Abstract
Background/Objectives: Treating critically ill patients is complex and often subjective. This study investigates adherence to clinical guidelines for sepsis among different providers. Considering the strengths of the recommendations, we hypothesize that heterogeneity in the decision-making process will be low and independent of provider background and psychological makeup. Methods: This cohort study used two clinical vignettes of sepsis. Providers were given standardized treatment plans for 7 days, and their responses were recorded. Demographical, professional, and psychological (ambiguity tolerance, defensiveness, anxiety due to uncertainty, risk-taking behavior, decision styles, and optimism) variables were acquired. Results: Crystalloids were commonly used in both vignettes. Pressor engagement, especially norepinephrine, increased significantly after the third day. Providers recommended antibiotics and no provider stopped antibiotic therapy. Cluster analysis revealed no differences in therapy implementation among provider types, but some differences existed between the two vignettes. Cluster #1 was characterized by the implementation of early light bundle therapy combined with the use of pressors and a notable enhancement in therapies by the fifth day (Early Cluster). Cluster #2 (Minimalists) involved consistent engagement only in light bundle therapy throughout the treatment period. Cluster #3 (Escalation) comprised providers who rapidly escalated treatment using multiple different modalities. Cluster #3 stood out as most providers were female, non-MD, with significant ICU duties, and enhanced rational thinking. Conclusions: Providers differ in implementation styles of the sepsis treatment standard based on types of therapies selected not studied psychological variables.
1. Introduction
Treatment of critically ill patients demands skill, knowledge, and excellent decision-making abilities, all while making over 100 decisions per day [1,2]. These decisions are made under significant uncertainty, as most clinical data are imperfect and uncertain [3]. On the other hand, sepsis is an example of a situation where emphasis is placed on early implementation of several treatments even when the suspicion threshold is low [4,5,6]. Initial treatment should consist of early antibiotics implementation and fluid resuscitation. If these measures fail, pressors should be engaged. These steps are considered a gold standard in accordance with the Surviving Sepsis Campaign by the Society of Critical Care Medicine [7,8,9]. Considering that mortality increases exponentially with a treatment delay, this represents a situation where a particular combination of risk and ambiguity tolerance may interfere with providing optimal care [4]. Considering that sepsis treatment is highly standardized and the risk of error carries a significant penalty, implementation should be straightforward and thorough in cases where the level of suspicion is low. However, in some providers, the threshold for most medical decisions is not determined by simple Bayesian methods of risk-benefit ratio, but rather by their static and dynamic psychological makeup [5,10,11,12,13,14,15,16,17,18]. The literature is inconclusive on whether patient data or provider characteristics prevail, especially if the pre-test probability for the test is low.
Consequently, we embarked on testing, using clinical vignettes, how adherence to treatment varies over time and across providers. We juxtaposed a sepsis vignette with another one with less certainty, expecting increased variability in treatment. Considering the strengths of the recommendation, we hypothesize that heterogeneity in the decision-making process will be low, implementation will occur early, and it will be independent of provider background and psychological makeup [13].
2. Materials and Methods
2.1. Participants and Study Setting
This is a convenience cohort study using surveys and clinical vignettes.
This study is a part of a larger research project conducted between March 2017 and March 2018, in an academic multicenter hospital. Approved by the IRB (826741; 3 January 2018) for the University of Pennsylvania Health System, survey invitations were emailed to providers from surgery, anesthesia, and internal medicine using a pre-existing distribution list for the ICU staff. The self-administered surveys were completed via the REDCAP™ web-based survey tool. An initial recruitment email was followed by a second request sent only to those who completed the first part. We sent 1078 invitations to ICU staff and received 258 responses, although 120 questionnaires had missing variables. Only physicians and allied health professionals were included in this study, resulting in the exclusion of 55 records. The characteristics of all participants (n = 83) are presented in Table 1. AXIS Tool (Appraisal Tool for Cross-Sectional Studies) was utilized for assessment of potential bias [19].

Table 1.
Demographic and professional characteristics of the studied group demonstrated differences, as expected from our study groups across different professionals.
2.2. Study Design
The participants were presented with two clinical scenarios of sepsis derived from the literature, based on real patients; however, the clinical vignettes were edited for simplification. (Appendix A Figure A1A,B) [20,21,22]. Several treatments were offered every day, which included standards for sepsis treatment by the Surviving Sepsis Campaign issued by the Society of Critical Care Medicine [7,8,9]. The combined use of antibiotics was defined as a light bundle in accordance with the existing Sepsis Campaign recommendations. Several other treatments were also presented to providers (Appendix A Figure A1A,B). The providers could choose any number of them each day. (Appendix A Figure A1A,B)
2.3. Psychological Variables
We utilized a few psychological tools. The Tolerance of Ambiguity Scale (TOA) examined the ability to cope with clinical uncertainty [23,24]. The Rationality/Emotional Defensiveness Scale (R/ED) assessed repression/denial [25,26]. The Anxiety Due to Uncertainty (ADU) 13-item subscale of the Physician Uncertainty Reaction measures anxiety related to ambiguity [27,28]. Propensity for risk-taking behavior was assessed using the Modified Jackson Personality Index (JPI), a six-item scale [29]. The Decision Style Survey (DSS) measured the decision-making process on two dimensions: rational and intuitive processes [30,31]. Life Orientation Test (LOT) estimates optimism [32,33]. Psychometric values of these tests are presented in the attached literature.
2.4. Risk of Bias Assessment
The study’s methodological quality was assessed using the AXIS tool for cross-sectional studies [19,34]. Each of the 20 domains was independently evaluated by two authors (K.L. and H.G.), and discrepancies were resolved by consensus. A summary of the AXIS assessment is provided in Appendix A Table A1.
2.5. Statistical Analysis
The Shapiro–Wilk W test and distribution plots were used to test the normality of distribution variables. Parametric variables will be expressed as mean ± SD and compared using t-Student. For non-parametric variables, median (Me) and interquartile ranges (IR) will be shown with U-Mann–Whitney statistics employed to compare such variables. We utilized k-means clustering with the center of initial clustering established automatically, considering we did not specify a research hypothesis for clustering analysis. The final clusters were defined after 10 iterations. A double-sided p-value of less than 0.05 will be considered statistically significant for all tests. Statistical analyses will be performed with Statistica 11.0 (StatSoft Inc., Tulsa, OK, USA).
3. Results
3.1. Variability in Sepsis Bundle Implementation
Our findings clearly demonstrate that the use of crystalloids was the predominant practice in both vignettes, underscoring their central role in initial treatment. While colloids and blood products were introduced later and in only limited amounts, their minimal use highlights a cautious approach. Notably, although fluid utilization showed a decline over time, this change was not statistically significance. It is also important to note that a substantial number of providers did not recommend fluid administration even at the initial stages, especially in Vignette #2 (Figure 1), raising questions about early intervention strategies. Additionally, variations in therapeutic approaches across different provider types were observed, albeit sporadically (Appendix A Figure A2). Antibiotic initiation rates varied significantly across vignettes, with less than 40% of participants starting antibiotics initially. By day 3 in vignette 2, all participants had begun full antibiotic treatment, highlighting a strong tendency toward early intervention (Figure 2). Daily adjustments to therapy were frequently made, even in less clear cases, demonstrating proactive management (Figure 2). Meanwhile, few providers discontinued treatment, indicating a potential area for optimizing antibiotic use. The implementation of protocols was consistent across provider types (Appendix A Figure A3), warrants further attention.
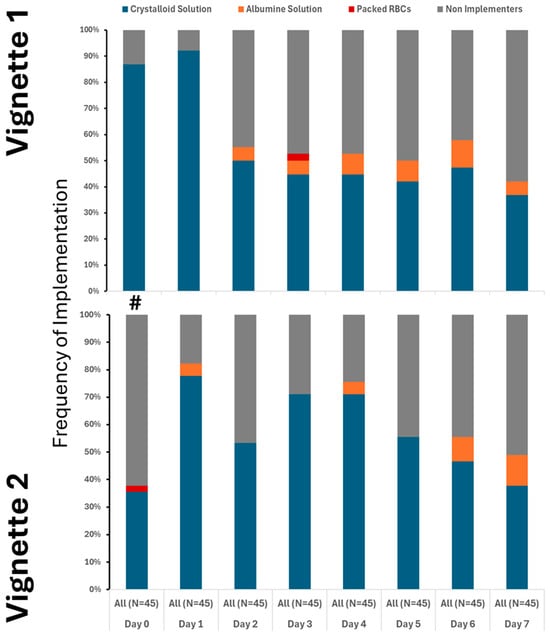
Figure 1.
Utilization of crystalloid varied between the cases, but only on 1st day. # denotes differences in frequency of measured therapy between cases.
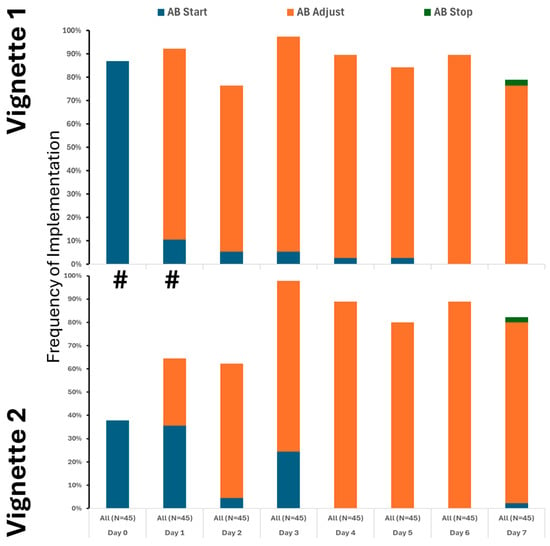
Figure 2.
Utilization of antibiotics varied between the cases, but only on the 1st and 2nd day. # denotes differences in frequency of measured therapy between cases.
Pressor use increased notably after day three, with norepinephrine as the primary medication. Many providers also used dopamine and phenylephrine (Figure 3). Epinephrine was not used, and dobutamine was rarely administered (Figure 3). Approaches to pressor therapy varied slightly among providers.
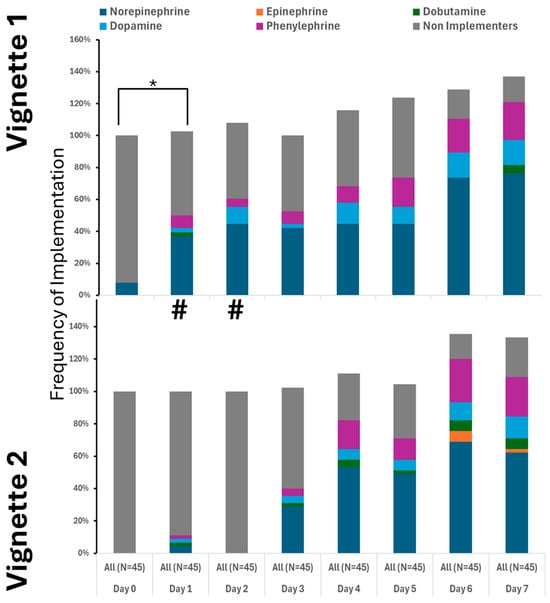
Figure 3.
Utilization of pressors was similar between the cases. # denotes differences in frequency of measured therapy between cases. * Denotes the difference in utilized therapy to the 1st day.
Engagement with other therapies varied considerably, especially later in the treatment period when the clinical scenario for the fictional patient’s condition remained unchanged (Figure 4).
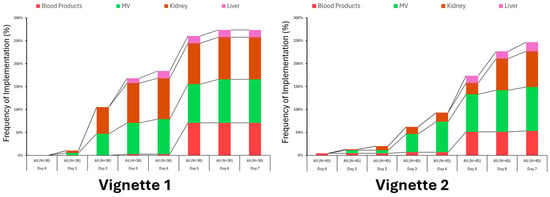
Figure 4.
Utilization of ancillary therapies varied somewhat between vignettes but not significantly.
3.2. Clustering of Treatment
By assessing the engagement of various therapies, three clusters of recommended therapy were identified (Figure 5).
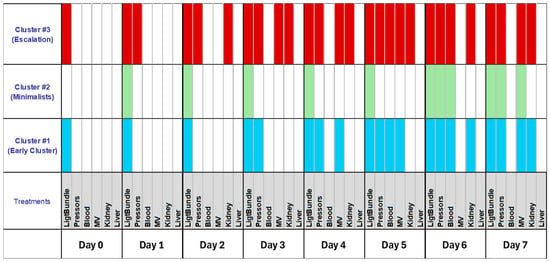
Figure 5.
Unsupervised cluster analysis revealed three distinct clusters when treatment for sepsis was taken into consideration. The light bundle was characterized as the use of fluid resuscitation and antibiotics. MV denotes engagement of mechanical ventilation to treat evolving in a clinical scenario.
Cluster #1 featured early fluid resuscitation and pressors, with enhanced therapies by day five. Cluster #2 (Minimalists) consistently used only light bundle therapy. Cluster #3 (Escalation) providers quickly escalated treatment with multiple modalities and differed from those in clusters #1 and #2. Most providers were female, non-MD, with significant ICU duties (Table 2). Their psychological traits showed more pronounced rational thinking (Table 3).

Table 2.
The distribution of psychological variables across the studied population revealed a significant difference in risk-taking between MDs and APPs.

Table 3.
Basic demographic and professional characteristics of the studied group demonstrated differences as expected across different clusters. The distribution of psychological variables across the various clusters revealed a significant difference in rational decision-making.
4. Discussion
This study demonstrated that ICU sepsis treatment decisions displayed considerable variation, which did not correlate with providers’ demographics, professional backgrounds, or psychological characteristics. Although many practitioners followed recommended therapies, few implemented the complete sepsis bundle. Such heterogeneity was unexpected, especially given the straightforward nature of sepsis guidelines, which were anticipated to promote more uniform practices. Providers could be organized into three distinct clusters based on their implementation strategies: Cluster #1 (Early Cluster) was marked by early initiation of light bundle therapy combined with pressor use and notable intensification of treatments by day five; Cluster #2 (Minimalists) maintained consistent light bundle therapy throughout the course; and Cluster #3 (Escalation) comprised those who rapidly intensified treatment using diverse modalities. Notably, providers in Cluster #3 were predominantly female, non-physician doctors, had substantial ICU responsibilities, and exhibited enhanced critical thinking.
The evolution of guidelines since the initial edition has not resulted in significant breakthroughs [7,8,9,35,36,37]. Standard practices, such as effective infection source control with antibiotics, have remained consistent over time, along with the use of fluids and pressors to manage sepsis-related hypotension. Consistently, our findings demonstrate a low utilization of steroids, colloids, and blood products, aligning with earlier recommendations. Notably, there was a marked willingness to initiate antibiotic therapy, despite infrequent modification or discontinuation. Although current guidelines advise reassessment within 48 h, our participants typically delayed this process until the sixth or seventh day [7,38,39,40]. Achieving alignment between provider practices and established guidelines remains a challenge [37,41]. This is in stark contrast to the guidelines’ recommendations on how to treat sepsis [7]. However, one must keep in mind that we did not test how familiar these recommendations were to the providers. Also, in different countries, recommendations vary slightly, introducing a confounding variable.
Clinical guidelines are designed to deliver evidence-based recommendations for specific conditions, thereby reducing practice variability and improving mortality outcomes [37]. Previous research highlights ongoing discrepancies in ICU decision-making, particularly concerning end-of-life care, patient triage, and admission policies [42,43,44]. This variation is also evident in the management of sepsis, a well-studied condition with established guidelines aimed at reducing mortality. Implementation science suggests that this may stem from factors such as limited knowledge, disjointed handoffs, and challenges in delivering antibiotics promptly [45,46]. Although our study was theoretical and surveyed experienced critical care providers, these barriers may have still influenced their choices. While psychological traits do influence decision-making, their distribution among clinicians is generally consistent and unlikely to explain the observed variation. Existing research supports the complexity of these interactions but has yet to find definitive links.
The study’s findings should be interpreted cautiously, as it is a single-center study with unique characteristics, including a provider mix and care delivery. The study is also limited to the American healthcare providers. Generalization of these results requires validation in larger, multicenter studies. Selection bias may have occurred due to participant location and recruitment method, and the responses from non-responders remain unknown. Response rates were low, yet similar to those in comparable studies. Still, selection bias can affect our measured variables [47]. Furthermore, cluster analysis is also very limited by a relatively low response rate. These methodological issues are not uncommon, but they do caution against the generalization of our findings. We also lack insight into how participants interpreted the vignettes; conducting semi-quantitative interviews could clarify this understanding. Additionally, cognitive biases may also influence the results [48]. We did not measure knowledge of the Surviving Sepsis recommendations. The absence of correlations between the studied psychological traits and the standard implementation of sepsis is vexing, suggesting that future research should employ semi-quantitative methods to investigate the underlying decision-making processes.
The study employs established methods, including standardized psychological tests, surveys, and clinical vignettes, to assess decision-making, thereby supporting its validity [20]. The sample is broad and representative of U.S. medical professionals, with demographics comparable to those in similar research, which strengthens the internal validity.
This study clarifies how ICU providers make decisions about the sepsis bundle’s three treatment clusters. It found no association between psychological factors and decisions; however, further research is required to verify and generalize these findings.
5. Conclusions
Providers differ in implementation styles of the sepsis treatment standard based on types of therapies selected not studied psychological variables.
Author Contributions
Conceptualization, K.L.; Methodology, K.L.; Validation, K.L.; Formal analysis, K.L. and H.G.; Investigation, K.L., H.G. and A.E.; Resources, K.L.; Writing—original draft preparation, K.L.; Writing—review and editing, K.L., H.G. and A.E.; Visualization, K.L., H.G. and A.E.; Supervision, K.L.; Project administration, K.L.; Funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board at the University of Pennsylvania (#826741; 3 January 2018). This is a reanalysis of the previously published study with a limited set of data [20].
Informed Consent Statement
Electronic informed consent was obtained from all participants before study enrollment.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy and ethical restrictions.
Acknowledgments
K.L. would like to acknowledge the participation of the nursing staff of the University of Pennsylvania Medical Center and several members at the Department of Anesthesiology and Critical Care and their help with the study. Grace Ibitamuno, Mayo Clinic, provided scientific review of the introduction.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| ADU | Anxiety Due to Uncertainty |
| DSS | Decision Style Survey |
| ICU | Intensive Care Unit |
| IR | Interquartile Range |
| IRB | Institutional Review Board |
| JPI | Jackson Personality Index |
| LOT | Life Orientation Test |
| MD | Doctor of Medicine |
| Me | Median |
| R/ED | Rationality/Emotional Defensiveness Scale |
| REDCap™ | Research Electronic Data Capture |
| SD | Standard Deviation |
| TOA | Tolerance of Ambiguity Scale |
Appendix A
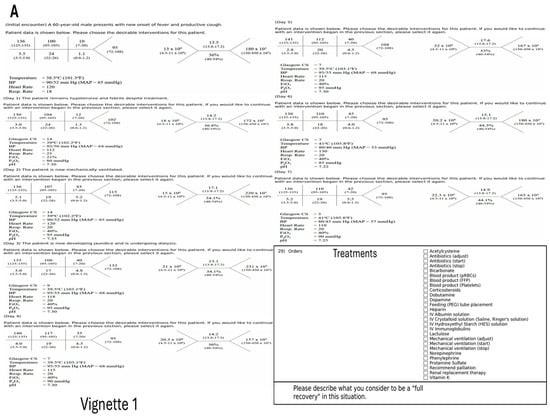

Figure A1.
(A,B) Layout of the clinical vignettes showing evolution of the illness over time.

Table A1.
Assessment of the risk for bias using AXIS tool.
Table A1.
Assessment of the risk for bias using AXIS tool.
| # | AXIS Item | Response | Justification |
|---|---|---|---|
| 1 | Were the aims/objectives of the study clear? | Yes | Clearly stated in the Introduction section. |
| 2 | Was the study design appropriate for the stated aim(s)? | Yes | Cross-sectional vignette-based survey appropriate for assessing provider variability. |
| 3 | Was the sample size justified? | No | This is pilot study |
| 4 | Was the target/reference population clearly defined? | Yes | ICU providers (physicians, APPs) defined as study population. |
| 5 | Was the sample frame taken from an appropriate population base so that it closely represented the target/reference population under investigation? | Yes | Invitations sent to ICU staff across multiple specialties at a large academic center. |
| 6 | Was the selection process likely to select subjects/participants representative of the target/reference population? | Partial | Convenience sampling used; some representativeness concern. |
| 7 | Were measures undertaken to address and categorize non-responders? | No | Non-responders not analyzed or described. |
| 8 | Were the risk factor and outcome variables measured appropriate to the aims of the study? | Yes | Psychological and behavioral measures aligned with study aims. |
| 9 | Were the risk factor and outcome variables measured correctly using instruments/measurements that had been trialed, piloted or published previously? | Yes | All psychological scales (TOA, R/ED, ADU, JPI, DSS, LOT) are validated tools. |
| 10 | Is it clear what was used to determine statistical significance and/or precision estimates? (e.g., p values, CIs) | Yes | Statistical significance defined (p < 0.05); parametric/non-parametric tests specified. |
| 11 | Were the methods (including statistical methods) sufficiently described to enable them to be repeated? | Yes | Detailed methodology and statistical procedures reported. |
| 12 | Were the basic data adequately described? | Yes | Participant demographics and psychological variables detailed in Table 1, Table 2 and Table 3. |
| 13 | Does the response rate raise concerns about non-response bias? | Yes | Response rate (~24%) low; potential bias noted in Discussion. |
| 14 | If appropriate, was information about non-responders described? | No | Non-responder characteristics not provided. |
| 15 | Were the results internally consistent? | Yes | Tables and figures consistent with described results. |
| 16 | Were the results for the analyses described in the methods presented? | Yes | All analyses mentioned in Methods appear in Results. |
| 17 | Were the authors’ discussions and conclusions justified by the results? | Yes | Discussion aligns with results and acknowledges limitations. |
| 18 | Were the limitations of the study discussed? | Yes | Detailed discussion of selection bias, generalizability, and response rate limitations. |
| 19 | Were there any funding sources or conflicts of interest that may affect the authors’ interpretation of the results? | No | No funding or conflicts declared; transparently reported. |
| 20 | Was ethical approval or consent of participants attained? | Yes | IRB approval and informed electronic consent explicitly stated. |
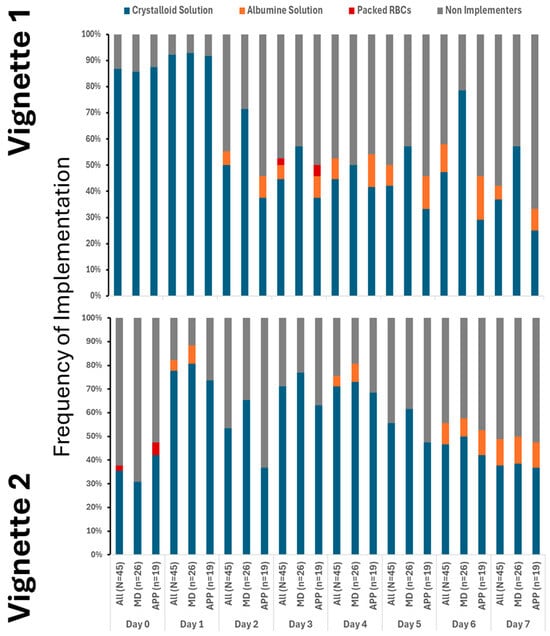
Figure A2.
Variation between providers with respect to fluid resuscitation was minimal between vignettes.
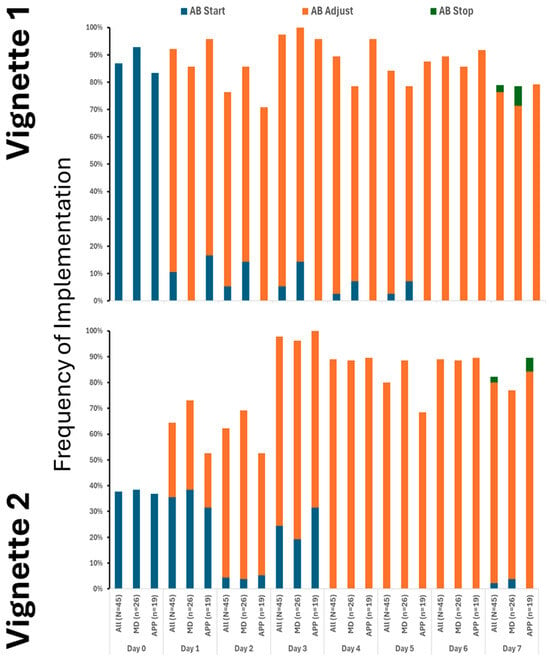
Figure A3.
Variation in antibiotic implementation was minimal between providers across vignettes.
References
- Preuschoff, K.; Mohr, P.M.C.; Hsu, M. Decision Making under uncertainity. Frontiers 2015, 7, 218. [Google Scholar]
- Dzeng, E.; Colaianni, A.; Roland, M.; Chander, G.; Smith, T.J.; Kelly, M.P.; Barclay, S.; Levine, D. Influence of Institutional Culture and Policies on Do-Not-Resuscitate Decision Making at the End of Life. JAMA Intern. Med. 2015, 175, 812–819. [Google Scholar] [CrossRef]
- Tinghog, G.; Andersson, D.; Bonn, C.; Johannesson, M.; Kirchler, M.; Koppel, L.; Vastfjall, D. Intuition and Moral Decision-Making-The Effect of Time Pressure and Cognitive Load on Moral Judgment and Altruistic Behavior. PLoS ONE 2016, 11, e0164012. [Google Scholar] [CrossRef] [PubMed]
- Reitz, K.M.; Kennedy, J.; Li, S.R.; Handzel, R.; Tonetti, D.A.; Neal, M.D.; Zuckerbraun, B.S.; Hall, D.E.; Sperry, J.L.; Angus, D.C.; et al. Association Between Time to Source Control in Sepsis and 90-Day Mortality. JAMA Surg. 2022, 157, 817–826. [Google Scholar] [CrossRef] [PubMed]
- Rudd, K.E.; Mair, C.F.; Angus, D.C. Applying Syndemic Theory to Acute Illness. JAMA 2022, 327, 33–34. [Google Scholar] [CrossRef] [PubMed]
- SCCM. Sepsis Definition. 2021. Available online: https://www.sepsis.org/sepsis-basics/what-is-sepsis/ (accessed on 1 September 2020).
- Evans, L.; Rhodes, A.; Alhazzani, W.; Antonelli, M.; Coopersmith, C.M.; French, C.; Machado, F.R.; McIntyre, L.; Ostermann, M.; Prescott, H.C.; et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock 2021. Crit. Care Med. 2021, 49, e1063–e1143. [Google Scholar] [CrossRef]
- Weiss, S.L.; Peters, M.J.; Alhazzani, W.; Agus, M.S.D.; Flori, H.R.; Inwald, D.P.; Nadel, S.; Schlapbach, L.J.; Tasker, R.C.; Argent, A.C.; et al. Surviving sepsis campaign international guidelines for the management of septic shock and sepsis-associated organ dysfunction in children. Intensive Care Med. 2020, 46, 10–67. [Google Scholar] [CrossRef]
- Alhazzani, W.; Moller, M.H.; Arabi, Y.M.; Loeb, M.; Gong, M.N.; Fan, E.; Oczkowski, S.; Levy, M.M.; Derde, L.; Dzierba, A.; et al. Surviving Sepsis Campaign: Guidelines on the management of critically ill adults with Coronavirus Disease 2019 (COVID-19). Intensive Care Med. 2020, 46, 854–887. [Google Scholar] [CrossRef]
- Hozo, I.; Schell, M.J.; Djulbegovic, B. Decision-making when data and inferences are not conclusive: Risk-benefit and acceptable regret approach. Semin. Hematol. 2008, 45, 150–159. [Google Scholar] [CrossRef]
- Angus, D.C.; Bindman, A.B. Achieving Diagnostic Excellence for Sepsis. JAMA 2022, 327, 117–118. [Google Scholar] [CrossRef]
- Wilcox, M.E.; Vaughan, K.; Chong, C.; Neumann, P.J.; Bell, C.M. Cost-Effectiveness Studies in the ICU: A Systematic Review. Crit. Care Med. 2019, 47, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Alexis Ruiz, A.; Wyszynska, P.K.; Laudanski, K. Narrative Review of Decision-Making Processes in Critical Care. Anesth. Analg. 2019, 128, 962–970. [Google Scholar] [CrossRef]
- Djulbegovic, B.; Elqayam, S.; Dale, W. Rational decision making in medicine: Implications for overuse and underuse. J. Eval. Clin. Pract. 2018, 24, 655–665. [Google Scholar] [CrossRef] [PubMed]
- Treadwell, J.R.; Lenert, L.A. Health values and prospect theory. Med. Decis. Mak. 1999, 19, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Trope, Y.; Liberman, N. Construal-level theory of psychological distance. Psychol. Rev. 2010, 117, 440–463. [Google Scholar] [CrossRef]
- Wu, G. The strengths and limitations of expected utility theory. Med. Decis. Mak. 1996, 16, 9–10. [Google Scholar] [CrossRef]
- Djulbegovic, B.; Hozo, I. Hybrid and Dual-Processing Threshold Decision Models. Cancer Treat. Res. 2023, 189, 85–92. [Google Scholar]
- Ma, L.L.; Wang, Y.Y.; Yang, Z.H.; Huang, D.; Weng, H.; Zeng, X.T. Methodological quality (risk of bias) assessment tools for primary and secondary medical studies: What are they and which is better? Mil. Med. Res. 2020, 7, 7. [Google Scholar] [CrossRef]
- Fleck, D.; Gad, H.; Hogan Quigley, B.; Antar, M.; Sayed Ahmed, A.; Mahmoud, M.A.; Laudanski, K. The Effect of Clinical Ambiguity on the Decision-Making Process Among Intensive Care Unit Providers in Northern America Using Clinical Vignettes in Mixed Methods Study. J. Multidiscip. Health 2025, 18, 3091–3104. [Google Scholar] [CrossRef]
- Veloski, J.; Tai, S.; Evans, A.S.; Nash, D.B. Clinical vignette-based surveys: A tool for assessing physician practice variation. Am. J. Med. Qual. 2005, 20, 151–157. [Google Scholar] [CrossRef]
- Barnes, J.A.; Abramson, J.S.; Scott, J.A.; Sohani, A.R. Case 35-2013—A 77-Year-Old Man with Confusion and Malaise. N. Engl. J. Med. 2013, 369, 1946–1957. [Google Scholar] [CrossRef] [PubMed]
- Callahan, P.; Young-Cureton, G.; Zalar, M.; Wahl, S. Relationship between tolerance/intolerance of ambiguity and perceived environmental uncertainty in hospitals. J. Psychosoc. Nurs. Ment. Health Serv. 1997, 35, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Geller, G.; Tambor, E.S.; Chase, G.A.; Holtzman, N.A. Measuring physicians’ tolerance for ambiguity and its relationship to their reported practices regarding genetic testing. Med. Care 1993, 31, 989–1001. [Google Scholar] [CrossRef]
- Swan, G.E.; Carmelli, D.; Dame, A.; Rosenman, R.H.; Spielberger, C.D. The Rationality/Emotional Defensiveness Scale—I. Internal structure and stability. J. Psychosom. Res. 1991, 35, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Swan, G.E.; Carmelli, D.; Dame, A.; Rosenman, R.H.; Spielberger, C.D. The Rationality/Emotional Defensiveness Scale—II. Convergent and discriminant correlational analysis in males and females with and without cancer. J. Psychosom. Res. 1992, 36, 349–359. [Google Scholar] [CrossRef]
- Gerrity, M.S.; White, K.P.; DeVellis, R.F.; Dittus, R.S. Physicians’ Reactions to Uncertainty: Refining the constructs and scales. Motiv. Emot. 1995, 19, 175–191. [Google Scholar] [CrossRef]
- Gerrity, M.S.; DeVellis, R.F.; Earp, J.A. Physicians’ reactions to uncertainty in patient care. A new measure and new insights. Med. Care 1990, 28, 724–736. [Google Scholar] [CrossRef]
- Pearson, S.D.; Goldman, L.; Orav, E.J.; Guadagnoli, E.; Garcia, T.B.; Johnson, P.A.; Lee, T.H. Triage decisions for emergency department patients with chest pain: Do physicians’ risk attitudes make the difference? J. Gen. Intern. Med. 1995, 10, 557–564. [Google Scholar] [CrossRef]
- Hamilton, K.; Shih, S.I.; Mohammed, S. The Development and Validation of the Rational and Intuitive Decision Styles Scale. J. Pers. Assess. 2016, 98, 523–535. [Google Scholar] [CrossRef]
- Hamilton, K.; Shih, S.-I.; Mohammed, S. The predictive validity of the decision styles scale: An evaluation across task types. Personal. Individ. Differ. 2017, 119, 333–340. [Google Scholar] [CrossRef]
- Scheier, M.F.; Carver, C.S. Optimism, coping, and health: Assessment and implications of generalized outcome expectancies. Health Psychol. 1985, 4, 219–247. [Google Scholar] [CrossRef]
- Lyrakos, G.N.; Damigos, D.; Mavreas, V.; Georgia, K.; Dimoliatis, I.D.Κ. A Translation and Validation Study of the Life Orientation Test Revised in the Greek Speaking Population of Nurses among Three Hospitals in Athens and Ioannina. Soc. Indic. Res. 2010, 95, 129–142. [Google Scholar] [CrossRef]
- Downes, M.J.; Brennan, M.L.; Williams, H.C.; Dean, R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open 2016, 6, e011458. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Carlet, J.M.; Bion, J.; Parker, M.M.; Jaeschke, R.; Reinhart, K.; Angus, D.C.; Brun-Buisson, C.; Beale, R.; et al. Surviving Sepsis Campaign: International guidelines for management of severe sepsis and septic shock: 2008. Crit. Care Med. 2008, 36, 296–327. [Google Scholar] [CrossRef] [PubMed]
- Dellinger, R.P.; Levy, M.M.; Rhodes, A.; Annane, D.; Gerlach, H.; Opal, S.M.; Sevransky, J.E.; Sprung, C.L.; Douglas, I.S.; Jaeschke, R.; et al. Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock: 2012. Crit. Care Med. 2013, 41, 580–637. [Google Scholar] [CrossRef] [PubMed]
- Rababa, M.; Bani Hamad, D.; Hayajneh, A.A. Sepsis assessment and management in critically Ill adults: A systematic review. PLoS ONE 2022, 17, e0270711. [Google Scholar] [CrossRef]
- Venkatesh, B.; Schlapbach, L.; Mason, D.; Wilks, K.; Seaton, R.; Lister, P.; Irwin, A.; Lane, P.; Redpath, L.; Gibbons, K. Impact of 1-hour and 3-hour sepsis time bundles on patient outcomes and antimicrobial use: A before and after cohort study. Lancet Reg. Health–West. Pac. 2022, 18, 100305. [Google Scholar] [CrossRef]
- Gao, F.; Melody, T.; Daniels, D.F.; Giles, S.; Fox, S. The impact of compliance with 6-hour and 24-hour sepsis bundles on hospital mortality in patients with severe sepsis: A prospective observational study. Crit. Care 2005, 9, R764–R770. [Google Scholar] [CrossRef]
- Castellanos-Ortega, A.; Suberviola, B.; Garcia-Astudillo, L.A.; Ortiz, F.; Llorca, J.; Delgado-Rodriguez, M. Late compliance with the sepsis resuscitation bundle: Impact on mortality. Shock 2011, 36, 542–547. [Google Scholar] [CrossRef]
- Rhee, C.; Yu, T.; Wang, R.; Kadri, S.S.; Fram, D.; Chen, H.-C.; Klompas, M.; Program, C.P.E. Association between implementation of the severe sepsis and septic shock early management bundle performance measure and outcomes in patients with suspected sepsis in US hospitals. JAMA Netw. Open 2021, 4, e2138596. [Google Scholar] [CrossRef]
- James, F.R.; Power, N.; Laha, S. Decision-making in intensive care medicine–a review. J. Intensive Care Soc. 2018, 19, 247–258. [Google Scholar] [CrossRef]
- Mason, J.; Nixon, V. Clinical decision making. In Professional Practice in Paramedic, Emergency and Urgent Care; John Wiley & Sons: Hoboken, NJ, USA, 2013; pp. 50–76. [Google Scholar]
- Thakkar, J.J. Multi-Criteria Decision Making; Springer: Berlin/Heidelberg, Germany, 2021; Volume 336. [Google Scholar]
- Medeiros, D.N.M.; Shibata, A.O.; Pizarro, C.F.; Rosa, M.d.L.A.; Cardoso, M.P.; Troster, E.J. Barriers and proposed solutions to a successful implementation of pediatric sepsis protocols. Front. Pediatr. 2021, 9, 755484. [Google Scholar] [CrossRef]
- Lafary, C.W. Implementation of a One-Hour Sepsis Bundle in the Emergency Setting; Grand Canyon University: Phoenix, AZ, USA, 2025. [Google Scholar]
- Gloss, D. An Overview of Products and Bias in Research. Neurotherapeutics 2015, 12, 731–734. [Google Scholar] [CrossRef]
- Saposnik, G.; Redelmeier, D.; Ruff, C.C.; Tobler, P.N. Cognitive biases associated with medical decisions: A systematic review. BMC Med. Inform. Decis. Mak. 2016, 16, 138. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).