Comprehensive Evaluation of Non-Standard Inflammatory and Metabolic Indices in Obesity: A Single-Center Retrospective Study
Abstract
1. Introduction
2. Methods
3. Statistical Analysis
4. Results
5. Discussion
6. Limitations
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Obesity and Overweight. 2025. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 22 August 2025).
- Turkish Statistical Institute (TURKSTAT). Turkey Health Survey 2022. Available online: https://data.tuik.gov.tr/Bulten/Index?dil=2&p=Turkiye-Health-Survey-2022-49747 (accessed on 22 August 2025).
- World Health Organization. Obesity: Overview. 2025. Available online: https://www.who.int/health-topics/obesity (accessed on 22 August 2025).
- Liu, K.; Tang, S.; Liu, C.; Ma, J.; Cao, X.; Yang, X.; Zhu, Y.; Chen, K.; Liu, Y.; Zhang, C.; et al. Systemic immune-inflammatory biomarkers (SII, NLR, PLR and LMR) linked to non-alcoholic fatty liver disease risk. Front. Immunol. 2024, 15, 1337241. [Google Scholar] [CrossRef]
- Tang, Y.; Deng, Y.; Zhang, G.; Wang, Y.; Wang, J.; Wu, J.; Gu, M. Inflammatory markers as predictors of liver fibrosis in type 2 diabetes patients with metabolic dysfunction-associated fatty liver disease. Front. Endocrinol. 2025, 16, 1556646. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Zhao, Y.; Li, Z.; Li, D.; Zhao, F.; Hao, J.; Yang, C.; Song, J.; Gu, X.; Huang, R. Association between novel inflammatory markers and non-alcoholic fatty liver disease: A cross-sectional study. Eur. J. Gastroenterol. Hepatol. 2024, 36, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; He, X.; Tan, S.; Qu, X.; Huang, W.; Cai, J.; You, J.; Fu, X.; He, Y.; Yang, H. The association between immunoinflammatory biomarkers NLR, PLR and LMR and nonalcoholic fatty liver disease: A systematic review and meta-analysis. Clin. Exp. Med. 2025, 25, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, W.; Liu, Z.; Ou, J.; Sun, Y.; Zhang, L.; Ji, G. Association between systemic inflammation markers and cardiovascular mortality in adults with metabolic dysfunction-associated steatotic liver disease. Nutr. Metab. Cardiovasc. Dis. 2025, 35, 103781. [Google Scholar] [CrossRef]
- Li, Y.; Shao, X. Mediating role of inflammatory markers (NLR, PLR, SII, SIRI) in the association between 25(OH)D deficiency and obesity in children and adolescents. J. Health Popul. Nutr. 2025, 44, 215. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention (CDC). Adult BMI Categories. Atlanta (GA): Centers for Disease Control and Prevention. 2024. Available online: https://www.cdc.gov/bmi/adult-calculator/bmi-categories.html (accessed on 25 October 2025).
- World Health Organization (WHO). Obesity and Overweight: Fact Sheet. Geneva (CH): World Health Organization. 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 October 2025).
- Ishiba, H.; Sumida, Y.; Tanaka, S.; Yoneda, M.; Hyogo, H.; Ono, M.; Fujii, H.; Eguchi, Y.; Suzuki, Y.; Yoneda, M.; et al. The novel cutoff points for the FIB4 index categorized by age increase the diagnostic accuracy in NAFLD: A multi-center study. J. Gastroenterol. 2018, 53, 1216–1224. [Google Scholar] [CrossRef]
- Sugiyama, A.; Kurisu, A.; Bunthen, E.; Ouoba, S.; Ko, K.; Rakhimov, A.; Akita, T.; Harakawa, T.; Sako, T.; Koshiyama, M.; et al. Distribution of FIB-4 index in the general population: Analysis of 75,666 residents who underwent health checkups. BMC Gastroenterol. 2022, 22, 241. [Google Scholar] [CrossRef]
- Cataltepe, E.; Ceker, E.; Fadiloglu, A.; Gungor, F.; Karakurt, N.; Ulger, Z.; Varan, H.D. Association between the systemic immune-inflammation index and sarcopenia in older adults: A cross-sectional study. BMC Geriatr. 2025, 25, 28. [Google Scholar] [CrossRef]
- Wrona, M.V.; Ghosh, R.; Coll, K.; Chun, C.; Yousefzadeh, M.J. The 3 I’s of immunity and aging: Immunosenescence, inflammaging, and immune resilience. Front. Aging 2024, 5, 1490302. [Google Scholar] [CrossRef]
- Wu, L.; Han, D.; Xue, Y.; He, S.; Ma, Z.; Su, S.; Li, P.; Liu, S.; Zhou, H. Association between the C-reactive protein-albumin-lymphocyte index and metabolic syndrome: Evidence from the 2003–2010 National Health and Nutrition Examination Survey. Diabetol. Metab. Syndr. 2025, 17, 39. [Google Scholar] [CrossRef]
- Kılıç, Ş.; Aydın, E.; Genç, Ç.D. Evaluation of C-reactive protein/albumin ratio in diabetic retinopathy patients. Diagnostics 2025, 15, 2178. [Google Scholar] [CrossRef]
- Lim, T.; Lee, Y.J. C-reactive protein to albumin ratio and risk of incident metabolic syndrome in community-dwelling adults: Longitudinal findings over a 12-year follow-up period. Endocrine 2024, 86, 156–162. [Google Scholar] [CrossRef]
- Bilgin, S.; Kurtkulagi, O.; Tel, B.M.A.; Duman, T.T.; Kahveci, G.; Khalid, A.; Aktas, G. Does C-reactive protein to serum albumin ratio correlate with diabetic nephropathy in patients with type 2 diabetes mellitus? The CARE TIME study. Prim. Care Diabetes 2021, 15, 1071–1074. [Google Scholar] [CrossRef]
- Tani, S.; Imatake, K.; Suzuki, Y.; Yagi, T.; Takahashi, A.; Monden, M.; Matsumoto, N.; Okumura, Y. Triglyceride/high-density lipoprotein cholesterol ratio may be a better index of cardiometabolic risk in women than in men in Japan. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 868–881. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Tu, Z.; Duan, L.; Tu, R. Differential effects of systemic immune inflammation indices on hepatic steatosis and hepatic fibrosis: Evidence from NHANES 1999–2018. BMC Gastroenterol. 2024, 24, 463. [Google Scholar] [CrossRef] [PubMed]
- Anstee, Q.M.; Berentzen, T.L.; Nitze, L.M.; Jara, M.; Jensen, A.B.; Kjær, M.S.; Mangla, K.K.; Tarp, J.M.; Khunti, K. Prognostic utility of Fibrosis-4 index for risk of subsequent liver and cardiovascular events and all-cause mortality in individuals with obesity and/or type 2 diabetes: A longitudinal cohort study. Lancet Reg. Health Eur. 2024, 36, 100780. [Google Scholar] [CrossRef] [PubMed]
- Chew, N.W.; Ng, C.H.; Chan, K.E.; Chee, D.; Syn, N.; Tamaki, N.; Muthiah, M.; Noureddin, M. FIB-4 predicts MACE and cardiovascular mortality in patients with nonalcoholic fatty liver disease. Can. J. Cardiol. 2022, 38, 1779–1780. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Wu, T.; Zhang, A.; Li, Y. Association between obesity and systemic immune inflammation index, systemic inflammation response index among US adults: A population-based analysis. Lipids Health Dis. 2024, 23, 245. [Google Scholar] [CrossRef]
- Liao, Y.; Zhou, K.; Lin, B.; Deng, S.; Weng, B.; Pan, L. Associations between systemic immune-inflammatory index and visceral adipose tissue area: Results of a national survey. Front. Nutr. 2025, 11, 1517186. [Google Scholar] [CrossRef]
- Türkkan, E.; Dağ, N.Ç.; Arabacı, Ç.; Dikker, O.; Dağ, H. Evaluation of inflammatory hematological ratios (NLR, PLR, MLR and monocyte/HDL-cholesterol ratio) in obese adolescents. Iberoam. J. Med. 2022, 4, 11–17. [Google Scholar] [CrossRef]
- Soesilo, N.; Hendrianingtyas, M.; Rachma, D.E.; Limijadi, E.K.S. Monocyte-to-HDL cholesterol ratio (MHR) and monocyte-to-lymphocyte ratio (MLR) in overweight and obese women. Mod. Med. 2023, 30, 335–339. [Google Scholar] [CrossRef]
- Yuan, J.; He, X.; Lu, Y.; Pu, X.; Liu, L.; Zhang, X.; Liao, J.; Li, G.; Luo, Y.; Zhang, T. Triglycerides/high-density lipoprotein cholesterol ratio outperforms traditional lipid indicators in predicting metabolic dysfunction-associated steatotic liver disease among US adults. Front. Endocrinol. 2025, 16, 1591241. [Google Scholar] [CrossRef] [PubMed]
- Poochanasri, M.; Lertsakulbunlue, S.; Kookanok, C.; Rangsin, R.; Kaewput, W.; Mungthin, M.; Samakkarnthai, P. Triglyceride to high-density lipoprotein ratio as a predictor for 10-year cardiovascular disease in individuals with diabetes in Thailand. J. Health Popul. Nutr. 2025, 44, 147. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Zhang, H.; Ren, G.; Wang, Y.; Fu, S.; Liu, Y.; Zhang, Z.; Guo, L.; Ma, X. Association of the triglyceride glucose index with obesity indicators and hypertension in American adults based on NHANES 2013 to 2018. Sci. Rep. 2025, 15, 2443. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ning, Z.; Lu, Q.; Huang, J.; Yuan, G.; Chen, J.; Liu, G. Reference interval establishment for neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune-inflammation index in athletes: Analysis of sex and sport type impact. J. Clin. Lab. Anal. 2025, 39, e70005. [Google Scholar] [CrossRef]
- Holven, K.B.; van Lennep, J.R. Sex differences in lipids: A life course approach. Atherosclerosis 2023, 384, 117270. [Google Scholar] [CrossRef]
- Patro, S.; Choudhary, A.; Sharma, V.; Mahajan, A.; Sahoo, D.; Pattnaik, S.S. Evaluating platelet-to-lymphocyte ratio and systemic immune-inflammation index as distinctive biomarkers in type 2 diabetes mellitus patients with and without proteinuria: A retrospective study. Cureus 2025, 17, e79348. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Gu, W.; Cai, B.; Lei, M.; Luo, Y.; Zhang, N. Association of immune-inflammation indexes with incidence and prognosis of diabetic nephropathy: A systematic review and meta-analysis. Front. Endocrinol. 2025, 16, 1532682. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y. Associations of the TyG index with albuminuria and chronic kidney disease in patients with type 2 diabetes. PLoS ONE 2024, 19, e0312374. [Google Scholar] [CrossRef]
- Liu, J.; Lv, H.; Wang, J.; Zhu, Q.; Chen, G.; Jiang, Y.; Zhao, K.; Shao, L.; Shi, J.; Pan, X. Blood pressure stratification for predicting liver fibrosis risk in metabolic dysfunction associated fatty liver disease. Ann. Hepatol. 2023, 28, 100892. [Google Scholar] [CrossRef]
- Sachar, M.; Pan, J.J.; Park, J. A noninvasive scoring system for liver fibrosis in patients with metabolic dysfunction-associated fatty liver disease. Gastro Hep Adv. 2022, 1, 1006–1013. [Google Scholar] [CrossRef] [PubMed]
- Takase, H.; Kawakatsu, N.; Hayashi, K.; Kin, F.; Isogaki, T.; Dohi, Y. Relationship between fibrosis-4 index and new onset hypertension in the general population. Eur. Heart J. 2024, 45, ehae666.2540. [Google Scholar] [CrossRef]
- Afşin, A.; Asoğlu, R.; Kurtoğlu, E.; Kaya, H. Neutrophil to lymphocyte ratio as a predictor of left ventricular hypertrophy in patients with newly diagnosed hypertension. Hypertens. Manag. 2019, 5, 42. [Google Scholar] [CrossRef]
- Cao, Y.; Li, P.; Zhang, Y.; Qiu, M.; Li, J.; Ma, S.; Yan, Y.; Li, Y.; Han, Y. Association of systemic immune inflammatory index with all-cause and cause-specific mortality in hypertensive individuals: Results from NHANES. Front. Immunol. 2023, 14, 1087345. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Liao, L.; Liao, Y.; Fang, Y.; Shen, Y. The relationship between C-reactive protein/albumin ratio and mortality in hypertensive patients: A national cohort study. Nutr. Metab. Cardiovasc. Dis. 2024, 34, 1601–1609. [Google Scholar] [CrossRef]
- Li, C.; Zhang, Z.; Luo, X.; Xiao, Y.; Tu, T.; Liu, C.; Liu, Q.; Wang, C.; Dai, Y.; Zhang, Z.; et al. The triglyceride–glucose index and its obesity-related derivatives as predictors of all-cause and cardiovascular mortality in hypertensive patients: Insights from NHANES data with machine learning analysis. Cardiovasc. Diabetol. 2025, 24, 47. [Google Scholar] [CrossRef]
- Sato, S.; Kawai, H.; Sato, S.; Iwasaki, H.; Omori, M.; Kita, Y.; Ikeda, Y.; Awatsu, T.; Murata, A.; Taniguchi, G.; et al. Hypertension and diabetes mellitus are associated with high FIB-4 index in a health checkup examination cohort without known liver disease. BMC Gastroentrol. 2022, 22, 478. [Google Scholar] [CrossRef]
- Tamaki, S.; Nagai, Y.; Shutta, R.; Masuda, D.; Yamashita, S.; Seo, M.; Yamada, T.; Nakagawa, A.; Yasumura, Y.; Nakagawa, Y.; et al. Combination of neutrophil-to-lymphocyte and platelet-to-lymphocyte ratios as a novel predictor of cardiac death in patients with acute decompensated heart failure with preserved left ventricular ejection fraction: A multicenter study. J. Am. Heart Assoc. 2023, 12, e026326. [Google Scholar] [CrossRef]
- Ying, Y.; Ji, Y.; Ju, R.; Chen, J.; Chen, M. Association between the triglyceride-glucose index and liver fibrosis in adults with metabolism-related fatty liver disease in the United States: A cross-sectional study of NHANES 2017–2020. BMC Gastroenterol. 2025, 25, 3. [Google Scholar] [CrossRef]
| Variables | n or Median (Min−Max) | % or Mean ± SD |
|---|---|---|
| Age (Years) | 38.50 (18.0–68.0) | 38.92 ± 11.48 |
| Age group | ||
| 18–30 years | 87 | 29.2 |
| 31–40 years | 79 | 26.5 |
| 41–50 years | 77 | 25.8 |
| >50 years | 55 | 18.5 |
| Sex | ||
| Female | 161 | 54.0 |
| Male | 137 | 46.0 |
| Height (cm) | 167.0 (142.0–190.0) | 167.57 ± 9.48 |
| Weight (kg) | 90.0 (44.0–170.0) | 92.80 ± 21.41 |
| Waist circumference (cm) | 106.0 (30.0–149.0) | 106.07 ± 14.77 |
| BMI | 32.98 (17.19–57.07) | 32.98 ± 6.51 |
| BMI group | ||
| Normal/Underweight | 50 | 16.8 |
| Overweight | 55 | 18.5 |
| Class I obesity | 91 | 30.6 |
| Class II obesity | 53 | 17.8 |
| Class III obesity | 48 | 16.3 |
| Smoking status | ||
| No | 218 | 73.2 |
| Yes | 80 | 26.8 |
| Alcohol consumption | ||
| No | 286 | 96.0 |
| Yes | 12 | 4.0 |
| Marital status | ||
| Single/Widowed | 95 | 31.9 |
| Married | 203 | 68.1 |
| Educational level | ||
| Primary school | 40 | 13.4 |
| Secondary school | 39 | 13.1 |
| High school | 62 | 20.8 |
| University | 101 | 33.9 |
| Postgraduate | 56 | 18.8 |
| Diabetes | ||
| No | 280 | 94.0 |
| Yes | 18 | 6.0 |
| Hypertension | ||
| No | 279 | 93.6 |
| Yes | 19 | 6.4 |
| Hyperlipidemia | ||
| No | 287 | 96.3 |
| Yes | 11 | 3.7 |
| Variables | Min. | Max | Mean | SD |
|---|---|---|---|---|
| FIB-4 Score | 0.11 | 7.46 | 0.71 | 0.56 |
| SII | 55.73 | 2964.11 | 521.95 | 313.81 |
| TG/Glucose | 0.00 | 7.74 | 1.51 | 0.96 |
| TG/HDL | 0.35 | 16.58 | 3.14 | 2.47 |
| NLR | 0.37 | 7.51 | 1.88 | 0.85 |
| MLR | 0.04 | 0.85 | 0.19 | 0.08 |
| CRP/Alb | 0.00 | 29.76 | 1.07 | 2.01 |
| PLR | 0.01 | 1.14 | 0.12 | 0.07 |
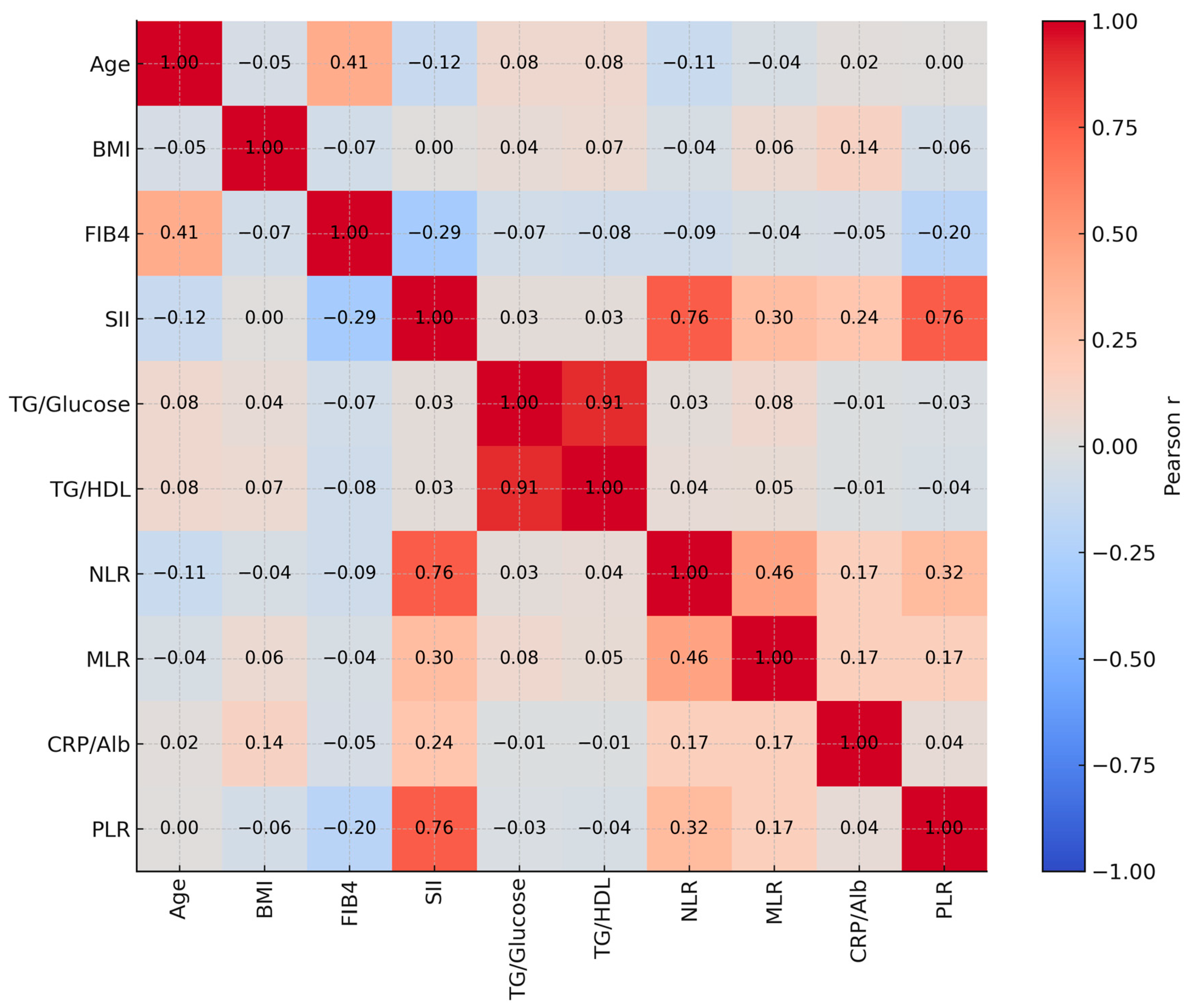
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1-Age (Years) | r | 1 | |||||||||
| p | |||||||||||
| 2-BMI | r | −0.050 | 1 | ||||||||
| p | 0.392 | ||||||||||
| 3-FIB-4 Score | r | 0.409 ** | −0.067 | 1 | |||||||
| p | <0.001 | 0.247 | |||||||||
| 4-SII | r | −0.117 * | 0.002 | −0.294 ** | 1 | ||||||
| p | 0.044 | 0.977 | <0.001 | ||||||||
| 5-TG/Glucose | r | 0.078 | 0.044 | −0.069 | 0.034 | 1 | |||||
| p | 0.179 | 0.454 | 0.237 | 0.561 | |||||||
| 6-TG/HDL | r | 0.079 | 0.072 | −0.079 | 0.034 | 0.911 ** | 1 | ||||
| p | 0.175 | 0.214 | 0.177 | 0.559 | <0.001 | ||||||
| 7-NLR | r | −0.106 | −0.044 | −0.086 | 0.763 ** | 0.031 | 0.043 | 1 | |||
| p | 0.068 | 0.446 | 0.138 | <0.001 | 0.590 | 0.461 | |||||
| 8-MLR | r | −0.045 | 0.056 | −0.039 | 0.303 ** | 0.081 | 0.049 | 0.456 ** | 1 | ||
| p | 0.441 | 0.334 | 0.502 | <0.001 | 0.163 | 0.398 | <0.001 | ||||
| 9-CRP/Alb | r | 0.02 | 0.145 * | −0.053 | 0.236 ** | −0.015 | −0.007 | 0.173 ** | 0.168 ** | 1 | |
| p | 0.735 | 0.012 | 0.361 | <0.001 | 0.800 | 0.907 | 0.003 | 0.004 | |||
| 10-PLR | r | 0.002 | −0.062 | −0.203 ** | 0.757 ** | −0.033 | −0.037 | 0.320 ** | 0.171 ** | 0.045 | 1 |
| p | 0.971 | 0.287 | <0.001 | <0.001 | 0.573 | 0.528 | <0.001 | 0.003 | 0.441 |
| Variables | n | FIB-4 Score | SII | TG/Glucose | TG/HDL | NLR | MLR | CRP/Alb | PLR |
|---|---|---|---|---|---|---|---|---|---|
| Degree of steatosis | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | |
| 0 | 52 | 0.65 (0.45) | 413.05 (283.66) | 1.16 (0.72) | 2.29 (2.1) | 1.51 (0.84) | 0.17 (0.05) | 0.64 (0.95) | 0.16 (0.05) |
| 1 | 95 | 0.64 (0.35) | 469.56 (278.57) | 1.17 (1.06) | 2.13 (2.46) | 1.71 (0.61) | 0.18 (0.06) | 0.53 (0.76) | 0.12 (0.5) |
| 2 | 110 | 0.61 (0.4) | 455.24 (336) | 1.23 (0.97) | 2.46 (2.31) | 1.81 (0.98) | 0.18 (0.07) | 0.83 (0.90) | 0.11 (0.05) |
| 3 | 41 | 0.56 (0.42) | 435.71 (294.84) | 1.36 (1.11) | 2.86 (3.57) | 1.63 (1) | 0.19 (0.09) | 0.6 (0.7) | 0.12 (0.05) |
| p= | 0.915 | 0.723 | 0.564 | 0.610 | 0.459 | 0.333 | 0.071 | 0.798 | |
| Sex | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | |
| Female | 161 | 0.57 (0.4) | 466.85 (349.35) | 1.20 (0.8) | 2.01 (1.66) | 1.71 (0.9) | 0.17 (0.06) | 0.77 (1.0) | 0.12 (0.05) |
| Male | 137 | 0.64 (0.41) | 433.56 (273.16) | 1.45 (1.14) | 3.32 (3.2) | 1.70 (0.8) | 0.19 (0.07) | 0.58 (0.7) | 0.11 (0.04) |
| Z= | 1.35 | −1.41 | 3.39 | 4.97 | 0.07 | 3.20 | −2.01 | −3.74 | |
| p= | 0.179 | 0.158 | <0.001 | <0.001 | 0.947 | 0.010 | 0.045 | <0.001 | |
| BMI Group | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | |
| (1) Normal/Underweight | 50 | 0.59 (0.37) | 475.03 (295.16) | 0.88 (0.44) | 1.35 (1.10) | 1.93 (0.91) | 0.18 (0.1) | 0.22 (0.41) | 0.12 (0.04) |
| (2) Overweight | 55 | 0.67 (0.39) | 454.03 (265.99) | 1.47 (1.17) | 2.42 (3.52) | 1.71 (0.70) | 0.17 (0.09) | 0.44 (0.57) | 0.12 (0.04) |
| (3) Class I obesity | 91 | 0.65 (0.42) | 403.96 (304.40) | 1.34 (1.03) | 2.73 (2.76) | 1.59 (0.81) | 0.18 (0.07) | 0.8 (0.65) | 0.10 (0.04) |
| (4) Class II obesity | 53 | 0.55 (0.40) | 428.99 (287.50) | 1.49 (0.93) | 3.06 (1.89) | 1.62 (0.94) | 0.19 (0.05) | 0.75 (0.88) | 0.11 (0.04) |
| (5) Class III obesity | 48 | 0.55 (0.41) | 533.70 (360.82) | 1.22 (0.61) | 2.63 (1.67) | 1.88 (0.72) | 0.18 (0.04) | 1.15 (1.01) | 0.12 (0.035) |
| p= | 0.374 | 0.089 | <0.001 | <0.001 | 0.025 | 0.967 | <0.001 | 0.028 | |
| Post Hoc= | - | - | 1 < 2.3.4.5 | 1 < 2.3.4.5 | 1 < 3 | - | 1 < 2.3.4.5 | - | |
| Smoking status | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | |
| No | 218 | 0.64 (0.44) | 448.76 (296.99) | 1.22 (0.99) | 2.177 (2.297) | 1.70 (0.84) | 0.17 (0.07) | 0.66 (0.08) | 0.11 (0.05) |
| Yes | 80 | 0.61 (0.35) | 472.75 (346.35) | 1.303 (1.049) | 2.854 (2.767) | 1.87 (0.81) | 0.19 (0.05) | 0.67 (0.91) | 0.11 (0.04) |
| Z= | −0.72 | 0.54 | 0.63 | +1.77 | 1.37 | 1.52 | 0.74 | −1.78 | |
| p= | 0.475 | 0.592 | 0.530 | 0.077 | 0.171 | 0.130 | 0.458 | 0.075 | |
| Alcohol consumption | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | |
| No | 286 | 0.63 (0.42) | 451.14 (310.58) | 1.23 (1.03) | 2.38 (2.44) | 1.72 (0.87) | 0.18 (0.07) | 0.67 (0.87) | 0.12 (0.05) |
| Yes | 12 | 0.53 (0.28) | 415.01 (213.25) | 1.18 (0.68) | 2.48 (3.07) | 1.66 (0.52) | 0.19 (0.11) | 0.91 (1.13) | 0.09 (0.05) |
| Z= | −0.701 | −0.530 | −0.289 | −0.033 | −0.393 | −0.147 | −0.604 | −1.125 | |
| p= | 0.483 | 0.596 | 0.773 | 0.974 | 0.694 | 0.883 | 0.546 | 0.261 | |
| Diabetes | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | |
| No | 280 | 0.62 (0.42) | 450.28 (308.87) | 1.22 (0.98) | 2.36 (2.39) | 1.72 (0.84) | 0.18 (0.07) | 0.65 (0.79) | 0.12 (0.05) |
| Yes | 18 | 0.66 (0.38) | 448.27 (322.72) | 1.6 (1.35) | 3.2 (4.77) | 1.61 (1.06) | 0.17 (0.06) | 1.21 (0.93) | 0.11 (0.07) |
| Z= | −1.273 | −0.147 | −1.126 | −1.104 | −0.595 | −0.937 | −2.097 | −0.322 | |
| p= | 0.203 | 0.883 | 0.260 | 0.269 | 0.552 | 0.349 | 0.036 | 0.748 | |
| Hypertension | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | |
| No | 279 | 0.60 (0.4) | 450.88 (311.18) | 1.23 (1.04) | 2.39 (2.5) | 1.72 (0.86) | 0.19 (0.07) | 0.68 (0.89) | 0.12 (0.05) |
| Yes | 19 | 0.95 (0.48) | 384.57 (308.03) | 1.23 (0.93) | 2.41 (1.97) | 1.5 (0.76) | 0.17 (0.1) | 0.64 (0.48) | 0.11 (0.05) |
| Z= | −3.570 | −0.769 | −0.256 | −0.348 | −0.659 | −0.802 | −0.502 | −1.198 | |
| p= | <0.001 | 0.442 | 0.798 | 0.728 | 0.510 | 0.423 | 0.616 | 0.231 | |
| Hyperlipidemia | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | M(IQR) | |
| No | 287 | 0.61 (0.41) | 449.69 (310.85) | 1.22 (1.01) | 2.35 (2.37) | 1.71 (0.86) | 0.18 (0.07) | 0.67 (0.89) | 0.12 (0.05) |
| Yes | 11 | 0.90 (0.43) | 498.86 (286.32) | 1.84 (1.32) | 3.9 (4.13) | 1.85 (1.51) | 0.17 (0.13) | 0.74 (0.52) | 0.11 (0.07) |
| Z= | −2.490 | −0.123 | −1.200 | −1.639 | −0.258 | −0.111 | −0.206 | −0.358 | |
| p= | 0.013 | 0.902 | 0.230 | 0.101 | 0.796 | 0.912 | 0.836 | 0.720 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yildiz, L.M.; Kızılkaya, B.; Cüre, O. Comprehensive Evaluation of Non-Standard Inflammatory and Metabolic Indices in Obesity: A Single-Center Retrospective Study. Healthcare 2025, 13, 2946. https://doi.org/10.3390/healthcare13222946
Yildiz LM, Kızılkaya B, Cüre O. Comprehensive Evaluation of Non-Standard Inflammatory and Metabolic Indices in Obesity: A Single-Center Retrospective Study. Healthcare. 2025; 13(22):2946. https://doi.org/10.3390/healthcare13222946
Chicago/Turabian StyleYildiz, Latife Merve, Bayram Kızılkaya, and Osman Cüre. 2025. "Comprehensive Evaluation of Non-Standard Inflammatory and Metabolic Indices in Obesity: A Single-Center Retrospective Study" Healthcare 13, no. 22: 2946. https://doi.org/10.3390/healthcare13222946
APA StyleYildiz, L. M., Kızılkaya, B., & Cüre, O. (2025). Comprehensive Evaluation of Non-Standard Inflammatory and Metabolic Indices in Obesity: A Single-Center Retrospective Study. Healthcare, 13(22), 2946. https://doi.org/10.3390/healthcare13222946







