The Effect of Intradialytic Exercise on Cognition in Renal Patients Undergoing Hemodialysis: An Updated Systematic Review of Randomized Controlled Trials
Abstract
1. Introduction
2. Materials and Methods
2.1. Eligibility Criteria
2.2. Information Sources
2.3. Search Strategy
2.4. Study Selection and Data Extraction
2.5. Study Quality and Risk of Bias
3. Results
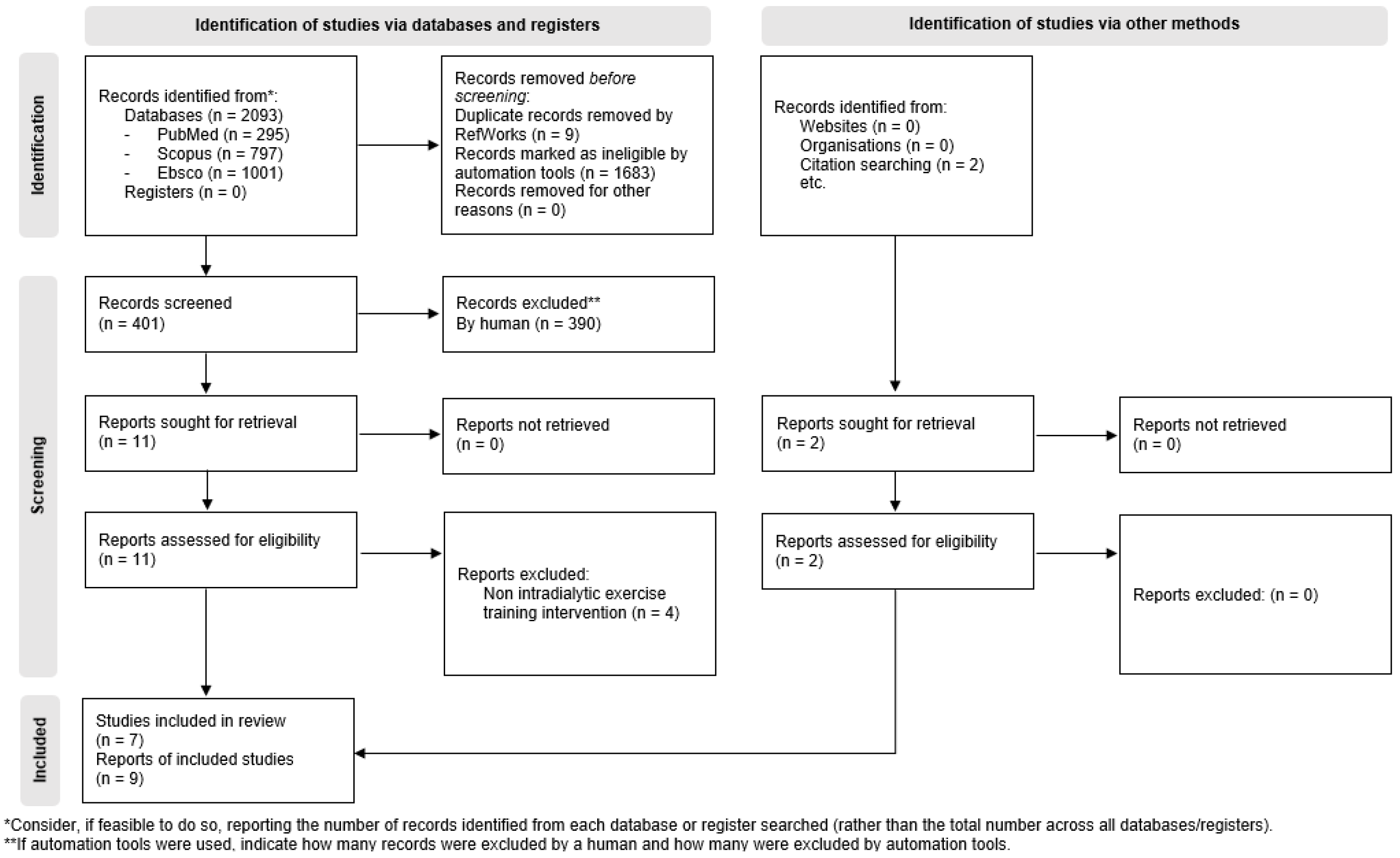
3.1. Literature Search and Characteristics of Included RCTs
3.2. Study Characteristics
4. Discussion
4.1. Limitations
4.2. Implications for Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kidney Disease: Improving Global Outcomes. KDIGO 2024 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int. 2024, 105, S117–S314. [Google Scholar] [CrossRef]
- Thurlow, J.S.; Joshi, M.; Yan, G.; Norris, K.C.; Agodoa, L.Y.; Yuan, C.M.; Nee, R. Global Epidemiology of End-Stage Kidney Disease and Disparities in Kidney Replacement Therapy. Am. J. Nephrol. 2021, 52, 98–107. [Google Scholar] [CrossRef]
- Bikbov, B.; Purcell, C.A.; Levey, A.S.; Smith, M.; Abdoli, A.; Abebe, M.; Adebayo, O.M.; Afarideh, M.; Agarwal, S.K.; Agudelo-Botero, M.; et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2020, 395, 709–733. [Google Scholar] [CrossRef]
- Vanholder, R.; Gryp, T.; Glorieux, G. Urea and chronic kidney disease: The comeback of the century? (in uraemia research). Nephrol. Dial. Transplant. 2018, 33, 4–12. [Google Scholar] [CrossRef]
- Mitsides, N.; Keane, D.F.; Lindley, E.; Mitra, S. Technology innovation for patients with kidney disease. J. Med. Eng. Technol. 2014, 39, 424–433. [Google Scholar] [CrossRef]
- Hornik, B.; Dulawa, J. Frailty, Quality of Life, Anxiety, and Other Factors Affecting Adherence to Physical Activity Recommendations by Hemodialysis Patients. Int. J. Environ. Res. Public Health 2019, 16, 1827. [Google Scholar] [CrossRef] [PubMed]
- Kurella Tamura, M.; Yaffe, K. Dementia and cognitive impairment in ESRD: Diagnostic and therapeutic strategies. Kidney Int. 2011, 79, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Nicholas, P.; Green, T.; Purtell, L.; Bonner, A. A cross-sectional study exploring cognitive impairment in kidney failure. J. Ren. Care 2022, 48, 93–101. [Google Scholar] [CrossRef]
- Murray, A.M. Cognitive impairment in the aging dialysis and chronic kidney disease populations: An occult burden. Adv. Chronic Kidney Dis. 2008, 15, 123–132. [Google Scholar] [CrossRef]
- Prince, M.; Ali, G.C.; Guerchet, M.; Prina, A.M.; Albanese, E.; Wu, Y.T. Recent global trends in the prevalence and incidence of dementia, and survival with dementia. Alzheimers Res. Ther. 2016, 8, 23. [Google Scholar] [CrossRef]
- Olczyk, P.; Kusztal, M.; Golebiowski, T.; Letachowicz, K.; Krajewska, M. Cognitive Impairment in End Stage Renal Disease Patients Undergoing Hemodialysis: Markers and Risk Factors. Int. J. Environ. Res. Public Health 2022, 19, 2389. [Google Scholar] [CrossRef]
- Viggiano, D.; Wagner, C.A.; Martino, G.; Nedergaard, M.; Zoccali, C.; Unwin, R.; Capasso, G. Mechanisms of cognitive dysfunction in CKD. Nat. Rev. Nephrol. 2020, 16, 452–469. [Google Scholar] [CrossRef] [PubMed]
- Angermann, S.; Schier, J.; Baumann, M.; Steubl, D.; Hauser, C.; Lorenz, G.; Gunthner, R.; Braunisch, M.C.; Kemmner, S.; Satanovskij, R.; et al. Cognitive Impairment is Associated with Mortality in Hemodialysis Patients. J. Alzheimers Dis. 2018, 66, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Capasso, G.; Franssen, C.F.M.; Perna, A.F.; Massy, Z.A.; Menzies, R.I.; Zoccali, C.; Tessitore, A.; Nedergaard, M.; Okusa, M.D.; Ortiz, A.; et al. Drivers and mechanisms of cognitive decline in chronic kidney disease. Nat. Rev. Nephrol. 2025, 21, 536–552. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Nakagawa, S. Physical activity for cognitive health promotion: An overview of the underlying neurobiological mechanisms. Ageing Res. Rev. 2023, 86, 101868. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Hadjigeorgiou, G.M.; Karatzaferi, C.; Pantzaris, M.C.; Stefanidis, I.; Sakkas, G.K. Epidemiology, impact, and treatment options of restless legs syndrome in end-stage renal disease patients: An evidence-based review. Kidney Int. 2014, 85, 1275–1282. [Google Scholar] [CrossRef]
- Koufaki, P.; Greenwood, S.; Painter, P.; Mercer, T. The BASES expert statement on exercise therapy for people with chronic kidney disease. J. Sports Sci. 2015, 33, 1902–1907. [Google Scholar] [CrossRef]
- Wilkinson, T.J.; Shur, N.F.; Smith, A.C. “Exercise as medicine” in chronic kidney disease. Scand. J. Med. Sci. Sports 2016, 26, 985–988. [Google Scholar] [CrossRef]
- Chu, N.M.; McAdams-DeMarco, M.A. Exercise and cognitive function in patients with end-stage kidney disease. Semin. Dial. 2019, 32, 283–290. [Google Scholar] [CrossRef]
- Krase, A.A.; Terzis, G.; Giannaki, C.D.; Stasinaki, A.N.; Wilkinson, T.J.; Smith, A.C.; Zorz, C.; Karatzaferi, C.; Stefanidis, I.; Sakkas, G.K. Seven months of aerobic intradialytic exercise training can prevent muscle loss in haemodialysis patients: An ultrasonography study. Int. Urol. Nephrol. 2022, 54, 447–456. [Google Scholar] [CrossRef]
- Mitrou, G.I.; Grigoriou, S.S.; Konstantopoulou, E.; Theofilou, P.; Giannaki, C.D.; Stefanidis, I.; Karatzaferi, C.; Sakkas, G.K. Exercise training and depression in ESRD: A review. Semin. Dial. 2013, 26, 604–613. [Google Scholar] [CrossRef]
- Gomes Neto, M.; de Lacerda, F.F.R.; Lopes, A.A.; Martinez, B.P.; Saquetto, M.B. Intradialytic exercise training modalities on physical functioning and health-related quality of life in patients undergoing maintenance hemodialysis: Systematic review and meta-analysis. Clin. Rehabil. 2018, 32, 1189–1202. [Google Scholar] [CrossRef]
- Grigoriou, S.S.; Krase, A.A.; Karatzaferi, C.; Giannaki, C.D.; Lavdas, E.; Mitrou, G.I.; Bloxham, S.; Stefanidis, I.; Sakkas, G.K. Long-term intradialytic hybrid exercise training on fatigue symptoms in patients receiving hemodialysis therapy. Int. Urol. Nephrol. 2021, 53, 771–784. [Google Scholar] [CrossRef]
- Pu, J.; Jiang, Z.; Wu, W.; Li, L.; Zhang, L.; Li, Y.; Liu, Q.; Ou, S. Efficacy and safety of intradialytic exercise in haemodialysis patients: A systematic review and meta-analysis. BMJ Open 2019, 9, e020633. [Google Scholar] [CrossRef] [PubMed]
- Giannaki, C.D.; Stefanidis, I.; Karatzaferi, C.; Liakos, N.; Roka, V.; Ntente, I.; Sakkas, G.K. The effect of prolonged intradialytic exercise in hemodialysis efficiency indices. ASAIO J. 2011, 57, 213–218. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, R.L.M.; Micali, P.N.; do Carmo, E.G.; Orlandi, F.S.; Costa, J.L.R. Cognitive abilities and physical activity in chronic kidney disease patients undergoing hemodialysis. Dement. Neuropsychol. 2019, 13, 329–334. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Bennett, H.; Miatke, A.; Dumuid, D.; Curtis, R.; Ferguson, T.; Brinsley, J.; Szeto, K.; Petersen, J.M.; Gough, C.; et al. Effectiveness of exercise for improving cognition, memory and executive function: A systematic umbrella review and meta-meta-analysis. Br. J. Sports Med. 2025, 59, 866–876. [Google Scholar] [CrossRef]
- Garber, C.E.; Blissmer, B.; Deschenes, M.R.; Franklin, B.A.; Lamonte, M.J.; Lee, I.M.; Nieman, D.C.; Swain, D.P.; American College of Sports, M. American College of Sports Medicine position stand. Quantity and quality of exercise for developing and maintaining cardiorespiratory, musculoskeletal, and neuromotor fitness in apparently healthy adults: Guidance for prescribing exercise. Med. Sci. Sports Exerc. 2011, 43, 1334–1359. [Google Scholar] [CrossRef]
- Levin, O.; Netz, Y.; Ziv, G. The beneficial effects of different types of exercise interventions on motor and cognitive functions in older age: A systematic review. Eur. Rev. Aging Phys. Act. 2017, 14, 20. [Google Scholar] [CrossRef]
- Mann, T.; Lamberts, R.P.; Lambert, M.I. Methods of prescribing relative exercise intensity: Physiological and practical considerations. Sports Med. 2013, 43, 613–625. [Google Scholar] [CrossRef]
- Yang, J.; Dong, Y.; Yan, S.; Yi, L.; Qiu, J. Which Specific Exercise Models Are Most Effective on Global Cognition in Patients with Cognitive Impairment? A Network Meta-Analysis. Int. J. Environ. Res. Public Health 2023, 20, 2790. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, D.; Green, S.; Higgins, J.P. Defining the review question and developing criteria for including studies. In Cochrane Handbook for Systematic Reviews of Interventions: Cochrane Book Series; Wiley: Hoboken, NJ, USA, 2008. [Google Scholar]
- Jadad, A.R.; Moore, R.A.; Carroll, D.; Jenkinson, C.; Reynolds, D.J.; Gavaghan, D.J.; McQuay, H.J. Assessing the quality of reports of randomized clinical trials: Is blinding necessary? Control. Clin. Trials 1996, 17, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Olivo, S.A.; Macedo, L.G.; Gadotti, I.C.; Fuentes, J.; Stanton, T.; Magee, D.J. Scales to assess the quality of randomized controlled trials: A systematic review. Phys. Ther. 2008, 88, 156–175. [Google Scholar] [CrossRef] [PubMed]
- Ghildayal, N.; Liu, Y.; Hong, J.; Li, Y.; Chen, X.; Fernandez, M.G.; Carlson, M.C.; Fine, D.M.; Appel, L.J.; Diener-West, M.; et al. Intradialytic Cognitive and Aerobic Exercise Training to Preserve Cognitive Function: IMPCT, a Multi-Dialysis Center 2 × 2 Factorial Block-Randomized Controlled Trial. Am. J. Nephrol. 2025. [Google Scholar] [CrossRef]
- Feng, X.; Sun, J.; Wang, Z.; Zhang, N.; Liu, Y.; Wang, Z.; Wang, N.; Jian, G.; Cheng, D.; Sheng, X.; et al. The impact of intradialytic elastic band exercise on physical and cognitive abilities in patients on maintenance hemodialysis: A randomized controlled trial. Ren. Fail. 2025, 47, 2482124. [Google Scholar] [CrossRef]
- Saputrana, A.S.; Pawana, I.P.A.; Arfianti, L.; Thaha, M.; Atika, A. The effect of low-intensity intradialytic cycling aerobic exercise on cognitive function in patients undergoing continuous hemodialysis. Rom. J. Neurol. 2024, 23, 261–266. [Google Scholar] [CrossRef]
- Bogataj, S.; Pajek, M.; Mesaric, K.K.; Kren, A.; Pajek, J. Twelve weeks of combined physical and cognitive intradialytic training preserves alertness and improves gait speed: A randomized controlled trial. Aging Clin. Exp. Res. 2023, 35, 2119–2126. [Google Scholar] [CrossRef]
- Kren, A.; Bogataj, S. The Impact of Intradialytic Cognitive and Physical Training Program on the Physical and Cognitive Abilities in End-Stage Kidney Disease Patients: A Randomized Clinical Controlled Trial. Brain Sci. 2023, 13, 1228. [Google Scholar] [CrossRef]
- Bogataj, S.; Pajek, M.; Kren, A.; Kurnik Mesaric, K.; Pajek, J. Randomized Controlled Trial of Intradialytic Cognitive and Physical Training to Enhance Functional Capacity. Kidney Int. Rep. 2024, 9, 2028–2036. [Google Scholar] [CrossRef]
- Nakamura-Taira, N.; Horikawa, N.; Oka, F.; Igarashi, Y.; Kobayashi, S.; Kato, S.; Enomoto, T.; Kimura, H.; Watanabe, Y.; Kumada, T.; et al. Quasi-cluster randomized trial of a six-month low-intensity group-based resistance exercise for hemodialysis patients on depression and cognitive function: A 12-month follow-up. Health Psychol. Behav. Med. 2021, 9, 741–760. [Google Scholar] [CrossRef]
- McAdams-DeMarco, M.A.; Konel, J.; Warsame, F.; Ying, H.; Fernandez, M.G.; Carlson, M.C.; Fine, D.M.; Appel, L.J.; Segev, D.L. Intradialytic Cognitive and Exercise Training May Preserve Cognitive Function. Kidney Int. Rep. 2018, 3, 81–88. [Google Scholar] [CrossRef]
- Stringuetta Belik, F.; Oliveira, E.S.V.R.; Braga, G.P.; Bazan, R.; Perez Vogt, B.; Costa Teixeira Caramori, J.; Barretti, P.; de Souza Goncalves, R.; Fortes Villas Boas, P.J.; Hueb, J.C.; et al. Influence of Intradialytic Aerobic Training in Cerebral Blood Flow and Cognitive Function in Patients with Chronic Kidney Disease: A Pilot Randomized Controlled Trial. Nephron 2018, 140, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bedirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef]
- Teng, E.L.; Chui, H.C. The Modified Mini-Mental State (3MS) examination. J. Clin. Psychiatry 1987, 48, 314–318. [Google Scholar]
- Benedict, R.H.; DeLuca, J.; Phillips, G.; LaRocca, N.; Hudson, L.D.; Rudick, R.; Multiple Sclerosis Outcome Assessments, C. Validity of the Symbol Digit Modalities Test as a cognition performance outcome measure for multiple sclerosis. Mult. Scler. 2017, 23, 721–733. [Google Scholar] [CrossRef]
- Zalonis, I.; Kararizou, E.; Triantafyllou, N.I.; Kapaki, E.; Papageorgiou, S.; Sgouropoulos, P.; Vassilopoulos, D. A normative study of the trail making test A and B in Greek adults. Clin. Neuropsychol. 2008, 22, 842–850. [Google Scholar] [CrossRef]
- Zimmermann, P.; Fimm, B. Test of Attentional Performance 2.3.1. 2021. Available online: https://www.psytest.de/index.php?page=TAP-2-2&hl=en_US (accessed on 15 September 2025).
- Loring, D.W.; Bowden, S.C.; Staikova, E.; Bishop, J.A.; Drane, D.L.; Goldstein, F.C. NIH Toolbox Picture Sequence Memory Test for Assessing Clinical Memory Function: Diagnostic Relationship to the Rey Auditory Verbal Learning Test. Arch. Clin. Neuropsychol. 2019, 34, 268–276. [Google Scholar] [CrossRef]
- Elwood, R.W. The Wechsler Memory Scale-Revised: Psychometric characteristics and clinical application. Neuropsychol. Rev. 1991, 2, 179–201. [Google Scholar] [CrossRef]
- Bogataj, S.; Mesaric, K.K.; Pajek, M.; Petrusic, T.; Pajek, J. Physical exercise and cognitive training interventions to improve cognition in hemodialysis patients: A systematic review. Front. Public Health 2022, 10, 1032076. [Google Scholar] [CrossRef]
- Liu, H.; Song, Y.; Zhao, D.; Zhan, M. Effect of exercise on cognitive impairment in patients undergoing haemodialyses: A systematic review and meta-analysis of randomised controlled trials. J. Ren. Care 2022, 48, 243–252. [Google Scholar] [CrossRef]
- Bogataj, S.; Roelands, B.; Pajek, M.; Pajek, J. Intradialytic cycling and cognitive training to mitigate decreased functional and physiological status in haemodialysis patients. Nephrol. Dial. Transplant. 2024, 39, 1198–1200. [Google Scholar] [CrossRef]
- Deus, L.A.; Correa, H.L.; Neves, R.V.P.; Reis, A.L.; Honorato, F.S.; Silva, V.L.; Souza, M.K.; de Araujo, T.B.; de Gusmao Alves, L.S.; Sousa, C.V.; et al. Are Resistance Training-Induced BDNF in Hemodialysis Patients Associated with Depressive Symptoms, Quality of Life, Antioxidant Capacity, and Muscle Strength? An Insight for the Muscle-Brain-Renal Axis. Int. J. Environ. Res. Public Health 2021, 18, 11299. [Google Scholar] [CrossRef]
- Giannaki, C.D.; Sakkas, G.K.; Hadjigeorgiou, G.M.; Manconi, M.; Bargiotas, P. Unfolding the role of exercise in the management of sleep disorders. Eur. J. Appl. Physiol. 2024, 124, 2547–2560. [Google Scholar] [CrossRef]
| PICOS Item | Keyword |
|---|---|
| Patient population | Hemodialysis patients |
| Interventions (exposures) | Exercise |
| Comparator (control) group | Standard care |
| Outcomes | Cognitive function |
| Study design | Randomized controlled trial |
| Database | Final Search Algorithm |
|---|---|
| PubMed | (((((hemodialysis [Title/Abstract]) OR (haemodialysis [Title/Abstract])) OR (dialysis [Title/Abstract])) OR (dialy* [Title/Abstract])) AND (((((“physical activity” [Title/Abstract]) OR (“physical exercise” [Title/Abstract])) OR (exercise* [Title/Abstract])) OR (activit* [Title/Abstract])) OR (fitness [Title/Abstract]))) AND (((cognition [Title/Abstract]) OR (cognitive [Title/Abstract])) OR (cognit* [Title/Abstract])) |
| EBSCO | AB (hemodialysis or haemodialysis or dialysis or dialy*) AND AB (physical activity or exercise or fitness or physical exercise or activit*) AND AB (cognition or cognitive function or cognitive performance or cognitive abilities or cognitive ability or memory or cognit*) |
| SCOPUS | ((TITLE-ABS-KEY (hemodialysis) OR TITLE-ABS-KEY (haemodialysis) OR TITLE-ABS-KEY (dialysis) OR TITLE-ABS-KEY (dialy*))) AND ((TITLE-ABS-KEY (“physical activity”) OR TITLE-ABS-KEY (“physical exercise”) OR TITLE-ABS-KEY (exercise*) OR TITLE-ABS-KEY (activit*) OR TITLE-ABS-KEY (fitness)))AND ((TITLE-ABS-KEY (cognition)OR TITLE-ABS-KEY (cognitive) OR TITLE-ABS-KEY (cognit*))) |
| Study | Randomization | Blinding | Withdrawals/Dropouts | Score (0–5) |
|---|---|---|---|---|
| Ghildayal et al., 2025 [35] | ++ | 0 | + | 3 |
| Feng et al., 2025 [36] | ++ | 0 | + | 3 |
| Saputrana et al., 2024 [37] | 0 | 0 | + | 1 |
| Bogataj trial: Bogataj et al., 2023 [38] (A); Kren & Bogataj, 2023 [39] (B); Bogataj et al., 2024 [40] (C) * | ++ (A, B, C) | 0 (A, B, C) | + (A, B, C) | 3 (A, B, C) |
| Nakamura-Taira et al., 2021 [41] | + | 0 | + | 2 |
| McAdams-DeMarco et al., 2018 [42] | + | 0 | + | 2 |
| Stringuetta Belik et al., 2018 [43] | ++ | 0 | + | 3 |
| Study ID | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | QR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ghildayal et al., 2025 [35] | + | + | - | - | + | - | + | NR | + | NR | + | + | + | + | F |
| Feng et al., 2025 [36] | + | + | + | - | + | + | + | + | + | CD | + | + | - | CD | G |
| Saputrana et al., 2024 [37] | + | - | - | NR | NR | + | + | + | + | NR | + | - | - | - | F |
| Bogataj et al., 2023 [38] (A); Kren & Bogataj, 2023 [39] (B); Bogataj et al., 2024 [40] (C) * | + (A, B, C) | + (A, B, C) | NR (A, B, C) | - (A, B, C) | + (A, B, C) | + (A, B, C) | + (A, B, C) | + (A, B, C) | + (A, B, C) | NR (A, B, C) | + (A, B, C) | + (A, B, C) | + (A) NR (B, C) | + (A, B, C) | G (A, B, C) |
| Nakamura-Taira et al., 2021 [41] | + | NR | NR | - | NR | + | - | + | + | + | + | + | NR | + | F |
| Stringuetta-Belik et al., 2018 [42] | + | + | + | - | NR | + | + | + | NR | + | + | - | NR | + | F |
| McAdams-DeMarco et al., 2018 [43] | + | NR | NR | NR | NR | + | + | + | + | NR | + | - | + | + | F |
| Study | Year | Country | Sample Size | Age (Mean ± SD)/(IQR) | Sex (%Male) | Design | Intervention vs. Control | Measures | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| Ghildayal et al., 2025 [35] | 2025 | USA | ET: (n = 29) CT: (n = 31) ET + CT: (n = 35) SC: (n = 26) | ET: 60.4 (38.3–66.5) CT: 64.2 (56.2–69.9) ET + CT: 60.4 (52.0–70.3) SC: 61.5 (52.0–67.5) | ET: 66 CT: 65 ET + CT: 60 SC: 62 | RCT | ET, CT, ET + CT vs. SC | TMTB-A TMT-A TMT-B MoCA DSST RAVLT | Between-group differences at 3 months compared to SC, presented as difference and (95% CI) TMTB-A (s) (n = 95) SC: reference ET: −15.8 (−54.7, 23.1) CT: 6.4 (−33.6, 46.4) ET + CT: −5.8 (−43.6,31.9) TMT-A (s) (n = 95) SC: reference ET: 11.4 (−2.7, 25.5) CT: 0.8 (−13.5, 15.1) ET + CT: 3.9 (−9.6, 17.5) TMT-B (s) (n = 95): SC: reference ET: −1.9 (−43.9, 40.2) CT: 11.4 (−31.7, 54.6) ET + CT: 4.1 (−36.4, 44.7) MoCA (n = 77) SC: reference ET: 0.7 (−1.1, 2.6) CT: 0.2 (−1.7, 2.0) ET + CT: 2.1 (0.4, 3.9) DSST (n = 77) SC: reference ET:7.4 (−4.8, 19.6) CT:3.8 (−8.5, 16.1) ET + CT: 2.9 (−8.8, 14.5) RAVLT (n = 79) SC: reference ET: −0.3 (−1.1, 0.6) CT: 0.2 (−0.6, 1.1) CT + ET: 0.3 (−0.5, 1.1) |
| Feng et al., 2025 [36] | 2025 | China | ET: (n = 28) SC: (n = 30) | ET: 57.50 ± 11.6 SC: 56.10 ± 12.07 | ET: 61 SC: 67 | RCT | ET vs. SC | MoCA | Within-group change (mean ± SD) ET: from 24.21 ± 3.17 to 28.36 ± 1.75; t = −9.257, p < 0.001 SC: from 23.37 ± 4.19 to 24.37 ± 3.94; t = −1.888, p = 0.069 Interaction group x time: t = 5.047, p < 0.001 |
| Saputrana et al., 2024 [37] | 2024 | Indonesia | ET: (n = 9) SC: (n = 9) | ET: 36.56 ± 4.61 SC: 39.44 ± 5.36 | ET: 56 SC: 44 | RCT | ET vs. SC | MoCA | Within-group change ET: 22.56 ± 2.65 to 27.56 ± 2.29; ES = 1.88, p < 0.001 SC: 23.44 ± 2.29 to 24.00 ± 2.64; p = 0.468 Between-group differences ET: 4 ± 2.64 SC: 1 ± 2.18 p = 0.001 |
| Bogataj et al., 2023 [38] (A); Kren & Bogataj, 2023 [39] (B); Bogataj et al., 2024 [40] (C) * | 2023/24 | Slovenia | EXP (n = 22) CON (n = 21) | EXP: 65.7 ± 9.7 CON: 67.2 ± 12.5 | EXP: 54 CON: 77 | Single-Blind RCT | ET + CT vs. SC | (A) TAP Test | Within-group changes in (mean and 95% CI) Alertness: EXP: 43.3 (−8.7 to 95.3), p = 0.098, ES: +0.37 CON: −26.7 (−52 to −1.4), p = 0.040, ES: −0.48 Selective attention: EXP: 20.4 (−22.1 to 62.9), p = 0.329, ES: +0.21 CON: −32.1 (−90 to 26.6), p = 0.267, ES: −0.25 Divided attention: EXP: 15.2 (−18.1 to 48.5), p = 0.352, ES: +0.21 CON: 16.1 (−36.6 to 68.7), p = 0.530, ES: +0.12 Between-group changes in Alertness: (F(1,41) = 6.15, p = 0.017, η2 = 0.13) Selective attention: (F(1,41) = 2.31, p = 0.136, η2 = 0.53) Divided attention: (F(1,41) = 0.001, p = 0.977, η2 = 0.00) |
| (B) SDMT MoCA | Time × group interaction effect in favor of EXP group SDMT: p < 0.001; η2 = 0.267 MoCA: p < 0.001; η2 = 0.266I | ||||||||
| (C) TMT-A TMT-B TMTB_A | Within-group changes: TMT-A (s): EXP: −3.6 ± 5.5 (−6.1 to −1.2), p = 0.006, ES: 0.12 CON: 6.6 ± 10.5 (1.8 to 11.3), p = 0.009, ES: 0.17 TMT-B (s): EXP: −14 ± 17.2 (−21.7 to −6.4), p < 0.001, ES: 0.19 CON: 7.0 ± 13.4 (0.9 to 13.1), p = 0.026. ES: 0.08 TMTB-TMTA (s): EXP: −10.4 ± 15.2 (−17.1 to −3.7), p = 0.004, ES: 0.20 CON: 0.4 ± 17.9 (−7.7 to 8.6), p = 0.914, ES: 0.01 Time × group interaction effect for EXP group: TMT-A: (F [1,43] = 16.218, p < 0.001, η2 = 0.283) TMT-B: (F [1,43] = 19.944, p < 0.001, η2 = 0.327) TMT_B—TMT_A: (F [1,43] = 4.606, p = 0.038, η2 = 0.101) | ||||||||
| Nakamura-Taira et al., 2021 [41] | 2021 | Japan | ET (n = 21) SC (n = 21) | ET: 74.9 ± 2.23 SC: 72.57 ± 2.26 | ET: 33 SC: 71 | Quasi-Cluster RCT | ET vs. SC | MoCA | Within-group change (AMS ± SE) EXP: 18.45 ± 0.63 to 18.87 ± 0.71 CON: 18.48 ± 0.77 to 18.09 ± 0.94 Comparison between groups ES: −0.13 (95% CI: −0.74 to 0.48) p > 0.05 |
| Stringuetta Belik et al., 2018 [43] | 2018 | Brazil | ET (n = 15) SC (n = 15) | ET: 50.3 ± 17.24 SC: 57.8 ± 15.01 | ET: 47 SC: 53 | RCT | ET vs. SC | MMSE | Within group × time change EXP: 24.0 ± 3.00 (baseline) to 26.4 ± 2.92 (post intervention); p < 0.001 CON: 22.4 ± 4.98 (baseline) to 23.0 ± 5.09 (post intervention); p > 0.050 Interaction between group × time p = 0.001 |
| McAdams-DeMarco et al., 2018 [42] | 2018 | USA | ET: (n = 6) CT: (n = 7) SC: (n = 7) | ET: 48.0 ± 7.0 CT: 48.9 ± 12.2 SC: 55.0 ± 9.7 | ET: 67 CT: 29 SC: 100 | RCT | ET, CT vs. SC | 3MS TMT-A TMT-B TMTB-A | Within-group change (mean ± SD) 3MS SC: −0.1 (7.1); p = 0.96 ET: 4.3 (5.4); p = 0.17 TMTA SC: 15.0 (25.8); p = 0.055 ET: −2.5 (9.3); p = 0.77 TMTB SC: 47.4 (45.7); p = 0.006 ET: −8.9 (24.4); p = 0.63 TMTB—TMTA SC: 31.7 (47.8); p = 0.052 ET: −3.2 (31.2); p = 0.86 Between-group change (mean and 95% CI) (SC vs. ET) 3MS: 4.48 (95% CI: −4.27 to 13.22; p = 0.30 TMTA: −17.48 (95% CI: −41.18 to 6.22; p = 0.14) TMTB: −56.21 (95% CI: −105.86 to −6.56; p = 0.03) TMTB-TMTA: −34.93 (95% CI: −85.43 to 15.56; p = 0.16) |
| Study | F.I.T.T. Principles: Frequency (Sessions/Week), Intensity (Rating), Time (Training Session Duration and Intervention Length), and Type (Exercise Modality) | |||
|---|---|---|---|---|
| Frequency | Intensity | Time | Type | |
| Ghildayal et al., 2025 [35] | 3 | Moderate–High | 30 min/12 wks. | Aerobic |
| Feng et al., 2025 [36] | 3 | Low–Moderate | 30–40 min/12 wks. | Resistance |
| Saputrana et al., 2024 [37] | 2 | Low–Moderate | 30 min/12 wks. | Aerobic |
| Bogataj et al., 2023/24 [38,40] | 3 | Moderate–High | 30 min/12 wks. | Aerobic |
| Nakamura-Taira et al., 2021 [41] | 3 | Low | 25–30 min/24 wks. | Resistance |
| Stringuetta Belik et al., 2018 [43] | 3 | Moderate–High | 30–45 min/16 wks. | Aerobic |
| McAdams De-Marco et al., 2018 [42] | 3 | CD | 20 min/12 wks. | Aerobic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mavrommatis, A.; Mitsides, N.; Pieri, M.; Andreou, E.P.; Sakkas, G.K.; Dimitriou, K.; Spartalis, M.; Arsali, M.; Christofi, T.; Theophanous, T.; et al. The Effect of Intradialytic Exercise on Cognition in Renal Patients Undergoing Hemodialysis: An Updated Systematic Review of Randomized Controlled Trials. Healthcare 2025, 13, 3016. https://doi.org/10.3390/healthcare13233016
Mavrommatis A, Mitsides N, Pieri M, Andreou EP, Sakkas GK, Dimitriou K, Spartalis M, Arsali M, Christofi T, Theophanous T, et al. The Effect of Intradialytic Exercise on Cognition in Renal Patients Undergoing Hemodialysis: An Updated Systematic Review of Randomized Controlled Trials. Healthcare. 2025; 13(23):3016. https://doi.org/10.3390/healthcare13233016
Chicago/Turabian StyleMavrommatis, Andreas, Nicos Mitsides, Myrtani Pieri, Eleni P. Andreou, Giorgos K. Sakkas, Kyproula Dimitriou, Michalis Spartalis, Maria Arsali, Themis Christofi, Theophanis Theophanous, and et al. 2025. "The Effect of Intradialytic Exercise on Cognition in Renal Patients Undergoing Hemodialysis: An Updated Systematic Review of Randomized Controlled Trials" Healthcare 13, no. 23: 3016. https://doi.org/10.3390/healthcare13233016
APA StyleMavrommatis, A., Mitsides, N., Pieri, M., Andreou, E. P., Sakkas, G. K., Dimitriou, K., Spartalis, M., Arsali, M., Christofi, T., Theophanous, T., Wollesen, B., Hadjigeorgiou, G. M., & Giannaki, C. D. (2025). The Effect of Intradialytic Exercise on Cognition in Renal Patients Undergoing Hemodialysis: An Updated Systematic Review of Randomized Controlled Trials. Healthcare, 13(23), 3016. https://doi.org/10.3390/healthcare13233016











