Influence of Rehabilitation Aid Use on Obstacle Height During Gait in Patients with Foot Drop: A Case Series Study
Highlights
- In patients with foot drop, assisted gait (AG) improved dorsiflexion and frontal plane stabilization, as well as knee flexion and frontal plane alignment, thereby contributing to toe clearance and initial contact stabilization.
- The use of rehabilitation aids improved mechanisms for initial contact and propulsion transition during obstacle crossing, thereby contributing to shock absorption and enhanced dynamic stability.
- As the required clearance angle increases with obstacle height, AG is considered advantageous for safe walking in everyday environments with uneven surfaces, curbs, and thresholds.
- Rehabilitation aids provide support for patients with foot drop, with reduced toe tripping and improved gait safety.
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
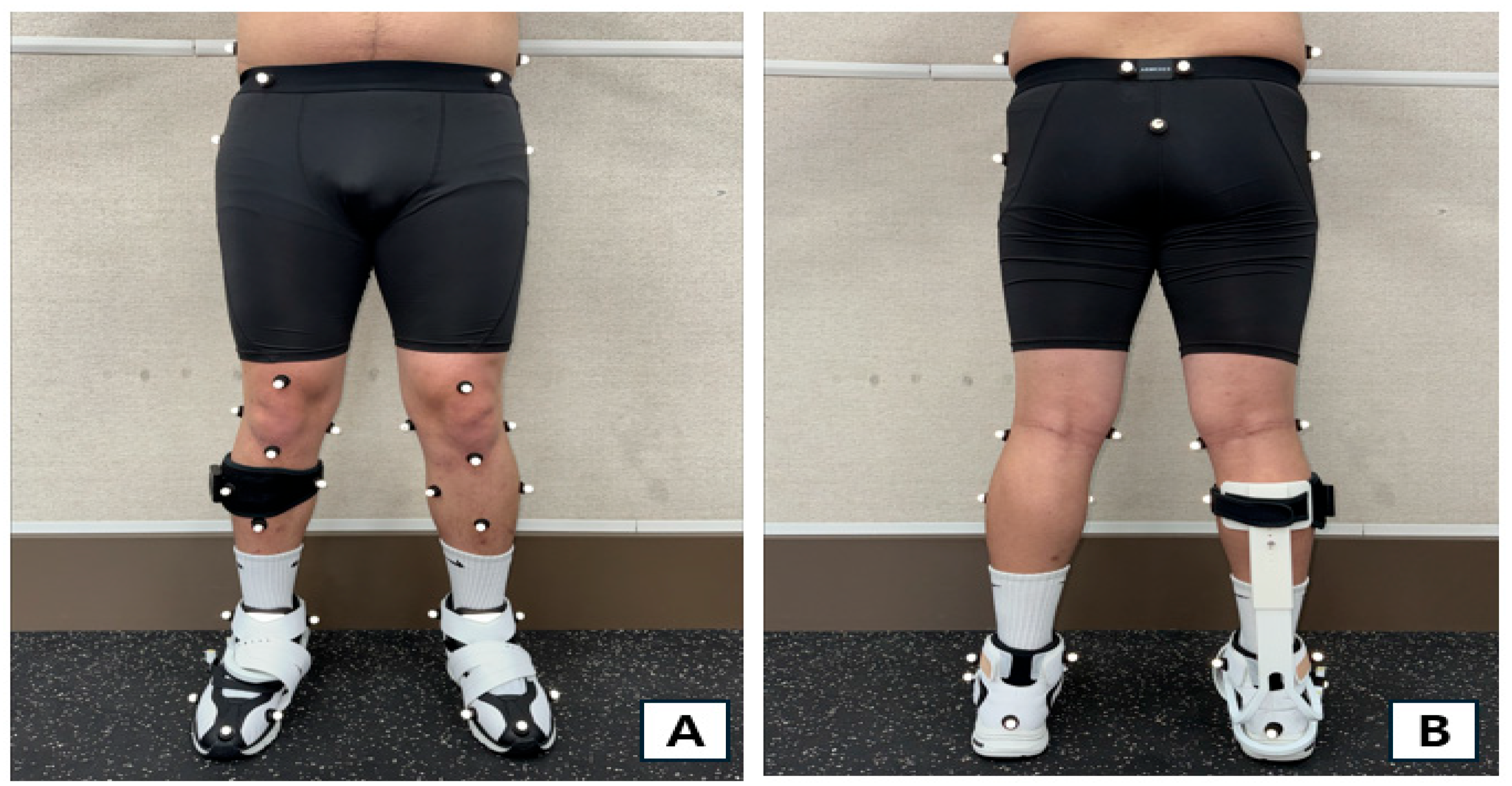
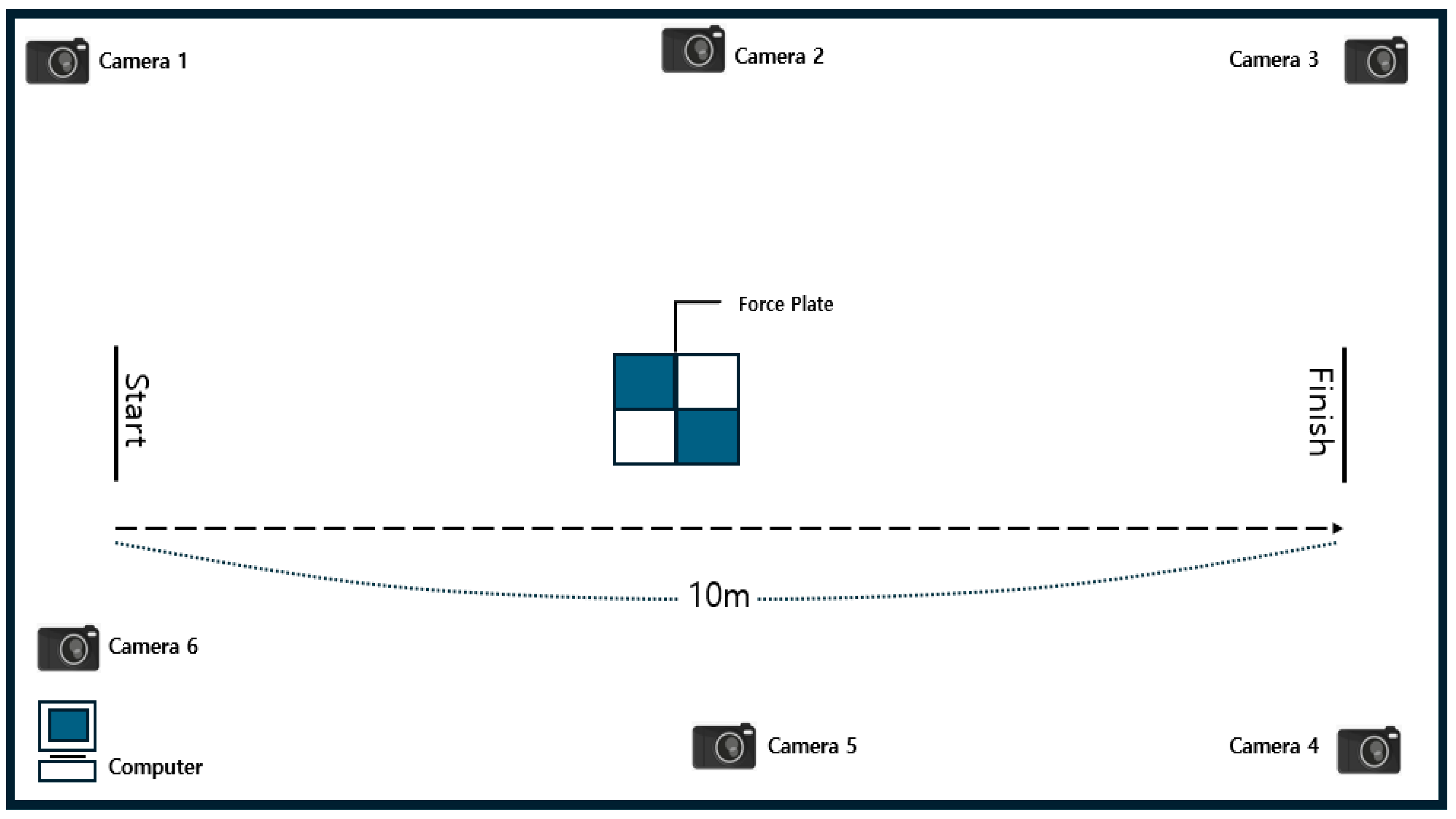
2.3. Measurement Procedure
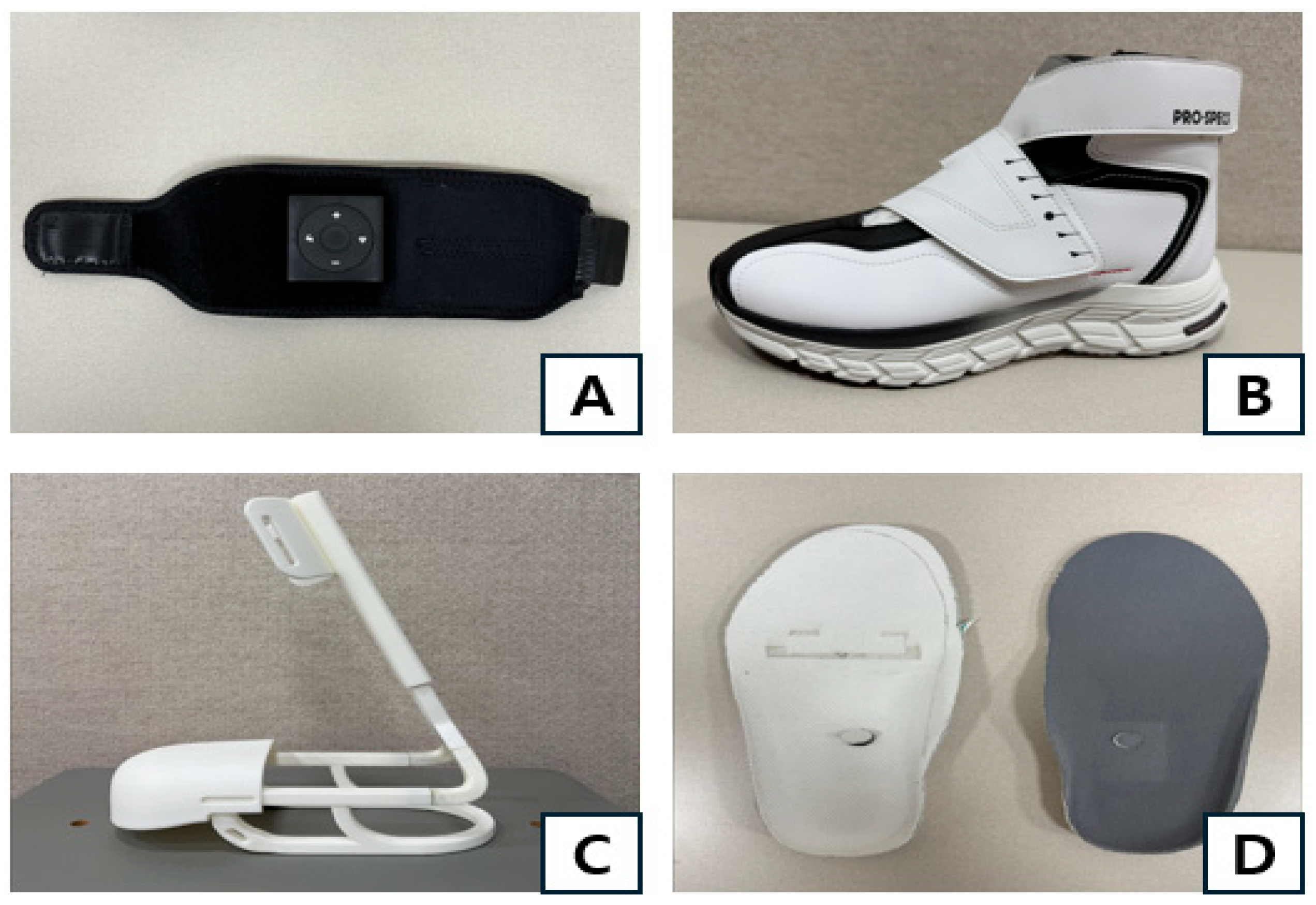
2.4. Measurement Devices
2.5. Data Collection and Processing Methods
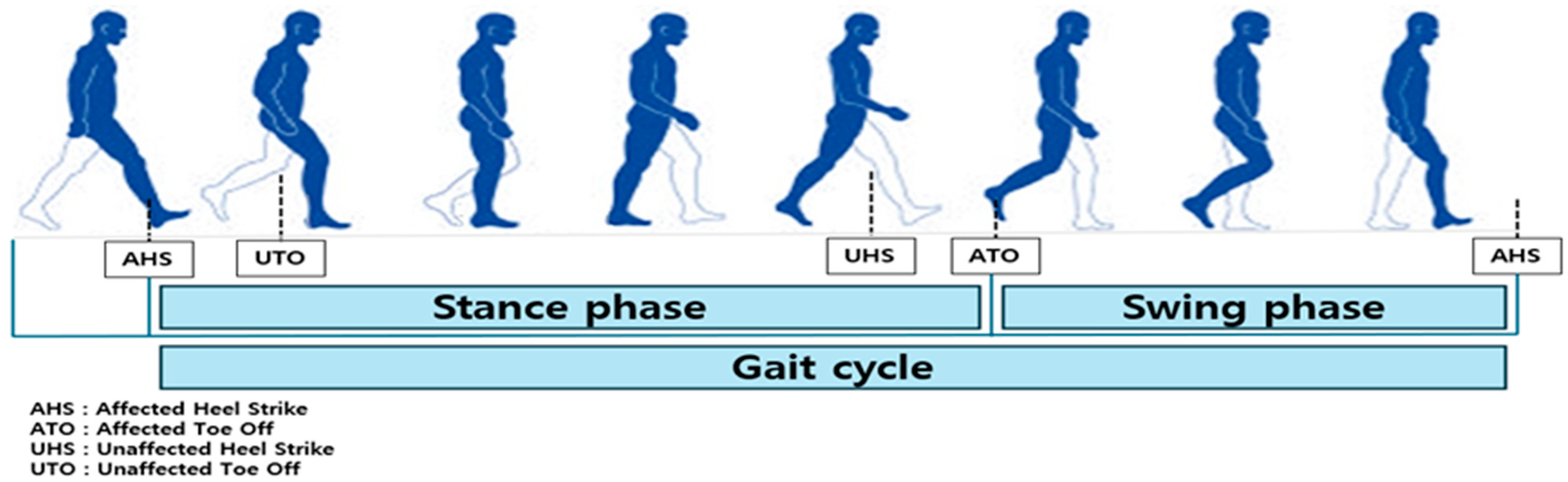
2.6. Definitions of Gait Analysis Intervals and Events
2.7. Definition of Joint Angles of the Lower Limb
2.8. Statistical Processing
3. Results
3.1. Spatiotemporal Gait Variables
3.2. GRF and CoP
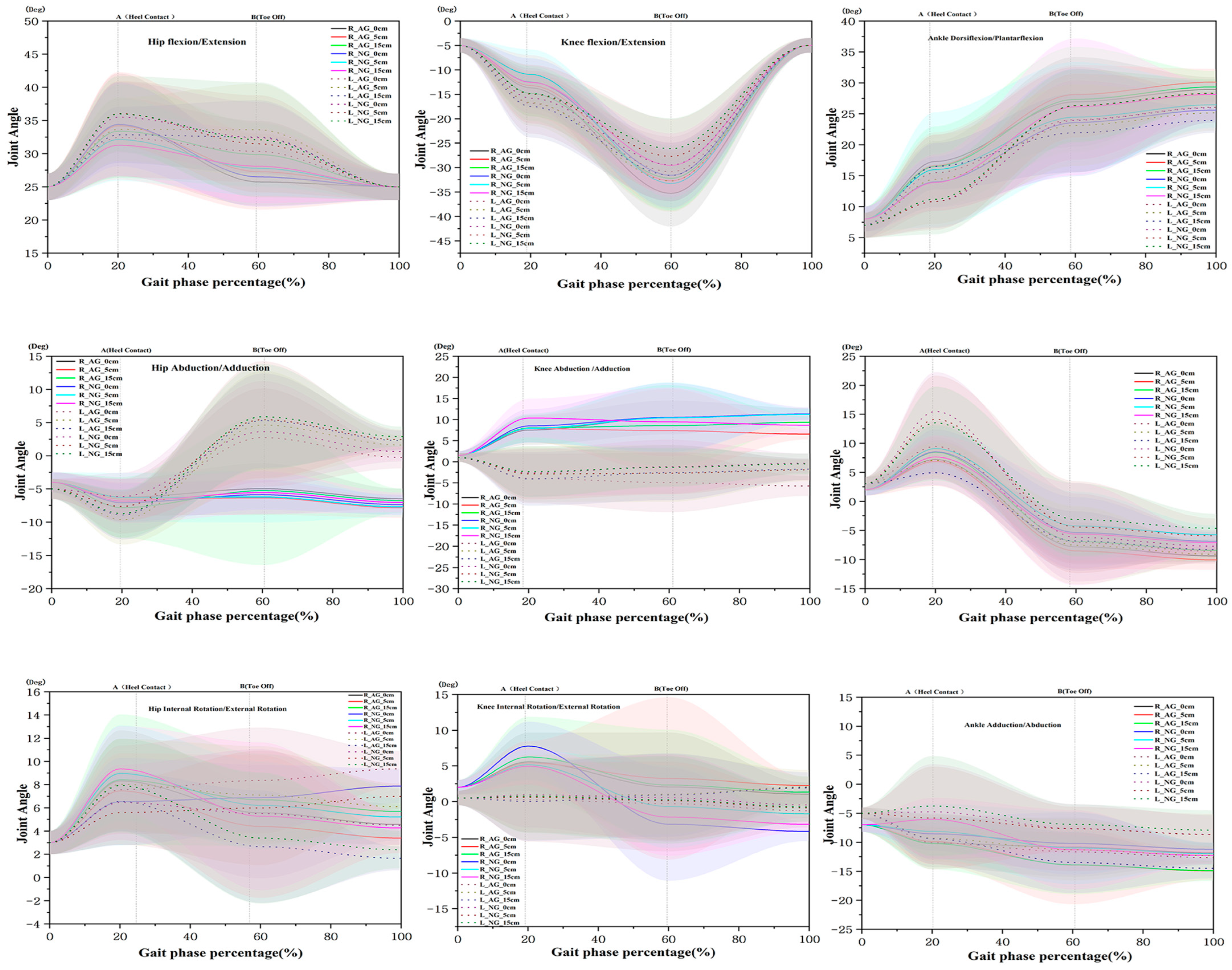
3.3. Joint Angles of the Lower Limb at HC
3.4. Joint Angles of the Lower Limb at TO
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AFO | ankle–foot orthosis |
| COP | center of pressure |
| HS | heel strike |
| TO | toe-off |
References
- World Health Organization. Global Health Estimates 2021: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2021; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Shahid, J.; Kashif, A.; Shahid, M.K.A. comprehensive review of physical therapy interventions for stroke rehabilitation: Impairment-based approaches and functional goals. Brain Sci. 2023, 13, 717. [Google Scholar] [CrossRef]
- Krishnan, C.; Augenstein, T.E.; Claflin, E.S.; Hemsley, C.R.; Washabaugh, E.P.; Ranganathan, R. Rest the brain to learn new gait patterns after stroke. J. Neuroeng. Rehabil. 2024, 21, 192. [Google Scholar] [CrossRef]
- Kennedy, C.; Bernhardt, J.; Churilov, L.; Collier, J.M.; Ellery, F.; Rethnam, V.; Carvalho, L.B.; Donnan, G.A.; Hayward, K.S. Factors associated with time to independent walking recovery post-stroke. J. Neurol. Neurosurg. Psychiatry 2021, 92, 702–708. [Google Scholar] [CrossRef]
- Smith, M.C.; Barber, A.P.; Scrivener, B.J.; Stinear, C.M. The TWIST tool predicts when patients will recover independent walking after stroke: An observational study. Neurorehabilit. Neural Repair 2022, 36, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Montané, E.; Lopez, L.; Scandella, M.; Gasq, D.; Cormier, C. Kinematic biomarkers of limb shortening and compensations in hemiparetic gait: A systematic review. Sensors 2025, 25, 4598. [Google Scholar] [CrossRef]
- Mendes-Andrade, I.; Reis E Silva, M.; Jacinto, J. Initial contact with forefoot or rearfoot in spastic patients after stroke—Three-dimensional gait analysis. Neurol. Int. 2025, 17, 10. [Google Scholar] [CrossRef]
- Johnston, T.E.; Keller, S.; Denzer-Weiler, C.; Brown, L.A. clinical practice guideline for the use of ankle-foot orthoses and functional electrical stimulation post-stroke. J. Neurol. Phys. Ther. 2021, 45, 112–196. [Google Scholar] [CrossRef]
- Bek, L.M.; E Hellemons, M.; Berentschot, J.C.; Visser, M.M.; Huijts, S.M.; van Bommel, J.; E van Genderen, M.; Aerts, J.G.; Ribbers, G.M.; Berg-Emons, R.J.v.D.; et al. Botulinum toxin type A improves gait spatiotemporal parameters in chronic stroke patients: A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2023, 66, 101737. [Google Scholar] [CrossRef]
- Daryabor, A.; Kobayashi, T.; Yamamoto, S.; Lyons, S.M.; Orendurff, M.; Akbarzadeh Baghban, A. Effect of ankle-foot orthoses on functional outcome measurements in individuals with stroke: A systematic review and meta-analysis. Disabil. Rehabil. 2022, 44, 6566–6581. [Google Scholar] [CrossRef]
- Choo, Y.J.; Chang, M.C. Effectiveness of an ankle–foot orthosis on walking in patients with stroke: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 15879. [Google Scholar] [CrossRef]
- Nascimento, L.R.; da Silva, L.A.; Barcellos, J.V.M.A.; Teixeira-Salmela, L.F. Ankle-foot orthoses and continuous functional electrical stimulation improve walking speed after stroke: A systematic review and meta-analyses. Physiotherapy 2020, 109, 43–53. [Google Scholar] [CrossRef]
- da Cunha, M.J.; Rech, K.D.; Salazar, A.P.; Pagnussat, A.S. Functional electrical stimulation of the peroneal nerve improves post-stroke gait speed when combined with physiotherapy. A systematic review and meta-analysis. Ann. Phys. Rehabil. Med. 2021, 64, 101388. [Google Scholar] [CrossRef] [PubMed]
- Koh, S.; Choi, W.; Lee, S. The effects of stair climbing training with functional electrical stimulation on muscle strength, balance, and gait in patients with chronic stroke. Phys. Ther. Rehabil. Sci. 2021, 10, 32–39. [Google Scholar] [CrossRef]
- Kim, E.; Min, K.; Song, C. The effects of balance training with functional electrical stimulation on balance and gait in patients with chronic stroke. Phys. Ther. Rehabil. Sci. 2021, 10, 55–63. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Liu, W.; Liu, Y.; Liang, W.; Zhang, L.; Zhou, F.; Zhang, Z.; Yuan, X. The effect of electromyographic-feedback functional electrical stimulation on plantar pressure in stroke patients with foot drop. Front. Neurosci. 2024, 18, 1377702. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.; Seary, C.; Beare, B.; Stevenson, V.L. Functional electrical stimulation for walking in adults with cerebral palsy: A service evaluation. J. Neuroeng. Rehabil. 2025, 22, 41. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Functional Electrical Stimulation for Drop Foot of Central Neurological Origin (Interventional Procedures Guidance IPG278); National Institute for Health and Care Excellence: London, UK, 2009; ISBN 1-84629-847-4. [Google Scholar]
- Thies, S.B.; Price, C.; Kenney, L.P.J.; Baker, R. Effects of shoe sole geometry on toe clearance and walking stability in older adults. Gait Posture 2015, 42, 105–109. [Google Scholar] [CrossRef]
- Hemmati, F.; Karimi, M.T.; Hosseini, S.I.; Mardani, M.A.; Fadayevatan, R. The effect of toe-only rocker sole shoes on gait variability of the elderly. Med. Biol. Eng. Comput. 2022, 60, 2493–2498. [Google Scholar] [CrossRef]
- Royal College of Physicians/Stroke Association. National Clinical Guideline for Stroke. 2023 Edition. Available online: www.strokeguideline.org (accessed on 1 May 2024).
- Shin, J.M.; Song, W.J.; Kim, K.C. Analysis of factors affecting obstacle crossing in stroke patients capable of independent walking. Kor. J. Neuromuscul. Rehabil. 2019, 9, 1–11. [Google Scholar]
- Lay, A.N.; Hass, C.J.; Richard Nichols, T.R.; Gregor, R.J. The effects of sloped surfaces on locomotion: An electromyographic analysis. J. Biomech. 2007, 40, 1276–1285. [Google Scholar] [CrossRef]
- Michel, J.; Benninger, D.; Dietz, V.; van Hedel, H.J.A. Obstacle stepping in patients with Parkinson’s disease. Complexity does influence performance. J. Neurol. 2009, 256, 457–463. [Google Scholar] [CrossRef]
- Keller Chandra, S.K.; Bockisch, C.J.; Dietz, V.; Hegemann, S.C.A.; Straumann, D.; van Hedel, H.J.A. Gaze strategies for avoiding obstacles: Differences between young and elderly subjects. Gait Posture 2011, 34, 340–346. [Google Scholar] [CrossRef]
- Mansfield, A.; Inness, E.L.; Komar, J.; Biasin, L.; Brunton, K.; Lakhani, B.; McIlroy, W.E. Training rapid stepping responses in an individual with stroke. Phys. Ther. 2011, 91, 958–969. [Google Scholar] [CrossRef]
- Chen, N.; Xiao, X.; Hu, H.; Chen, Y.; Song, R.; Li, L. Identify the alteration of balance control and risk of falling in stroke survivors during obstacle crossing based on kinematic analysis. Front. Neurol. 2019, 10, 813. [Google Scholar] [CrossRef]
- Jia, S.; Bello, U.M.; Manduchi, R.; Cheong, A.M.Y. Effect of visual searching and obstacle crossing on gait performance in older adults. Sci. Rep. 2025, 15, 22334. [Google Scholar] [CrossRef]
- Choi, Y.J.; Song, Y.G. Characteristics of obstacle avoidance strategies on the obstacle crossing in patients with chronic stroke. Korean J. Sport Psychol. 2022, 33, 1–10. [Google Scholar] [CrossRef]
- Fonseca, M.; Gasparutto, X.; Grouvel, G.; Bonnefoy-Mazure, A.; Dumas, R.; Armand, S. Evaluation of lower limb and pelvic marker placement precision among different evaluators and its impact on gait kinematics computed with the Conventional Gait Model. Gait Posture 2023, 104, 22–30. [Google Scholar] [CrossRef]
- Mügge, F.; Kleinholdermann, U.; Heun, A.; Ollenschläger, M.; Hannink, J.; Pedrosa, D.J. Subthalamic 85 Hz deep brain stimulation improves walking pace and stride length in Parkinson’s disease patients. Neurol. Res. Pract. 2023, 5, 33. [Google Scholar] [CrossRef]
- Wan, X.; Zhu, Z.; Xu, F. Association between gait characteristics during obstacle crossing and fall risk in stroke: A prospective cohort study. Gait Posture 2025, 120, 9–16. [Google Scholar] [CrossRef]
- Said, C.M.; Galea, M.; Lythgo, N. Obstacle crossing performance does not differ between the first and subsequent attempts in people with stroke. Gait Posture 2009, 30, 455–458. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Xu, F.; Li, Q.; Wan, X. Gait spatio-temporal characteristics during obstacle crossing as predictors of fall risk in stroke patients. BMC Neurol. 2025, 25, 111. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Yokogawa, M.; Nakagawa, T.; Ibune, M.; Ishiwatari, T.; Kawakita, S. Key function for obstacle crossing in hemiplegic persons with varied degrees of spasticity. J. Phys. Ther. Sci. 2017, 29, 1381–1386. [Google Scholar] [CrossRef]
- Begg, R.K.; Sparrow, W.A. Gait characteristics of young and older individuals negotiating a raised surface: Implications for the prevention of falls. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M147–M154. [Google Scholar] [CrossRef] [PubMed]
- Roelker, S.A.; Bowden, M.G.; Kautz, S.A.; Neptune, R.R. Paretic propulsion as a measure of walking performance and functional motor recovery post-stroke: A review. Gait Posture 2019, 68, 6–14. [Google Scholar] [CrossRef]
- Dean, J.C.; Bowden, M.G.; Kelly, A.L.; Kautz, S.A. Altered post-stroke propulsion is related to paretic swing phase kinematics. Clin. Biomech. 2020, 72, 24–30. [Google Scholar] [CrossRef]
- Yiou, E.; Artico, R.; Teyssedre, C.A.; Labaune, O.; Fourcade, P. Anticipatory postural control of stability during gait initiation over obstacles of different height and distance made under reaction-time and self-initiated instructions. Front. Hum. Neurosci. 2016, 10, 449. [Google Scholar] [CrossRef]
- Waterval, N.F.J.; Nollet, F.; Brehm, M.-A. Effect of stiffness-optimized ankle-foot orthoses on joint work in adults with neuromuscular diseases is related to severity of push-off deficits. Gait Posture 2024, 111, 162–168. [Google Scholar] [CrossRef]
- Kobayashi, T.; Orendurff, M.S.; Singer, M.L.; Gao, F.; Hunt, G.; Foreman, K.B. Effect of plantarflexion resistance of an ankle-foot orthosis on ankle and knee joint power during gait in individuals post-stroke. J. Biomech. 2018, 75, 176–180. [Google Scholar] [CrossRef]
- VA/DoD Clinical Practice Guideline. Management of Stroke Rehabilitation. 2024. Available online: https://www.healthquality.va.gov/guidelines/rehab/stroke/ (accessed on 1 May 2024).
- Bajelan, S.; Sparrow, W.A.; Begg, R. The ankle dorsiflexion kinetics demand to increase swing phase foot-ground clearance: Implications for assistive device design and energy demands. J. Neuroeng. Rehabil. 2024, 21, 105. [Google Scholar] [CrossRef]
- Neptune, R.R.; Kautz, S.A.; Zajac, F.E. Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking. J. Biomech. 2001, 34, 1387–1398. [Google Scholar] [CrossRef]
- Tyson, S.F.; Rogerson, L. Assistive walking devices in nonambulant patients undergoing rehabilitation after stroke: The effects on functional mobility, walking impairments, and patients’ opinion. Arch. Phys. Med. Rehabil. 2009, 90, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Skigen, J.T.; Koller, C.A.; Nigro, L.; Reisman, D.S.; McKee, Z.; Pinhey, S.R.; Henderson, A.; Wilken, J.M.; Arch, E.S. Customized passive-dynamic ankle–foot orthoses can improve walking economy and speed for many individuals post-stroke. J. Neuroeng. Rehabil. 2024, 21, 126. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.M.; Hong, J.; Yoo, J.; Rha, D.-W. Wearable Robots for Rehabilitation and Assistance of Gait: A Narrative Review. Ann. Rehabil. Med. 2025, 49, 187–195. [Google Scholar] [CrossRef]
- Orthotics Plus. Functional Electrical Stimulation (FES)—Melbourne. Available online: https://Orthoticsplus.com.au (accessed on 12 November 2025).
| No. of People | Age (Year) | Height (m) | Body Weight (kg) | Skeletal Muscle Mass (kg) | Body Fat Mass (kg) | Affected Side |
|---|---|---|---|---|---|---|
| n = 10 | 42.7 ± 2.9 | 1.61 ± 0.98 | 58.1 ± 15.5 | 22.1 ± 7.7 | 17.8 ± 5.5 | Right |
| Parameters | NG | AG | Effect | F (P) | d | Post hoc | Parameters | NG | AG | Effect | F (P) | d | Post hoc | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M(SD) | M(SD) | M(SD) | M(SD) | |||||||||||||
| Step length (cm) | 0 cm a | 47.50 (7.40) | 48.80 (7.70) | H | 0.653 (0.525) | 0.310 | ns | Medio-lateral GRF (N/kg) | R | 0 cm a | 1.22 (0.34) | 1.41 (0.37) † | H | 0.247 (0.782) | 0.519 | c > a,b |
| 5 cm b | 48.10 (8.50) | 48.40 (6.60) | G | 1.264 (0.266) | 0.081 | 5 cm b | 1.44 (0.51) | 1.33 (0.35) | G | 1.051 (0.310) | 0.262 | |||||
| 15 cm c | 53.00 (6.30) | 48.50 (8.80) † | H×G | 3.406 (0.0410) * | 1.268 | 15 cm c | 1.44 (0.46) | 1.18 (0.32) † | H×G | 8.438 (0.001) *** | 0.675 | |||||
| Step time (s) | 0 cm a | 0.63 (0.10) | 0.58 (0.15) | H | 0.494 (0.613) | 0.408 | ns | L | 0 cm a | 1.45 (0.61) | 1.49 (0.61) | H | 0.280 (0.757) | 0.060 | ns | |
| 5 cm b | 0.60 (0.09) | 0.53 (0.14) | G | 13.094 (0.001) ** | 0.557 | 5 cm b | 1.45 (0.91) | 1.51 (0.65) | G | 0.029 (0.865) | 0.082 | |||||
| 15 cm c | 0.64 (0.16) | 0.56 (0.15) † | H×G | 0.478 (0.623) | 0.517 | 15 cm c | 1.68 (0.60) | 1.53 (0.50) | H×G | 0.830 (0.442) | 0.277 | |||||
| Cadence (min/step) | 0 cm a | 97.46 (11.71) | 75.80 (14.04) † | H | 4.115 (0.022) * | 1.682 | c > b | Antero-posterior GRF (N/kg) | R | 0 cm a | 11.05 (1.10) | 11.28 (1.33) | H | 1.167 (0.320) | 0.186 | ns |
| 5 cm b | 76.23 (13.38) | 82.97 (16.86) | G | 6.174 (0.016) * | 0.446 | 5 cm b | 11.80 (1.47) | 11.49 (0.88) | G | 0.144 (0.706) | 0.262 | |||||
| 15 cm c | 91.40 (13.68) | 86.94 (13.52) | H×G | 10.292 (0.000) *** | 0.328 | 15 cm c | 11.93 (3.36) | 11.63 (0.77) | H×G | 0.306 (0.737) | 0.145 | |||||
| Left Swing Phase (s) | 0 cm a | 0.58 (0.18) | 0.63 (0.21) | H | 0.224 (0.800) | 0.241 | ns | L | 0 cm a | 11.44 (1.51) | 11.31 (1.42) | H | 0.278 (0.759) | 0.092 | ns | |
| 5 cm b | 0.65 (0.38) | 0.58 (0.15) | G | 0.901 (0.347) | 0.289 | 5 cm b | 11.51 (1.76) | 11.44 (1.26) | G | 0.002 (0.963) | 0.045 | |||||
| 15 cm c | 0.69 (0.22) | 0.60 (0.13) | H×G | 1.026 (0.366) | 0.508 | 15 cm c | 11.63 (2.86) | 11.81 (1.63) | H×G | 0.192 (0.826) | 0.078 | |||||
| Right Swing Phase (s) | 0 cm a | 0.68 (0.18) | 0.81 (0.41) | H | 2.789 (0.071) | 0.425 | a > b | Vertical GRF (N/kg) | R | 0 cm a | 1.72 (0.43) | 1.81 (0.53) | H | 2.884 (0.065) | 0.194 | c > a |
| 5 cm b | 0.62 (0.11) | 0.60 (0.18) | G | 0.008 (0.927) | 0.096 | 5 cm b | 2.19 (0.65) | 1.92 (0.56) † | G | 4.564 (0.038) * | 0.450 | |||||
| 15 cm c | 0.77 (0.32) | 0.64 (0.15) | H×G | 2.231 (0.118) | 0.530 | 15 cm c | 2.37 (0.91) | 2.05 (0.62) | H×G | 3.092 (0.054) | 0.410 | |||||
| Left Stance Phase (s) | 0 cm a | 0.97 (0.50) | 1.17 (0.77) | H | 0.091 (0.914) | 0.309 | ns | L | 0 cm a | 2.11 (0.86) | 2.15 (0.83) | H | 0.705 (0.499) | 0.041 | c > a,b | |
| 5 cm b | 1.12 (0.96) | 1.18 (0.96) | G | 0.226 (0.637) | 0.060 | 5 cm b | 2.55 (1.31) | 2.09 (0.91) † | G | 2.172 (0.147) | 0.413 | |||||
| 15 cm c | 1.26 (1.15) | 1.09 (0.60) | H×G | 1.899 (0.160) | 0.191 | 15 cm c | 2.56 (1.37) | 2.51 (1.11) | H×G | 2.122 (0.130) | 0.035 | |||||
| Right Stance Phase (s) | 0 cm a | 1.06 (0.54) | 0.98 (0.36) | H | 0.681 (0.511) | 0.172 | CoP_X (mm) | 68.43 (24.14) | 72.19 (22.36) | 0.090 (0.736) | 0.162 | |||||
| 5 cm b | 1.33 (1.44) | 1.30 (1.17) | G | 0.016 (0.899) | 0.027 | CoP_Y (mm) | 47.50 (21.12) | 53.65 (17.00) | 0.736 (0.506) | 0.323 | ||||||
| 15 cm c | 1.12 (0.95) | 1.30 (1.24) | H×G | 0.220 (0.803) | 0.163 | |||||||||||
| Variables (Heel Contact) | Hip | Knee | Ankle | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NG | AG | Effect | F (P) | d | Post hoc | NG | AG | Effect | F (P) | d | Post hoc | NG | AG | Effect | F (P) | d | Post hoc | |||
| M(SD) | M(SD) | M(SD) | M(SD) | M(SD) | M(SD) | |||||||||||||||
| Sagittal (°) | R | 0 cm a | 34.41 (6.32) | 34.36 (7.52) | H | 0.741 (0.482) | 0.008 | ns | 12.49 (4.85) | 13.67 (4.07) | H | 1.439 (0.247) | 0.265 | ns | 10.82 (4.31) | 15.48 (5.99) † | H | 0.581 (0.563) | 0.905 | ns |
| 5 cm b | 32.15 (6.23) | 34.12 (8.10) | G | 1.223 (0.274) | 0.287 | 10.93 (5.10) | 13.21 (6.24) | G | 4.901 (0.031) * | 0.401 | 10.83 (4.60) | 14.42 (6.98) | G | 14.505 (0.000) *** | 0.619 | |||||
| 15 cm c | 31.27 (4.62) | 33.39 (7.05) | H×G | 0.341 (0.713) | 0.364 | 12.55 (2.43) | 14.81 (5.64) | H×G | 0.266 (0.768) | 0.556 | 11.20 (5.66) | 16.52 (8.57) † | H×G | 0.174 (0.841) | 0.747 | |||||
| L | 0 cm a | 35.51 (5.30) | 32.41 (5.95) † | H | 0.216 (0.806) | 0.550 | ns | 15.79 (3.14) | 16.84 (6.22) | H | 0.081 (0.922) | 0.224 | ns | 14.01 (4.11) | 17.30 (3.28) | H | 0.285 (0.753) | 0.891 | ns | |
| 5 cm b | 36.06 (4.87) | 33.65 (4.80) | G | 10.574 (0.002) * | 0.496 | 14.96 (5.98) | 16.69 (4.75) | G | 2.900 (0.095) | 0.322 | 15.98 (5.28) | 16.63 (5.37) | G | 4.15 (0.047) * | 0.088 | |||||
| 15 cm c | 36.00 (5.71) | 32.84 (4.21) | H×G | 0.063 (0.939) | 0.636 | 14.44 (4.66) | 17.49 (6.35) | H×G | 0.189 (0.828) | 0.555 | 13.89 (4.52) | 16.50 (6.47) | H×G | 0.562 (0.573) | 0.475 | |||||
| Frontal (°) | R | 0 cm a | 7.07 (2.91) | 6.25 (2.79) | H | 0.685 (0.509) | 0.288 | ns | 8.47 (3.97) | 7.50 (3.48) | H | 1.309 (0.279) | 0.262 | ns | 15.48 (4.78) | 9.45 (4.32) † | H | 2.556 (0.088) | 1.086 | a > c |
| 5 cm b | 6.28 (3.69) | 6.536 (2.53) | G | 0.126 (0.724) | 0.081 | 7.88 (3.25) | 7.85 (3.18) | G | 2.828 (0.099) | 0.010 | 14.13 (7.54) | 7.07 (2.95) † | G | 61.045 (0.000) *** | 1.345 | |||||
| 15 cm c | 6.88 (3.33) | 7.82 (4.61) | H×G | 1.204 (0.367) | 0.247 | 10.37 (4.55) | 8.01 (3.41) | H×G | 0.949 (0.394) | 0.592 | 13.53 (6.24) | 4.92 (2.15) † | H×G | 0.644 (0.529) | 2.050 | |||||
| L | 0 cm a | 6.16 (3.59) | 7.60 (4.12) | H | 3.525 (0.037) * | 0.374 | c > b,a | 2.62 (1.62) | 3.94 (1.84) | H | 0.040 (0.961) | 0.231 | ns | 8.53 (3.86) | 7.21 (3.29) | H | 0.076 (0.927) | 0.369 | ns | |
| 5 cm b | 7.67 (2.22) | 9.65 (3.73) | G | 2.388 (0.129) | 0.666 | 2.84 (1.08) | 2.96 (1.40) | G | 2.785 (0.101) | 0.019 | 9.09 (3.95) | 6.89 (4.25) | G | 0.961 (0.332) | 0.535 | |||||
| 15 cm c | 8.72 (3.63) | 8.70 (3.68) | H×G | 0.661 (0.521) | 0.008 | 2.34 (1.07) | 4.07 (1.35) | H×G | 0.670 (0.516) | 0.280 | 7.63 (3.76) | 8.43 (4.82) | H×G | 1.640 (0.204) | 0.186 | |||||
| Transverse (°) | R | 0 cm a | 6.35 (3.76) | 8.32 (4.22) | H | 0.669 (0.517) | 0.492 | ns | 7.78 (3.40) | 5.57 (3.07) | H | 1.217 (0.305) | 0.590 | ns | 10.15 (4.15) | 8.54 (2.92) | H | 0.375 (0.689) | 0.454 | ns |
| 5 cm b | 8.96 (4.14) | 7.46 (3.25) | G | 0.223 (0.639) | 0.407 | 5.13 (2.11) | 5.40 (3.07) | G | 0.027 (0.869) | 0.094 | 9.88 (3.91) | 8.12 (4.75) | G | 19.490 (0.000) *** | 0.403 | |||||
| 15 cm c | 9.36 (3.68) | 8.42 (5.62) | H×G | 2.381 (0.103) | 0.202 | 4.95 (2.55) | 6.25 (2.62) | H×G | 2.845 (0.068) | 0.318 | 10.21 (4.45) † | 6.04 (3.68) † | H×G | 2.726 (0.075) | 0.826 | |||||
| L | 0 cm a | 6.46 (3.24) | 7.56 (3.81) | H | 0.078 (0.925) | 0.313 | ns | 0.95 (0.58) | 0.26 (0.85) | H | 0.003 (0.997) | 0.133 | ns | 10.08 (2.67) | 4.74 (2.23) † | H | 0.232 (0.978) | 0.751 | ns | |
| 5 cm b | 5.59 (2.77) | 8.02 (3.92) † | G | 1.645 (0.206) | 0.726 | 0.67 (0.90) | 0.59 (0.82) | G | 0.401 (0.529) | 0.003 | 9.30 (3.93) | 8.81 (4.85) | G | 11.111 (0.002) ** | 0.545 | |||||
| 15 cm c | 7.01 (4.03) | 6.55 (2.56) | H×G | 4.566 (0.015) * | 0.139 | 0.75 (0.12) | 0.01 (0.50) | H×G | 0.101 (0.905) | 0.139 | 9.40 (4.34) | 3.74 (4.61) † | H×G | 0.252 (0.778) | 0.873 | |||||
| Variables (Toe Off) | Hip | Knee | Ankle | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NG | AG | Effect | F (P) | d | Post hoc | NG | AG | Effect | F (P) | d | Post hoc | NG | AG | Effect | F (P) | d | Post hoc | |||
| M(SD) | M(SD) | M(SD) | M(SD) | M(SD) | M(SD) | |||||||||||||||
| Sagittal (°) | R | 0 cm a | 26.49 (4.51) | 25.73 (3.58) | H | 2.457 (0.096) | 0.188 | c > a | 29.45 (11.06) | 30.85 (5.96) | H | 0.511 (0.603) | 0.164 | ns | 23.59 (8.68) | 26.94 (7.25) | H | 0.311 (0.734) | 0.420 | ns |
| 5 cm b | 27.78 (4.11) | 27.28 (5.72) | G | 0.019 (0.890) | 0.103 | 27.71 (7.66) | 32.75 (6.30) † | G | 10.559 (0.002) * | 0.722 | 24.46 (8.95) | 28.12 (4.75) | G | 2.517 (0.119) | 0.535 | |||||
| 15 cm c | 28.10 (5.56) | 29.85 (4.83) | H×G | 0.814 (0.449) | 0.337 | 26.13 (6.23) | 31.59 (5.29) † | H×G | 1.130 (0.331) | 0.946 | 26.14 (11.03) | 27.34 (8.51) | H×G | 0.202 (0.818) | 0.123 | |||||
| L | 0 cm a | 32.40 (5.39) | 30.34 (4.61) | H | 0.350 (0.706) | 0.412 | ns | 31.58 (6.63) | 35.26 (6.78) | H | 2.713 (0.124) | 0.550 | ns | 23.98 (6.40) | 24.05 (8.52) | H | 0.162 (0.851) | 0.009 | ns | |
| 5 cm b | 31.48 (7.32) | 33.60 (6.95) | G | 0.002 (0.963) | 0.297 | 33.24 (5.38) | 32.70 (5.71) | G | 1.954 (0.168) | 0.098 | 26.33 (5.14) | 23.14 (6.58) | G | 3.463 (0.069) | 0.544 | |||||
| 15 cm c | 32.09 (8.65) | 32.51 (5.52) | H×G | 0.801 (0.454) | 0.059 | 29.55 (6.55) | 32.18 (6.66) | H×G | 0.854 (0.432) | 0.398 | 26.32 (5.88) | 21.93 (6.34) | H×G | 0.993 (0.377) | 0.717 | |||||
| Frontal (°) | R | 0 cm a | 5.87 (2.25) | 4.97 (3.84) | H | 0.206 (0.814) | 0.297 | ns | 10.52 (4.16) | 8.32 (4.01) | H | 0.320 (0.728) | 0.243 | ns | 5.38 (6.12) | 7.84 (2.95) | H | 0.024 (0.977) | 0.543 | ns |
| 5 cm b | 6.21 (3.85) | 6.35 (2.54) | G | 0.048 (0.828) | 0.044 | 10.42 (6.39) | 7.36 (4.94) | G | 0.648 (0.424) | 0.334 | 4.29 (5.93) | 8.54 (3.06) † | G | 7.710 (0.008) * | 0.944 | |||||
| 15 cm c | 5.54 (3.21) | 5.25 (3.19) | H×G | 0.141 (0.869) | 0.041 | 9.45 (4.09) | 8.55 (4.66) | H×G | 0.455 (0.637) | 0.101 | 5.63 (8.79) | 6.93 (2.50) | H×G | 0.737 (0.484) | 0.230 | |||||
| L | 0 cm a | 7.41 (2.74) | 7.65 (3.62) | H | 0.820 (0.446) | 0.118 | ns | 1.15 (1.42) | 4.94 (2.02) † | H | 0.155 (0.857) | 0.564 | ns | 6.21 (7.56) | 7.10 (3.45) | H | 0.465 (0.631) | 0.160 | ns | |
| 5 cm b | 8.18 (4.62) | 8.81 (5.43) | G | 0.717 (0.401) | 0.095 | 5.71 (2.00) | 6.75 (4.74) | G | 3.498 (0.067) | 0.007 | 4.43 (7.89) | 7.56 (6.71) | G | 6.394 (0.015) * | 0.428 | |||||
| 15 cm c | 7.13 (5.29) | 7.94 (5.88) | H×G | 0.017 (0.983) | 0.078 | 4.58 (1.30) | 6.52 (2.56) | H×G | 1.274 (0.288) | 0.227 | 3.13 (2.26) | 6.81 (4.10) † | H×G | 0.712 (0.495) | 0.647 | |||||
| Transverse (°) | R | 0 cm a | 6.87 (2.83) | 5.57 (2.45) | H | 0.269 (0.765) | 0.493 | ns | 3.21 (1.90) | 2.05 (1.62) † | H | 0.405 (0.669) | 0.678 | ns | 10.83 (5.10) | 10.19 (5.40) | H | 1.575 (0.217) | 0.121 | ns |
| 5 cm b | 6.21 (3.75) | 6.14 (4.39) | G | 1.141 (0.291) | 0.367 | 0.70 (0.78) | 3.24 (1.38) | G | 11.007 (0.002) * | 0.460 | 13.91 (6.77) | 10.96 (6.27) | G | 3.062 (0.086) | 0.452 | |||||
| 15 cm c | 5.27 (6.37) | 6.28 (4.37) | H×G | 1.699 (0.193) | 0.187 | 1.59 (0.41) | 2.31 (1.78) | H×G | 0.070 (0.932) | 0.593 | 13.88 (5.09) | 11.29 (6.85) | H×G | 0.382 (0.685) | 0.434 | |||||
| L | 0 cm a | 5.49 (2.56) | 8.36 (2.56) † | H | 5.033 (0.010) * | 0.566 | c > a,b | 0.53 (0.11) | 0.28 (0.68) | H | 0.059 (0.943) | 0.127 | ns | 11.64 (3.31) | 7.63 (4.21) † | H | 0.731 (0.487) | 0.765 | ns | |
| 5 cm b | 5.98 (3.93) | 7.10 (3.22) | G | 1.729 (0.195) | 0.243 | 0.12 (0.47) | 1.04 (0.59) | G | 0.121 (0.730) | 0.167 | 11.30 (5.28) | 7.66 (3.80) † | G | 27.077 (0.000) *** | 0.800 | |||||
| 15 cm c | 3.39 (1.64) | 2.65 (1.84) | H×G | 2.562 (0.087) | 0.141 | 0.24 (0.11) | 0.92 (0.83) | H×G | 0.393 (0.677) | 0.115 | 13.49 (5.27) | 7.16 (3.44) † | H×G | 1.291 (0.284) | 0.852 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.; Shim, H.; Jang, C.; Yin, H.; Kim, J. Influence of Rehabilitation Aid Use on Obstacle Height During Gait in Patients with Foot Drop: A Case Series Study. Healthcare 2025, 13, 2984. https://doi.org/10.3390/healthcare13222984
Park J, Shim H, Jang C, Yin H, Kim J. Influence of Rehabilitation Aid Use on Obstacle Height During Gait in Patients with Foot Drop: A Case Series Study. Healthcare. 2025; 13(22):2984. https://doi.org/10.3390/healthcare13222984
Chicago/Turabian StylePark, Joonsung, Himchan Shim, Changho Jang, Hanyang Yin, and Jongbin Kim. 2025. "Influence of Rehabilitation Aid Use on Obstacle Height During Gait in Patients with Foot Drop: A Case Series Study" Healthcare 13, no. 22: 2984. https://doi.org/10.3390/healthcare13222984
APA StylePark, J., Shim, H., Jang, C., Yin, H., & Kim, J. (2025). Influence of Rehabilitation Aid Use on Obstacle Height During Gait in Patients with Foot Drop: A Case Series Study. Healthcare, 13(22), 2984. https://doi.org/10.3390/healthcare13222984






