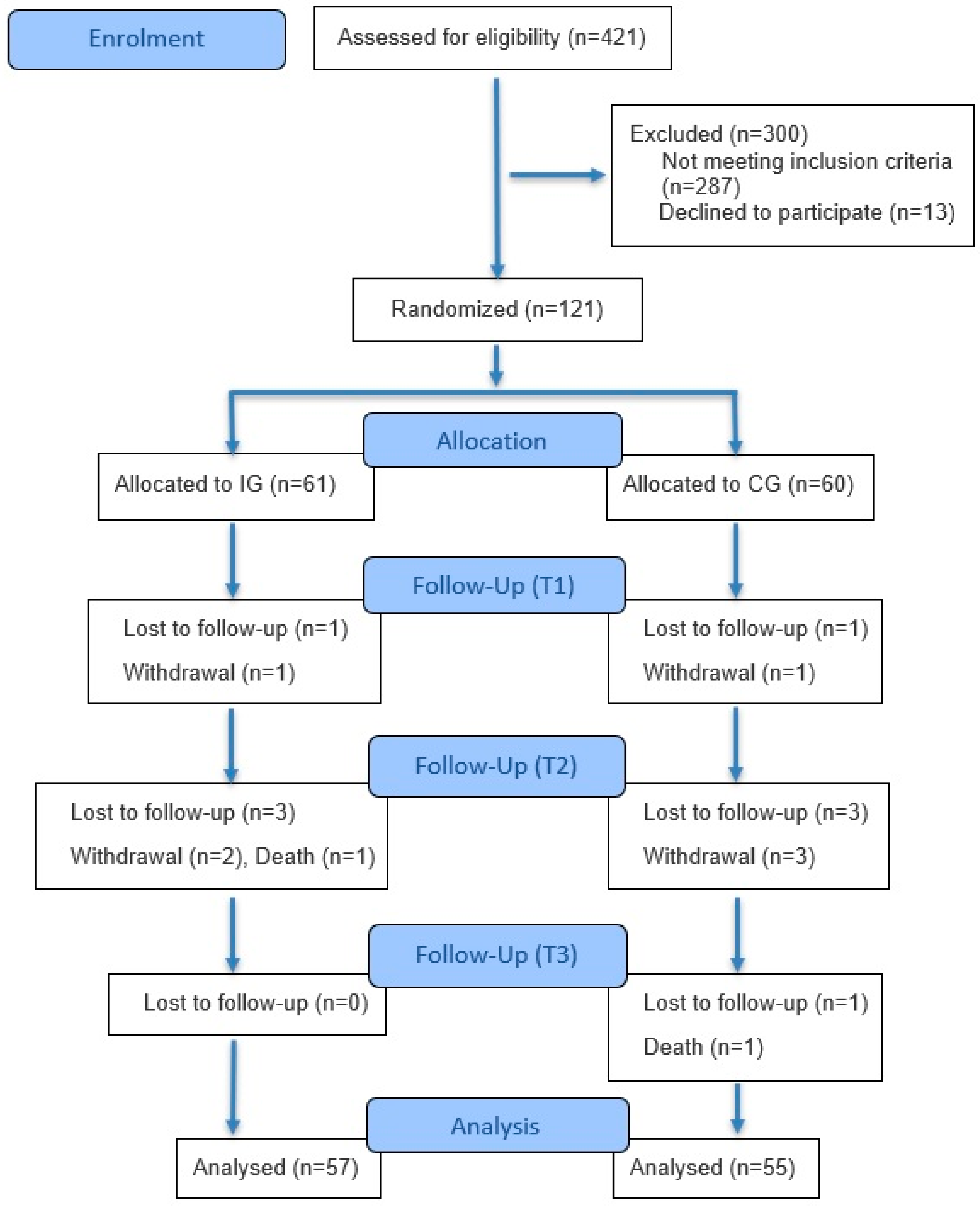
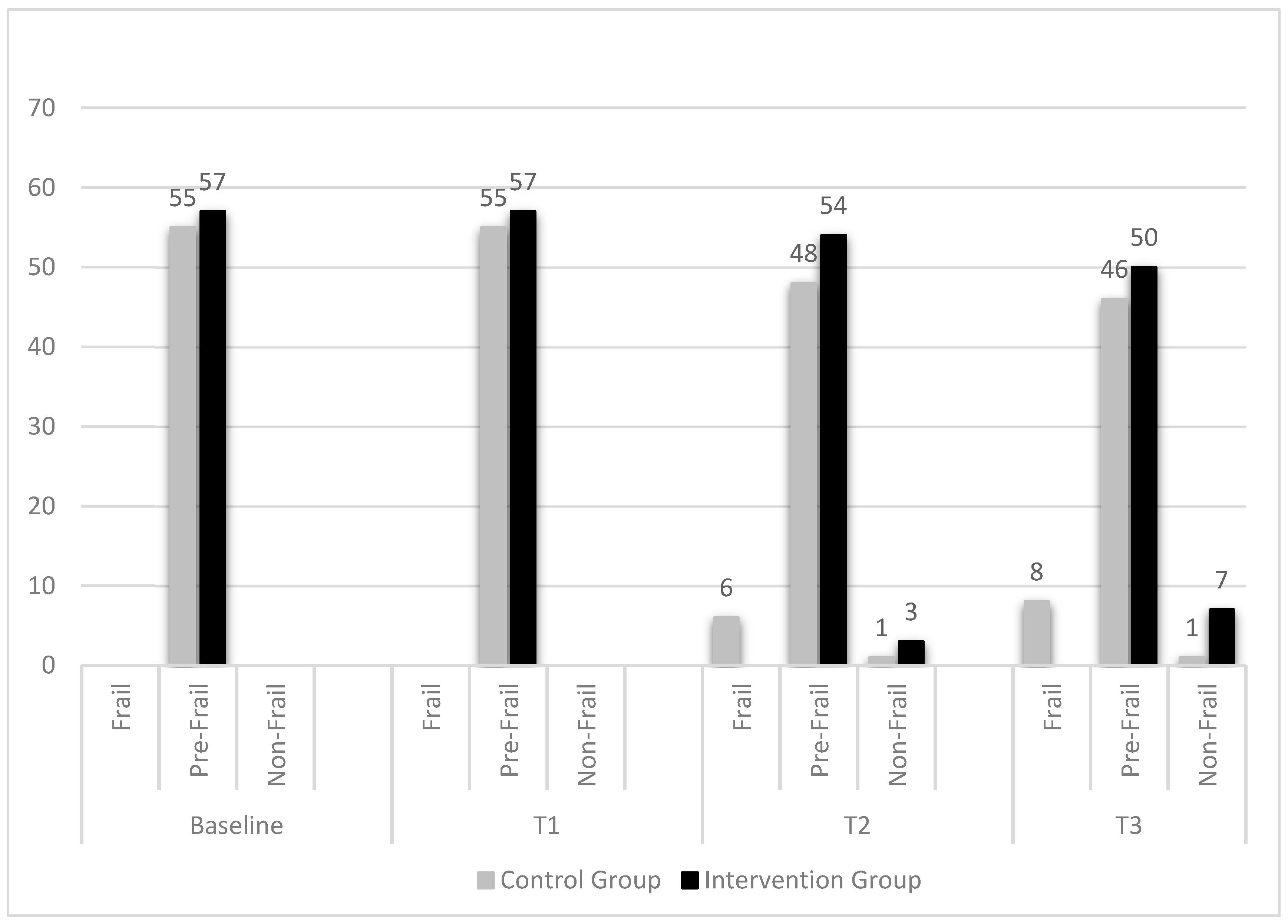
3. Results
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vermeiren, S.; Vella-Azzopardi, R.; Beckwée, D.; Habbig, A.K.; Scafoglieri, A.; Jansen, B.; Bautmans, I.; Bautmans, I.; Verté, D.; Beyer, I.; et al. Frailty and the Prediction of Negative Health Outcomes: A Meta-Analysis. J. Am. Med. Dir. Assoc. 2016, 17, 1163.e1–1163.e17. [Google Scholar] [CrossRef] [PubMed]
- Makizako, H.; Shimada, H.; Tsutsumimoto, K.; Lee, S.; Doi, T.; Nakakubo, S.; Hotta, R.; Suzuki, T. Social Frailty in Community-Dwelling Older Adults as a Risk Factor for Disability. J. Am. Med. Dir. Assoc. 2015, 16, 1003.e7–1003.e11. [Google Scholar] [CrossRef]
- Woolford, S.J.; Sohan, O.; Dennison, E.M.; Cooper, C.; Patel, H.P. Approaches to the diagnosis and prevention of frailty. Aging Clin. Exp. Res. 2020, 32, 1629–1637. [Google Scholar] [CrossRef]
- Ellmers, T.J.; Wilson, M.R.; Kal, E.C.; Young, W.R. The perceived control model of falling: Developing a unified framework to understand and assess maladaptive fear of falling. Age Ageing 2023, 52, afad093. [Google Scholar] [CrossRef]
- Delbaere, K.; Crombez, G.; Vanderstraeten, G.; Willems, T.; Cambier, D. Fear-related avoidance of activities, falls and physical frailty. A prospective community-based cohort study. Age Ageing 2004, 33, 368–373. [Google Scholar] [CrossRef]
- Di Lorito, C.; Long, A.; Byrne, A.; Harwood, R.H.; Gladman, J.R.F.; Schneider, S.; Logan, P.; Bosco, A.; van der Wardt, V. Exercise interventions for older adults: A systematic review of meta-analyses. J. Sport Health Sci. 2021, 10, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Hu, P.; Sun, W.; Wu, W.; Zhang, J.; Deng, H.; Huang, J.; Ukawa, S.; Lu, J.; Tamakoshi, A.; et al. Effect of physical activity on the risk of frailty: A systematic review and meta-analysis. PLoS ONE 2022, 17, e0278226. [Google Scholar] [CrossRef]
- Savvakis, I.; Adamakidou, T.; Kleisiaris, C. Physical-activity interventions to reduce fear of falling in frail and pre-frail older adults: A systematic review of randomized controlled trials. Eur. Geriatr. Med. 2024, 15, 333–344. [Google Scholar] [CrossRef] [PubMed]
- Navarrete-Villanueva, D.; Gómez-Cabello, A.; Marín-Puyalto, J.; Moreno, L.A.; Vicente-Rodríguez, G.; Casajús, J.A. Frailty and Physical Fitness in Elderly People: A Systematic Review and Meta-analysis. Sport. Med. 2020, 51, 143–160. [Google Scholar] [CrossRef]
- Travers, J.; Romero-Ortuno, R.; Langan, J.; Macnamara, F.; Mccormack, D.; Mcdermott, C.; Mcentire, J.; Mckiernan, J.; Lacey, S.; Doran, P.; et al. Building resilience and reversing frailty: A randomised controlled trial of a primary care intervention for older adults. Age Ageing 2023, 52, afad012. [Google Scholar] [CrossRef] [PubMed]
- Sadjapong, U.; Yodkeeree, S.; Sungkarat, S.; Siviroj, P. Multicomponent Exercise Program Reduces Frailty and Inflammatory Biomarkers and Improves Physical Performance in Community-Dwelling Older Adults: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2020, 17, 3760. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Lee, J.H.; Park, Y.H. Effects of a task-specific exercise program on balance, mobility, and muscle strength in the elderly. J. Phys. Ther. Sci. 2014, 26, 1693–1695. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Prommaban, A.; Moonkayaow, S.; Phinyo, P.; Siviroj, P.; Sirikul, W.; Lerttrakarnnon, P. The Effect of Exercise Program Interventions on Frailty, Clinical Outcomes, and Biomarkers in Older Adults: A Systematic Review. J. Clin. Med. 2024, 13, 6570. [Google Scholar] [CrossRef]
- Romero-ortuno, R.; Walsh, C.D.; Lawlor, B.A.; Kenny, R.A. A Frailty Instrument for primary care: Findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr. 2010, 10, 57. [Google Scholar] [CrossRef]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol.—Ser. A Biol. Sci. Med. Sci. 2001, 56, 146–157. [Google Scholar] [CrossRef] [PubMed]
- Guralnik, J.M.; Simonsick, E.M.; Ferrucci, L.; Glynn, R.J.; Berkman, L.F.; Blazer, D.G.; Scherr, P.A.; Wallace, R.B. A short physical performance battery assessing lower extremity function: Association with self-reported disability and prediction of mortality and nursing home admission. J. Gerontol. 1994, 49, M85–M94. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Berg, K.; Wood-Dauphine, S.L.; Williams, J.L.; Gayton, D. Measuring Balance in the Elderly: Validation of an Instrument. Can. J. Public Health 2014, 83, 7–11. [Google Scholar]
- Lampropoulou, S.I.; Billis, E.; Gedikoglou, I.A.; Point, P.; Nowicky, A. Cross Cultural Adaptation of Berg Balance Scale in Greek for Various Balance Impairments. J. Phys. Med. Rehabil. Disabil. 2016, 2, 1–13. [Google Scholar] [CrossRef]
- Yardley, L.; Beyer, N.; Hauer, K.; Kempen, G.; Piot-Ziegler, C.; Todd, C. Development and initial validation of the Falls Efficacy Scale-International (FES-I). Age Ageing 2005, 34, 614–619. [Google Scholar] [CrossRef]
- Billis, E.; Strimpakos, N.; Kapreli, E.; Sakellari, V.; Skelton, D.A.; Dontas, I.; Ioannou, F.; Filon, G.; Gioftsos, G. Cross-cultural validation of the Falls Efficacy Scale International (FES-I) in Greek community-dwelling older adults. Disabil. Rehabil. 2011, 33, 1776–1784. [Google Scholar] [CrossRef]
- Ginieri-Coccossis, M.; Triantafillou, E.; Tomaras, V.; Soldatos, C.; Mavreas, V.; Christodoulou, G. Psychometric properties of WHOQOL-BREF in clinical and health Greek populations: Incorporating new culture-relevant items. Psychiatriki 2012, 23, 130–142. [Google Scholar] [PubMed]
- Vahedi, S. World Health Organization Quality-of-Life Scale (WHOQOL-BREF): Analyses of Their Item Response Theory Properties Based on the Graded Responses Model. Iran. J. Psychiatry 2010, 5, 140–153. [Google Scholar] [PubMed]
- Yesavage, J.A.; Sheikh, J.I. Geriatric Depression Scale (GDS) Recent Evidence and Development of a Shorter Version. Clin. Gerontol. 1986, 5, 165–173. [Google Scholar] [CrossRef]
- Fountoulakis, K.N.; Tsolaki, M.; Iacovides, A.; Yesavage, J.; O’Hara, R.; Kazis, A.; Ierodiakonou, C. The validation of the short form of the Geriatric Depression Scale (GDS) in Greece. Aging Clin. Exp. Res. 1999, 11, 367–372. [Google Scholar] [CrossRef]
- Hopewell, S.; Chan, A.-W.; Collins, G.S.; Hróbjartsson, A.; Moher, D.; Schulz, K.F.; Tunn, R.; Aggarwal, R.; Berkwits, M.; Berlin, J.A.; et al. CONSORT 2025 statement: Updated guideline for reporting randomised trials. BMJ 2025, 389, e081123. [Google Scholar] [CrossRef]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 guidelines on physical activity and sedentary behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef]
- Chittrakul, J.; Siviroj, P.; Sungkarat, S.; Sapbamrer, R. Multi-system physical exercise intervention for fall prevention and quality of life in pre-frail older adults: A randomized controlled trial. Int. J. Environ. Res. Public Health 2020, 17, 3102. [Google Scholar] [CrossRef]
- Lim, H.; Jani, N.D.B.; Pang, W.T.; Lim, E.C.W. Community-based exercises improve health status in pre-frail older adults: A systematic review with meta-analysis. BioMed Cent. 2024, 24, 589. [Google Scholar] [CrossRef]
- López-López, S.; Abuín-Porras, V.; Berlanga, L.A.; Martos-Duarte, M.; Perea-Unceta, L.; Romero-Morales, C.; Pareja-Galeano, H. Functional mobility and physical fitness are improved through a multicomponent training program in institutionalized older adults. GeroScience 2024, 46, 1201–1209. [Google Scholar] [CrossRef]
- Monti, E.; Tagliaferri, S.; Zampieri, S.; Sarto, F.; Sirago, G.; Franchi, M.V.; Ticinesi, A.; Longobucco, Y.; Adorni, E.; Lauretani, F.; et al. Effects of a 2-year exercise training on neuromuscular system health in older individuals with low muscle function. J. Cachexia Sarcopenia Muscle 2023, 14, 794–804. [Google Scholar] [CrossRef]
- Tan, L.F.; Chan, Y.H.; Seetharaman, S.; Denishkrshna, A.; Au, L.; Kwek, S.C.; Chen, M.Z.; Ng, S.E.; Hui, R.J.Y. Impact of Exercise and Cognitive Stimulation Therapy on Physical Function, Cognition and Muscle Mass in Pre-Frail Older Adults in the Primary Care Setting: A Cluster Randomized Controlled Trial. J. Nutr. Health Aging 2023, 27, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Wu, Q.; Wang, D.; Li, Z.; Liu, H.; Liu, G.; Cui, Y.; Song, L. Effects of elastic band exercise on the frailty states in pre-frail elderly people. Physiother. Theory Pract. 2020, 36, 1000–1008. [Google Scholar] [CrossRef] [PubMed]
- García-gollarte, F.; Mora-concepción, A.; Pinazo-hernandis, S.; Segura-ortí, E.; Amer-cuenca, J.J.; Arguisuelas-martínez, M.D.; Lisón, J.F.; Benavent-caballer, V. Effectiveness of a Supervised Group-Based Otago Exercise Program on Functional Performance in Frail Institutionalized Older Adults: A Multicenter Randomized Controlled Trial. J. Geriatr. Phys. Ther. 2023, 46, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Millan-domingo, F.; Tarazona-santabalbina, F.J.; Carretero, A.; Olaso-gonzalez, G.; Viña, J.; Gomez-cabrera, M.C. Real-Life Outcomes of a Multicomponent Exercise Intervention in Community-Dwelling Frail Older Adults and Its Association with Nutritional-Related Factors. Nutrients 2022, 14, 5147. [Google Scholar] [CrossRef]
- Furtado, G.; Carvalho, H.M.; Patrício, M.; Uba-chupel, M.; Colado, J.C.; Hogervorst, E.; Ferreira, J.P.; Teixeira, A.M. Chair-based exercise programs in institutionalized older women: Salivary steroid hormones, disabilities and frailty changes. Exp. Gerontol. 2020, 130, 110790. [Google Scholar] [CrossRef]
- Fairhall, N.; Sherrington, C.; Kurrle, S.E.; Lord, S.R.; Lockwood, K.; Cameron, I.D. Effect of a multifactorial interdisciplinary intervention on mobility-related disability in frail older people: Randomised controlled trial. BMC Med. 2012, 10, 120. [Google Scholar] [CrossRef]
- Perera, S.; Mody, S.H.; Woodman, R.C.; Studenski, S.A. Meaningful change and responsiveness in common physical performance measures in older adults. J. Am. Geriatr. Soc. 2006, 54, 743–749. [Google Scholar] [CrossRef]
- Kp, N.; Anjupriya, D.; Nawed, A.; Nuhmani, S.; Khan, M.; Alghadir, A.H. Comparison of effects of Otago exercise program vs gaze stability exercise on balance and fear of fall in older adults: A randomized trial. Medicine 2024, 103, e38345. [Google Scholar] [CrossRef]
- Kekäläinen, T.; Kokko, K.; Sipilä, S.; Walker, S. Effects of a 9-month resistance training intervention on quality of life, sense of coherence, and depressive symptoms in older adults: Randomized controlled trial. Qual. Life Res. 2018, 27, 455–465. [Google Scholar] [CrossRef]
- Pucci, G.C.M.F.; Neves, E.B.; Santana, F.S.d.; Neves, D.d.A.; Saavedra, F.J.F. Efeito do Treinamento Resistido e do Pilates na Qualidade de vida de Idosas: Um ensaio clínico randomizado. Rev. Bras. Geriatr. E Gerontol. 2020, 23, e200283. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; Hayek, L.E.; Haidar, E.A.; Stringer, T.; Ulja, D. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-Hydroxybutyrate. Elife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xiao, H. Exercise for Mental Well-Being: Exploring Neurobiological Advances and Intervention Effects in Depression. Life 2023, 13, 1505. [Google Scholar] [CrossRef] [PubMed]
| Exercises | Beginner | Intermediate | Advanced |
|---|---|---|---|
| Hip abduction & extension | Standing with or without a light resistance band | Standing with a medium resistance band | Standing with a heavy resistance band |
| Knee extension | Seated with or without a light resistance band | Seated with a medium resistance band | Seated with a heavy resistance band |
| Shoulder abduction & external rotation | Standing with or without a light resistance band | Standing with a medium resistance band | Standing with a heavy resistance band |
| Standing calf raises | Holding onto a stable surface | Hands-free, slow, and controlled movements | Single-leg calf raises |
| Squats | Chair-assisted squats | Bodyweight squats | Weighted squats (holding an object) |
| Bridges | Assisted with arms pushing off the floor | Standard glute bridge | Single-leg bridge |
| Push-ups | Wall push-ups | Table push-ups | Standard or knee push-ups |
| Heel-to-toe standing/walking | Holding onto a stable surface | Hands-free standing | Walking heel-to-toe with a controlled pace |
| Single-leg standing | Holding onto support | Hands-free, eyes open | Hands-free, eyes closed |
| Stepping over obstacles | Small height obstacles | Moderate-height obstacles | Higher obstacles |
| Bending down—Lifting an object | Using a chair for support—Lightweight object | Hands-free, slow movement—Medium-weight object | Hands-free with feet together—Heavier object |
| Characteristics | Total (n = 112) | Control Group (n = 55) | Intervention Group (n = 57) |
|---|---|---|---|
| Age (years), mean (SD) | 79.3 (5.5) | 79.9 (5.2) | 78.7 (5.8) |
| Sex, n (%) | |||
| Male | 31 (27.7) | 17 (30.9) | 14 (24.6) |
| Female | 81 (72.3) | 38 (69.1) | 43 (75.4) |
| Marital status, n (%) | |||
| Married | 77 (68.8) | 36 (65.5) | 41 (71.9) |
| Widowed/divorced | 35 (31.3) | 19 (34.5) | 16 (28.1) |
| Ever smoked, n (%) | |||
| Yes | 81 (72.3) | 44 (80.0) | 37 (64.9) |
| No | 31 (27.7) | 11 (20.0) | 20 (35.1) |
| Profession, n (%) | |||
| Intellectual work | 10 (8.9) | 6 (10.9) | 4 (7.0) |
| Manual work | 96 (85.7) | 46 (83.6) | 50 (87.7) |
| Combination of manual and practical work | 6 (5.4) | 3 (5.5) | 3 (5.3) |
| Number of children, mean (SD) | 1.9 (0.9) | 1.9 (0.9) | 1.9 (0.8) |
| Live alone, n (%) | |||
| Yes | 33 (29.5) | 19 (34.5) | 14 (24.6) |
| No | 79 (70.5) | 36 (65.5) | 43 (75.4) |
| BMI (kg/cm2), mean (SD) | 25.7 (1.8) | 26.0 (2.1) | 25.5 (1.3) |
| Homebound, n (%) | |||
| Semi-confined | 53 (47.3) | 30 (54.5) | 23 (40.3) |
| Non-confined | 59 (52.7) | 25 (45.5) | 34 (59.6) |
| Baseline | Δ-T1 (6 Months) | Δ-T2 (12 Months) | Δ-T3 (18 Months) | p-Value Within Group | |
|---|---|---|---|---|---|
| SPPB | <0.001 $ | ||||
| Intervention Group | 8.0 ± 0.8 | 0.05 ± 0.35 | 0.16 ± 0.49 | 0.16 ± 0.49 | 0.012 # |
| Control Group | 8.0 ± 0.7 | −0.11 ± 0.31 | −0.31 ± 0.57 | −0.42 ± 0.63 | <0.001 # |
| p-value between Groups | 0.721 | 0.205 | 0.013 * | 0.002 * | |
| BBS | <0.001 $ | ||||
| Intervention Group | 48.1 ± 1.8 | 0.49 ± 1.34 | 0.75 ± 1.76 | 0.68 ± 1.97 | <0.001 # |
| Control Group | 48.5 ± 1.8 | −0.96 ± 1.91 | −1.49 ± 2.88 | −2.16 ± 3.29 | <0.001 # |
| p-value between Groups | 0.283 | 0.019 * | 0.002 * | <0.001 * | |
| TUG | <0.001 $ | ||||
| Intervention Group | 9.5 ± 1.3 | −0.2 ± 0.45 | −0.12 ± 0.55 | 0.05 ± 0.47 | <0.001 # |
| Control Group | 9.8 ± 1.8 | 0.42 ± 0.55 | 0.52 ± 0.63 | 0.6 ± 0.79 | <0.001 # |
| p-value between Groups | 0.677 | 0.009 * | 0.012 * | 0.045 * | |
| FES-I | <0.001 $ | ||||
| Intervention Group | 19.5 ± 2.7 | −0.37 ± 0.64 | −0.46 ± 0.95 | −0.37 ± 0.94 | <0.001 # |
| Control Group | 20.1 ± 2.8 | 0.05 ± 0.23 | 0.05 ± 0.36 | 0.05 ± 0.59 | 0.733 |
| p-value between Groups | 0.225 | 0.031 * | 0.019 * | 0.043 * |
| Baseline | Δ-T1 (6 Months) | Δ-T2 (12 Months) | Δ-T3 (18 Months) | p-Value Within Group | |
|---|---|---|---|---|---|
| Physical Health | 0.504 | ||||
| Intervention Group | 45.6 ± 13.7 | 3.26 ± 10.04 | 3.38 ± 10.2 | 2.19 ± 9.59 | 0.002 # |
| Control Group | 41.7 ± 10.1 | 2.27 ± 10.11 | 2.21 ± 10.14 | 2.4 ± 9.57 | 0.055 |
| p-value between Groups | 0.218 | 0.007 * | 0.005 * | 0.049 * | |
| Psychological Health | 0.440 | ||||
| Intervention Group | 45.8 ± 14.3 | 2.12 ± 5.85 | 2.27 ± 5.95 | 3.22 ± 7.64 | <0.001 # |
| Control Group | 41.1 ± 12.1 | 3.48 ± 6.45 | 3.48 ± 6.89 | 3.71 ± 7.97 | <0.001 # |
| p-value between Groups | 0.071 | 0.093 | 0.082 | 0.040 * | |
| Social Relationships | 0.704 | ||||
| Intervention Group | 41.7 ± 15.1 | 0 | 0.29 ± 1.55 | 0.15 ± 1.92 | 0.532 |
| Control Group | 40.3 ± 13.2 | 0.15 ± 1.96 | 0.15 ± 1.96 | 0 ± 1.6 | 0.881 |
| p-value between Groups | 0.550 | 0.613 | 0.532 | 0.488 | |
| Environment | 0.056 | ||||
| Intervention Group | 44.8 ± 12.5 | 0.05 ± 1.62 | 0.16 ± 1.91 | 1.48 ± 4.34 | 0.010 # |
| Control Group | 41.4 ± 10.9 | −0.06 ± 1.12 | −0.28 ± 1.24 | −0.17 ± 3.15 | 0.330 |
| p-value between Groups | 0.129 | 0.101 | 0.064 | 0.030 * | |
| Overall Quality of Life and General Health | 0.156 | ||||
| Intervention Group | 44.3 ± 17.7 | 1.1 ± 3.57 | 0.88 ± 3.22 | 2.41 ± 6.44 | 0.024 # |
| Control Group | 42.5 ± 15.3 | 0.23 ± 2.94 | 0 ± 3.4 | 0 ± 5.89 | 0.940 |
| p-value between Groups | 0.881 | 0.669 | 0.660 | 0.301 | |
| GDS | <0.001 $ | ||||
| Intervention Group | 5.23 ± 1.5 | −0.4 ± 0.53 | −0.61 ± 0.62 | −0.93 ± 0.88 | <0.001 # |
| Control Group | 5.07 ± 1.4 | −0.31 ± 0.57 | −0.29 ± 0.63 | −0.36 ± 0.65 | <0.001 # |
| p-value between Groups | 0.584 | 0.814 | 0.478 | 0.029 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savvakis, I.; Patelarou, A.; Mechili, E.A.; Stratidaki, E.; Patelarou, E.; Giakoumidakis, K. Targeted Physical Function Exercises for Frailty and Falls Management in Pre-Frail Community-Dwelling Older Adults: A Randomized Controlled Trial. Healthcare 2025, 13, 2486. https://doi.org/10.3390/healthcare13192486
Savvakis I, Patelarou A, Mechili EA, Stratidaki E, Patelarou E, Giakoumidakis K. Targeted Physical Function Exercises for Frailty and Falls Management in Pre-Frail Community-Dwelling Older Adults: A Randomized Controlled Trial. Healthcare. 2025; 13(19):2486. https://doi.org/10.3390/healthcare13192486
Chicago/Turabian StyleSavvakis, Ioannis, Athina Patelarou, Enkeleint A. Mechili, Eirini Stratidaki, Evridiki Patelarou, and Konstantinos Giakoumidakis. 2025. "Targeted Physical Function Exercises for Frailty and Falls Management in Pre-Frail Community-Dwelling Older Adults: A Randomized Controlled Trial" Healthcare 13, no. 19: 2486. https://doi.org/10.3390/healthcare13192486
APA StyleSavvakis, I., Patelarou, A., Mechili, E. A., Stratidaki, E., Patelarou, E., & Giakoumidakis, K. (2025). Targeted Physical Function Exercises for Frailty and Falls Management in Pre-Frail Community-Dwelling Older Adults: A Randomized Controlled Trial. Healthcare, 13(19), 2486. https://doi.org/10.3390/healthcare13192486






