Measuring Surgical Waiting Times in Breast Cancer: Admission to Surgery Versus Biopsy Result to Surgery
Abstract
1. Introduction
2. Materials and Methods
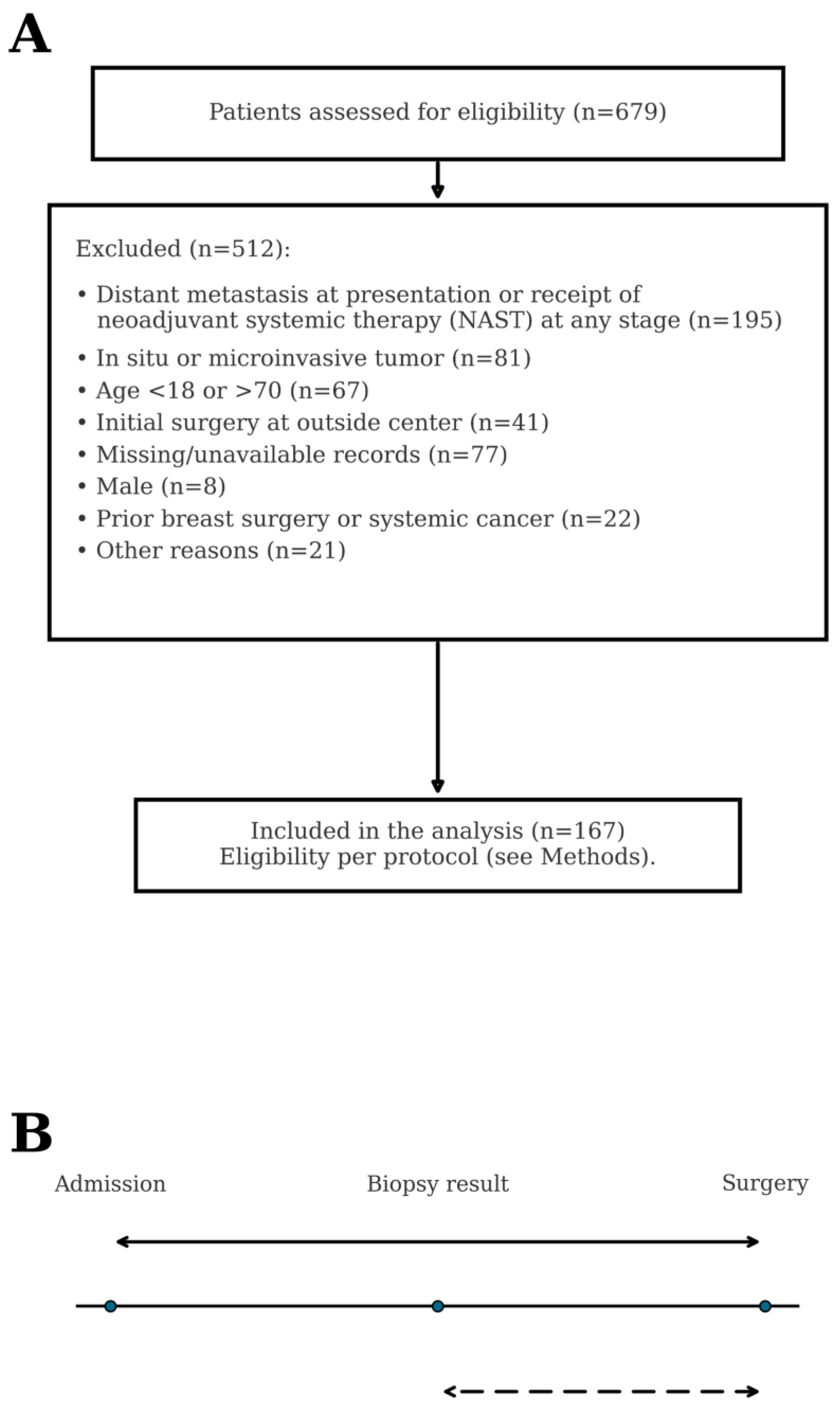
2.1. Study Design, Setting, and Participants
2.2. Data Sources and Variables
2.3. Diagnostic-to-Treatment Intervals
2.4. Outcomes and Follow-Up
2.5. Statistical Analysis
2.5.1. Agreement Between Timing Definitions
2.5.2. Recurrence Modeling
2.5.3. Overall Survival
2.5.4. Exploratory Subgroups
2.5.5. Software and Presentation
3. Results
3.1. Cohort and Outcomes
3.2. Diagnostic-to-Treatment Intervals
3.3. Recurrence
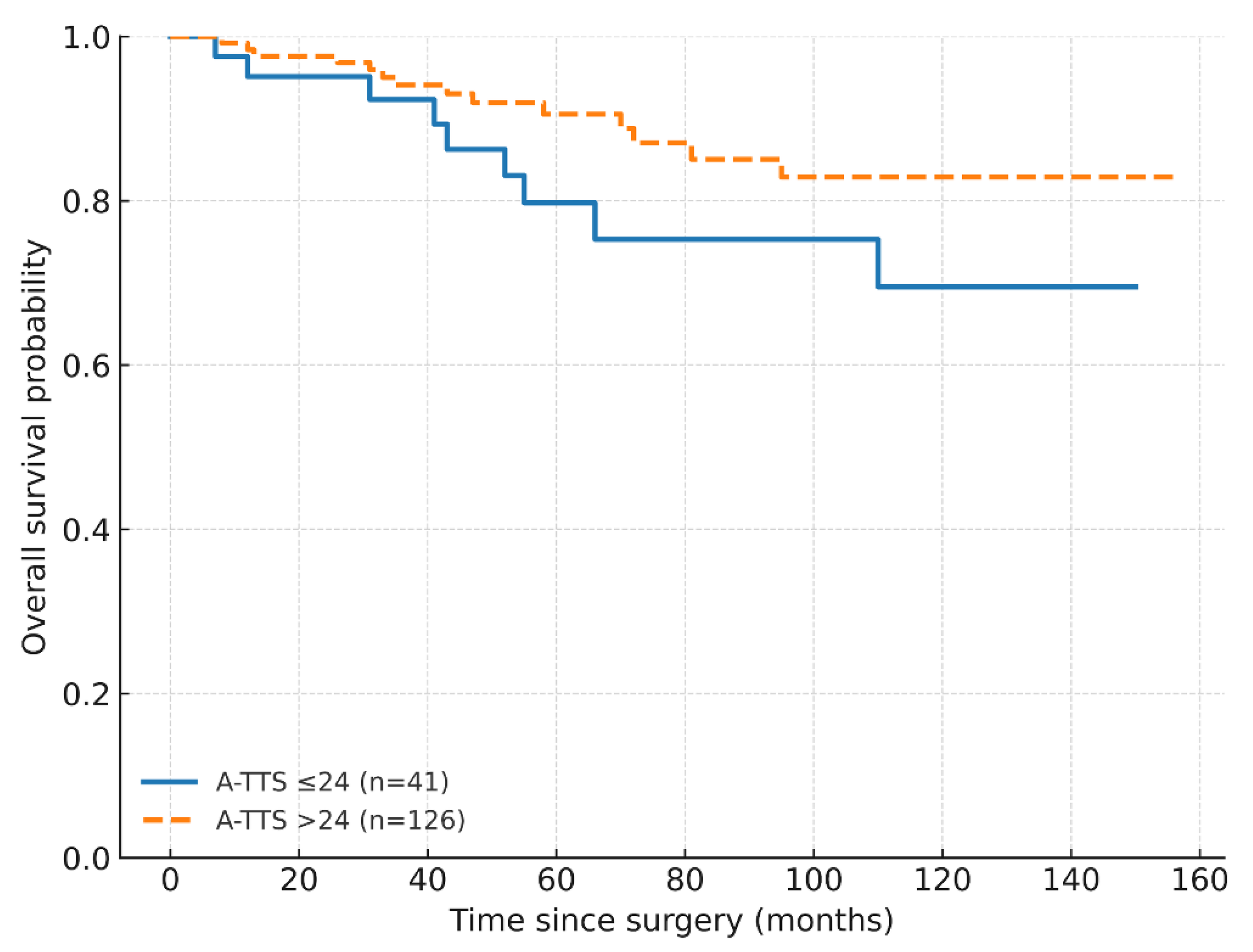
3.4. Overall Survival
3.5. Exploratory Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Loibl, S.; André, F.; Bachelot, T.; Barrios, C.H.; Bergh, J.; Burstein, H.J.; Cardoso, M.; Carey, L.; Dawood, S.; Del Mastro, L.; et al. Early breast cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann. Oncol. 2024, 35, 159–182. [Google Scholar] [CrossRef]
- Gilmour, A.; Cutress, R.; Gandhi, A.; Harcourt, D.; Little, K.; Mansell, J.; Murphy, J.; Pennery, E.; Tillett, R.; Vidya, R.; et al. Oncoplastic breast surgery: A guide to good practice. Eur. J. Surg. Oncol. 2021, 47, 2272–2285. [Google Scholar] [CrossRef]
- Cardoso, M.M.; Baixinho, C.L.; Silva, G.T.R.; Ferreira, Ó. Nursing Interventions in the Perioperative Pathway of the Patient with Breast Cancer: A Scoping Review. Healthcare 2023, 11, 1717. [Google Scholar] [CrossRef]
- Chagpar, A.B.; Howard-McNatt, M.; Chiba, A.; Levine, E.A.; Gass, J.S.; Gallagher, K.; Lum, S.; Martinez, R.; Willis, A.I.; Fenton, A.; et al. Factors affecting time to surgery in breast cancer patients. Am. Surg. 2022, 88, 648–652. [Google Scholar] [CrossRef]
- Freeman, H.D.; Burke, L.C.; Humphrey, J.G.; Wilbers, A.J.; Vora, H.; Khorfan, R.; Solomon, N.L.; Namm, J.P.; Ji, L.; Lum, S.S. Fragmentation of care in breast cancer: Greater than the sum of its parts. Breast Cancer Res. Treat. 2024, 208, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Tjoe, J.A.; Heslin, K.; Perez Moreno, A.C.; Thomas, S.; Kram, J.J.F. Factors associated with breast cancer surgery delay within a coordinated multihospital community health system: When does surgical delay impact outcome? Clin. Breast Cancer. 2022, 22, e91–e100. [Google Scholar] [CrossRef]
- Watanabe, T.; Rikitake, R.; Kakuwa, T.; Ichinose, Y.; Niino, M.; Mizushima, Y.; Ota, M.; Fujishita, M.; Tsukada, Y.; Higashi, T. Time to treatment initiation for six cancer types: An analysis of data from a nationwide registry in Japan. World J. Surg. 2023, 47, 877–886. [Google Scholar] [CrossRef]
- de Azambuja, E.; Trapani, D.; Loibl, S.; Delaloge, S.; Senkus, E.; Criscitiello, C.; Poortmans, P.; Gnant, M.; Di Cosimo, S.; Cortes, J.; et al. ESMO management and treatment adapted recommendations in the COVID-19 era: Breast cancer. ESMO Open 2020, 5 (Suppl. 3), e000793. [Google Scholar] [CrossRef]
- Ungvari, Z.; Fekete, M.; Buda, A.; Lehoczki, A.; Munkácsy, G.; Scaffidi, P.; Bonaldi, T.; Fekete, J.T.; Bianchini, G.; Varga, P.; et al. Quantifying the impact of treatment delays on breast cancer survival outcomes: A comprehensive meta-analysis. Geroscience 2025. [Google Scholar] [CrossRef]
- Wiener, A.A.; Hanlon, B.M.; Schumacher, J.R.; Vande Walle, K.A.; Wilke, L.G.; Neuman, H.B. Reexamining time from breast cancer diagnosis to primary breast surgery. JAMA Surg. 2023, 158, 485–492. [Google Scholar] [CrossRef]
- An, D.; Choi, J.; Lee, J.; Kim, J.Y.; Kwon, S.; Kim, J.; Lee, S.; Jeon, S.; Lee, C.; Lee, S.; et al. Time to surgery and survival in breast cancer. BMC Surg. 2022, 22, 388. [Google Scholar] [CrossRef] [PubMed]
- Leslie Salewon, M.; Pathak, R.; Dooley, W.C.; Squires, R.A.; Rui, H.; Chervoneva, I.; Tanaka, T. Surgical delay-associated mortality risk varies by subtype in loco-regional breast cancer patients in SEER-Medicare. Breast Cancer Res. 2024, 26, 191. [Google Scholar] [CrossRef]
- Murchie, P.; Raja, E.A.; Lee, A.J.; Brewster, D.H.; Campbell, N.C.; Gray, N.M.; Ritchie, L.; Robertson, R.; Samuel, L. Effect of longer health service provider delays on stage at diagnosis and mortality in symptomatic breast cancer. Breast 2015, 24, 248–255. [Google Scholar] [CrossRef]
- von Elm, E.; Altman, D.G.; Egger, M.; Pocock, S.J.; Gøtzsche, P.C.; Vandenbroucke, J.P.; STROBE Initiative. The STROBE statement: Guidelines for reporting observational studies. J. Clin. Epidemiol. 2008, 61, 344–349. [Google Scholar] [CrossRef]
- National Comprehensive Cancer Network (NCCN). Clinical Practice Guidelines in Oncology: Breast Cancer. Version 4. 2025. Available online: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 30 August 2025).
- Moran, M.S.; Schnitt, S.J.; Giuliano, A.E.; Harris, J.R.; Khan, S.A.; Horton, J.; Klimberg, S.; Chavez-MacGregor, M.; Freedman, G.; Houssami, N.; et al. SSO-ASTRO consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in stages I–II invasive breast cancer. Int. J. Radiat. Oncol. Biol. Phys. 2014, 88, 553–564. [Google Scholar] [CrossRef]
- Morrow, M.; Van Zee, K.J.; Solin, L.J.; Houssami, N.; Chavez-MacGregor, M.; Harris, J.R.; Horton, J.; Hwang, S.; Johnson, P.L.; Marinovich, M.L.; et al. SSO-ASTRO-ASCO consensus guideline on margins for breast-conserving surgery with whole-breast irradiation in DCIS. Pract. Radiat. Oncol. 2016, 6, 287–295. [Google Scholar] [CrossRef]
- Coeckelberghs, E.; Vanhaecht, K.; Seys, D.; Neven, P.; Smeets, A.; Boecxstaens, V. Variation in care for breast cancer surgery patients: A multicentre study. Eur. J. Surg. Oncol. 2024, 50, 107471. [Google Scholar] [CrossRef]
- Bleicher, R.J.; Ruth, K.; Sigurdson, E.R.; Beck, J.R.; Ross, E.; Wong, Y.N.; Patel, S.A.; Boraas, M.; Chang, E.I.; Topham, N.S.; et al. Time to surgery and breast cancer survival in the United States. JAMA Oncol. 2016, 2, 330–339. [Google Scholar] [CrossRef]
- Jeong, S.H.; Chun, S.M.; Lee, H.; Kim, M.; Leigh, J.H. Impact of diagnosis-to-treatment interval on mortality in early-stage breast cancer: A retrospective nationwide Korean cohort. BMC Women’s Health 2025, 25, 247. [Google Scholar] [CrossRef]
- National Cancer Database (NCDB). Quality Measure Improvements. Updated 17 July 2025. Available online: https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/quality-of-care-measures/ (accessed on 30 August 2025).
- Tortorello, G.N.; Shafique, N.; Keele, L.; Susman, C.G.; Dheer, A.; Fayanju, O.M.; Tchou, J.; Miura, J.T.; Karakousis, G.C. Longitudinal increases in time to surgery for patients with breast cancer: A national cohort study. Ann. Surg. Oncol. 2024, 31, 6804–6811. [Google Scholar] [CrossRef]
- Balic, M.; Thomssen, C.; Gnant, M.; Harbeck, N. St. Gallen/Vienna 2023: Optimization of treatment for patients with primary breast cancer—A brief summary of the consensus discussion. Breast Care 2023, 18, 213–222. [Google Scholar] [CrossRef]
- Roth, J.; Yu, B.Z.; Godek, M.; Fung, E.; Barrow, B.; Taub, P.J.; Henderson, P.W. Patient race independently predicts timeliness of breast cancer reconstructive care. J. Clin. Med. 2025, 14, 6532. [Google Scholar] [CrossRef]
- Lopez-Fernandez, O.; Aguilar Castillo, C.P.; Horrillo, B.; Sánchez de Molina Ramperez, M.L.; Guadalajara, H. The Implementation of Shared Decision-Making Using Patient Decision Aid Tools to Select Breast Cancer Treatment Options: A Systematic Review in the Time of Minimum Quality Standards. Healthcare 2025, 13, 748. [Google Scholar] [CrossRef] [PubMed]
- Tope, P.R.; Gonçalves, B.P.; El-Zein, M.; Franco, E.L. The health-related impact of disruptions in cancer care and the waiting time paradox. Am. J. Epidemiol. 2025, 194, 2928–2935. [Google Scholar] [CrossRef] [PubMed]
- Johnson, P. Updating the NHS cancer waiting time standards. BMJ Oncol. 2024, 3, e000337. [Google Scholar] [CrossRef]
- National Health Service (NHS). National Cancer Waiting Times Monitoring Dataset Guidance. Version 12.1; July 2025. Available online: https://www.england.nhs.uk/long-read/national-cancer-waiting-times-monitoring-dataset-guidance/ (accessed on 30 August 2025).
- Public Health Scotland. Cancer Waiting Times: 1 January to 31 March 2025. Available online: https://publichealthscotland.scot/publications/cancer-waiting-times/cancer-waiting-times-1-january-to-31-march-2025/ (accessed on 30 August 2025).
- Kuhn, E.; Gambini, D.; Despini, L.; Asnaghi, D.; Runza, L.; Ferrero, S. Updates on lymphovascular invasion in breast cancer. Biomedicines 2023, 11, 968. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Y.M.; Tong, F.; Shen, J. Lympho-vascular invasion impacts the prognosis in breast-conserving surgery: A systematic review and meta-analysis. BMC Cancer 2022, 22, 102. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, H.; Zhao, H.; He, X.; Wang, Y.; Wang, H. Prognostic significance and value of further classification of lymphovascular invasion in invasive breast cancer: A retrospective observational study. Breast Cancer Res. Treat. 2024, 206, 397–410. [Google Scholar] [CrossRef]
- Tope, P.; Farah, E.; Ali, R.; El-Zein, M.; Miller, W.H.; Franco, E.L. The impact of lag time to cancer diagnosis and treatment on clinical outcomes prior to the COVID-19 pandemic: A scoping review of systematic reviews and meta-analyses. eLife 2023, 12, e81354. [Google Scholar] [CrossRef]
- Lee, S.J.; Go, J.; Ahn, B.S.; Ahn, J.H.; Kim, J.Y.; Park, H.S.; Kim, S.I.; Park, B.-W.; Park, S. Lymphovascular invasion is an independent prognostic factor in breast cancer irrespective of axillary node metastasis and molecular subtypes. Front. Oncol. 2023, 13, 1269971. [Google Scholar] [CrossRef]

| Variable | Category | n (%) |
|---|---|---|
| Age category | <40 years | 32 (19.2) |
| ≥40 years | 135 (80.8) | |
| Family history of breast cancer | No | 131 (78.4) |
| Yes | 36 (21.6) | |
| MMG performed | No | 33 (19.8) |
| Yes | 134 (80.2) | |
| Breast MRI performed | No | 94 (56.3) |
| Yes | 73 (43.7) | |
| Histology (grouped) | NST and unfavorable subtypes | 137 (82.0) |
| ILC (classic) | 17 (10.2) | |
| Favorable subtypes | 13 (7.8) | |
| Pathologic tumor size (TNM) | ≤20 mm | 44 (26.3) |
| >20 to ≤50 mm | 101 (60.5) | |
| >50 mm | 22 (13.2) | |
| Histologic grade | Grade I | 10 (6.0) |
| Grade II | 74 (44.3) | |
| Grade III | 83 (49.7) | |
| DCIS component | No (absent) | 53 (31.7) |
| Yes (present) | 114 (68.3) | |
| Multifocality/multicentricity | No | 132 (79.0) |
| Yes | 35 (21.0) | |
| Lymphovascular invasion | Absent | 73 (43.7) |
| Present | 94 (56.3) | |
| Perineural invasion | Absent | 105 (62.9) |
| Present | 62 (37.1) | |
| N category (TNM) | N0 | 69 (41.3) |
| N1 (1–3 nodes) | 57 (34.1) | |
| N2 (4–9 nodes) | 27 (16.2) | |
| N3 (≥10 nodes) | 14 (8.4) | |
| Pathologic stage (anatomic) | Stage I | 41 (24.6) |
| Stage II | 106 (63.4) | |
| Stage III | 20 (12.0) | |
| Intrinsic subtype (IHC-based) | Luminal A | 104 (62.3) |
| Luminal B | 35 (21.0) | |
| HER2-enriched (non-luminal) | 18 (10.8) | |
| TNBC | 10 (6.0) | |
| HER2 status | HER2 negative | 138 (82.6) |
| HER2 positive | 29 (17.4) | |
| Surgery type | BCS + SLNB | 14 (8.4) |
| BCS + ALND | 11 (6.6) | |
| Mastectomy + SLNB | 55 (32.9) | |
| Mastectomy + ALND | 87 (52.1) | |
| Adjuvant chemotherapy | No | 16 (9.6) |
| Yes | 151 (90.4) | |
| Adjuvant radiotherapy | No | 56 (33.5) |
| Yes | 111 (66.5) | |
| Adjuvant endocrine therapy | No | 39 (23.4) |
| Yes | 128 (76.6) | |
| Recurrence during follow-up | No | 149 (89.2) |
| Yes | 18 (10.8) | |
| Death during follow-up | No | 144 (86.2) |
| Yes | 23 (13.8) | |
| Timing thresholds (A-TTS) | A-TTS ≤ 24 days: Yes | 41 (24.6) |
| A-TTS ≤ 24 days: No | 126 (75.4) | |
| A-TTS ≤ 30 days: Yes | 67 (40.1) | |
| A-TTS ≤ 30 days: No | 100 (59.9) | |
| Timing thresholds (B-TTS) | B-TTS ≤ 24 days: Yes | 123 (73.7) |
| B-TTS ≤ 24 days: No | 44 (26.3) | |
| B-TTS ≤ 30 days: Yes | 134 (80.2) | |
| B-TTS ≤ 30 days: No | 33 (19.8) |
| Variable | No Recurrence (n = 149) | Recurrence (n = 18) | p-Value |
|---|---|---|---|
| Age (years) | 50.11 ± 11.02 | 47.06 ± 8.93 | 0.196 |
| Tumor size at biopsy (mm) | 20.0 (14.0–25.0) | 30.0 (20.0–40.0) | 0.001 |
| Tumor size at pathology (mm) | 25.0 (18.0–37.0) | 45.0 (29.3–52.8) | 0.001 |
| Ki-67 (%) | 20.0 (10.0–30.0) | 15.0 (10.0–32.5) | 0.758 |
| A-TTS (days) | 35.0 (26.0–52.0) | 24.0 (17.5–41.3) | 0.017 |
| B-TTS (days) | 16.0 (9.5–26.5) | 11.0 (4.8–24.0) | 0.168 |
| A–B (days) | 16.0 (10.0–26.5) | 11.0 (9.0–14.0) | 0.017 |
| Surgery-to-adjuvant (days) | 42.0 (33.0–55.0) | 53.0 (33.5–62.0) | 0.274 |
| Variable | Odds Ratio | 95% CI | p-Value |
|---|---|---|---|
| A-TTS ≤ 24 days (yes vs. no) | 3.16 | 1.13–8.82 | 0.028 |
| Node-positive disease (N1–3 vs. N0) | 1.98 | 0.39–10.09 | 0.412 |
| LVI (yes vs. no) | 2.52 | 0.50–12.79 | 0.265 |
| Panel A. Cox Proportional Hazards (Baseline Model) | ||
| Variable | HR (95% CI) | p-Value |
| A-TTS ≤ 24 days (yes vs. no) | 1.73 (0.75–4.01) | 0.201 |
| Node-positive disease (N1–3 vs. N0) | 1.23 (0.36–4.21) | 0.737 |
| LVI (yes vs. no) | 3.93 (1.03–14.99) | 0.045 |
| Panel B. Extended Cox Model with Log–Time Interaction ln(time/24); the Main Effect Corresponds to the HR at 24 Months | ||
| Effect | HR (95% CI) | |
| A-TTS ≤ 24 days (HR at 24 months) | 22.83 (6.44–80.98) | |
| A-TTS ≤ 24 × log(time/24) | 0.06 (0.02–0.21) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tandoğan, C.; Berkeşoğlu, M.; Tuncel, F.; Derici Yıldırım, D.; Özcan, C.; Benli, S.; Güler, E.; Yılmaz, E.B. Measuring Surgical Waiting Times in Breast Cancer: Admission to Surgery Versus Biopsy Result to Surgery. Healthcare 2025, 13, 3010. https://doi.org/10.3390/healthcare13233010
Tandoğan C, Berkeşoğlu M, Tuncel F, Derici Yıldırım D, Özcan C, Benli S, Güler E, Yılmaz EB. Measuring Surgical Waiting Times in Breast Cancer: Admission to Surgery Versus Biopsy Result to Surgery. Healthcare. 2025; 13(23):3010. https://doi.org/10.3390/healthcare13233010
Chicago/Turabian StyleTandoğan, Cem, Mustafa Berkeşoğlu, Ferah Tuncel, Didem Derici Yıldırım, Cumhur Özcan, Sami Benli, Erkan Güler, and Eda Bengi Yılmaz. 2025. "Measuring Surgical Waiting Times in Breast Cancer: Admission to Surgery Versus Biopsy Result to Surgery" Healthcare 13, no. 23: 3010. https://doi.org/10.3390/healthcare13233010
APA StyleTandoğan, C., Berkeşoğlu, M., Tuncel, F., Derici Yıldırım, D., Özcan, C., Benli, S., Güler, E., & Yılmaz, E. B. (2025). Measuring Surgical Waiting Times in Breast Cancer: Admission to Surgery Versus Biopsy Result to Surgery. Healthcare, 13(23), 3010. https://doi.org/10.3390/healthcare13233010







