Development and Validation of the Knowledge of Human Papillomavirus Scale in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. Selection of Items for the HPV Knowledge Scale
2.2. Additional Questions
2.3. Translation
2.4. Back-Translation
2.5. Cognitive Debriefing
2.6. Participants
2.7. Study Procedure
2.8. Data Analysis
2.9. Validity Assessment of the Scale
2.10. Reliability Assessment of the Scale
2.11. Ethical Considerations
3. Results
3.1. Validity Analyses
3.2. Reliability Analyses
3.3. Participants’ Knowledge Score
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HPV | Human Papillomavirus |
| J-HPV-KS | Japan HPV Knowledge Scale |
| EFA | Exploratory Factor Analysis |
| CFA | Confirmatory Factor Analysis |
| KMO | Kaiser–Meyer–Olkin |
| GFI | Goodness-of-Fit Index |
| AGFI | Adjusted Goodness-of-Fit Index |
| CFI | Comparative Fit Index |
| RMSEA | Root Mean Square Error of Approximation |
| QR | Quick Response |
| SPSS | Statistical Package for the Social Sciences |
References
- World Health Organization. Human Papillomavirus (HPV) Vaccination Coverage. Available online: https://immunizationdata.who.int/global/wiise-detail-page/human-papillomavirus-(hpv)-vaccination-coverage (accessed on 12 September 2025).
- Han, J.; Zhang, L.; Chen, Y.; Zhang, Y.; Wang, L.; Cai, R.; Li, M.; Dai, Y.; Dang, L.; Chen, H.; et al. Global HPV vaccination programs and coverage rates: A systematic review. eClinicalMedicine 2025, 84, 103290. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare. Human Papillomavirus Infection—Cervical Cancer and the HPV Vaccine. Available online: https://www.mhlw.go.jp/bunya/kenkou/kekkaku-kansenshou28/index.html (accessed on 12 September 2025). (In Japanese)
- Ministry of Health, Labour and Welfare. HPV Vaccine. Available online: https://www.mhlw.go.jp/content/10601000/000854617.pdf (accessed on 12 September 2025). (In Japanese)
- Ministry of Health, Labour and Welfare. Learn About the HPV Vaccine: The Forefront of Cervical Cancer Prevention. Available online: https://www.mhlw.go.jp/stf/houdou_kouhou/kouhou_shuppan/magazine/202205_00001.html (accessed on 12 September 2025). (In Japanese)
- Japan Medical Association. Routine Vaccinations for Human Papillomavirus Infection and Cervical Cancer. Available online: https://www.med.or.jp/people/health/kansen/011756.html (accessed on 12 September 2025). (In Japanese).
- Ministry of Health, Labour and Welfare. HPV Vaccine Implementation Status. Available online: https://www.mhlw.go.jp/content/10601000/001126459.pdf (accessed on 12 September 2025). (In Japanese)
- World Health Organization. Global Strategy to Accelerate the Elimination of Cervical Cancer as a Public Health Problem; World Health Organization: Geneva, Switzerland, 2020; Available online: https://www.who.int/publications/i/item/9789240014107 (accessed on 12 September 2025).
- Zou, Z.; Fairley, C.K.; Ong, J.J.; Shen, M.; Chow, E.P.F.; Liu, H.; Xia, R.; Li, R.; Hocking, J.; Zhuang, G.; et al. Impact of achieving WHO’s 90-70-90 targets on cervical cancer elimination and potential benefits in preventing other HPV-related cancers in China: A modelling study. eClinicalMedicine 2024, 77, 102878. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.J.; Kavanagh, K.; Cuschieri, K.; Cameron, R.; Graham, C.; Wilson, A.; Roy, K. Invasive cervical cancer incidence following bivalent human papillomavirus vaccination: A population-based observational study of age at immunization, dose, and deprivation. JNCI J. Natl. Cancer Inst. 2024, 116, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Gargano, J.W.; Wilkinson, E.J.; Unger, E.R.; Steinau, M.; Watson, M.; Huang, Y.; Copeland, G.; Cozen, W.; Goodman, M.T.; Hopenhayn, C.; et al. Prevalence of human papillomavirus types in invasive vulvar cancers and vulvar intraepithelial neoplasia 3 in the United States before vaccine introduction. J. Low. Genit. Tract Dis. 2012, 16, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Steinau, M.; Unger, E.R.; Hernandez, B.Y.; Goodman, M.T.; Copeland, G.; Hopenhayn, C.; Cozen, W.; Saber, M.S.; Huang, Y.; Peters, E.S.; et al. Human papillomavirus prevalence in invasive anal cancers in the United States before vaccine introduction. J. Low. Genit. Tract Dis. 2013, 17, 397–403. [Google Scholar] [CrossRef]
- Moreira, E.D., Jr.; Giuliano, A.R.; Palefsky, J.; Flores, C.A.; Goldstone, S.; Ferris, D.; Hillman, R.J.; Moi, H.; Stoler, M.H.; Marshall, B.; et al. Incidence, clearance, and disease progression of genital human papillomavirus infection in heterosexual men. J. Infect. Dis. 2014, 210, 192–199. [Google Scholar] [CrossRef]
- Rosado, C.; Ângela, R.F.; Acácio, G.R.; Carmen, L. Impact of human papillomavirus vaccination on male disease: A systematic review. Vaccines 2023, 11, 1083. [Google Scholar] [CrossRef]
- Kotromanović Šimić, I.; Bilić-Kirin, V.; Miskulin, M.; Kotromanović, D.; Olujić, M.; Kovacevic, J.; Nujić, D.; Pavlovic, N.; Vukoja, I.; Miskulin, I. The Influence of Health Education on Vaccination Coverage and Knowledge of the School Population Related to Vaccination and Infection Caused by the Human Papillomavirus. Vaccines 2024, 12, 1222. [Google Scholar] [CrossRef]
- OECD; European Union. Beating Cancer Inequalities in the EU: Spotlight on Cancer Prevention and Early Detection; OECD Health Policy Studies; OECD Publishing: Paris, France, 2024. [Google Scholar] [CrossRef]
- Health Systems and Policy Monitor (HSPM). Estonia Further Expands HPV Vaccination, HSPM Network. 30 January 2025. Available online: https://eurohealthobservatory.who.int/monitors/health-systems-monitor/updates/hspm/hspm-estonia-2023/estonia-further-expands-hpv-vaccination (accessed on 12 September 2025).
- Bureau of Public Health; Tokyo Metropolitan Government. HPV Vaccination for Men. Available online: https://www.hokeniryo.metro.tokyo.lg.jp/kansen/info/hpv/hpvdansei (accessed on 12 September 2025). (In Japanese).
- Quinn, S.; Goldman, R.D. Human Papillomavirus Vaccination for Boys. Can. Fam. Physician 2015, 61, 43–46. [Google Scholar] [PubMed] [PubMed Central]
- Khawar, L.; McManus, H.; Vickers, T.; Chow, E.P.F.; Fairley, C.K.; Donovan, B.; Machalek, D.A.; Regan, D.G.; Grulich, A.E.; Guy, R.J.; et al. Genital warts trends in Australian and overseas-born people in Australia: A cross-sectional trend analysis to measure progress towards control and elimination. Lancet Reg. Health West. Pac. 2021, 16, 100251. [Google Scholar] [CrossRef]
- Kirchhoff, A.C.; Mann, K.; Warner, E.L.; Kaddas, H.K.; Fair, D.; Fluchel, M.; Knackstedt, E.D.; Kepka, D. HPV vaccination knowledge, intentions, and practices among caregivers of childhood cancer survivors. Hum. Vaccin. Immunother. 2019, 15, 1767–1775. [Google Scholar] [CrossRef]
- Petrosky, E.; Bocchini, J.A.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E.; Centers for Disease Control and Prevention (CDC). Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the Advisory Committee on Immunization Practices. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. Available online: https://www.cdc.gov/mmwr/preview/mmwrhtml/mm6411a3.htm (accessed on 12 September 2025). [PubMed]
- Japanese Law Translation. Immunization Act. Available online: https://www.japaneselawtranslation.go.jp/ja/laws/view/2964/en (accessed on 12 September 2025).
- Fukutori, K.; Oda, A.; Yamamoto, C.; Nukata, A.; Hirata, C.; Ito, M. A study on the HPV vaccination experience and factors behind it among junior high school girls. J. Health Welf. Stat. 2014, 61, 26–32. Available online: https://www.hws-kyokai.or.jp/images/ronbun/all/201401-04.pdf (accessed on 12 September 2025). (In Japanese).
- Furuta, K.; Yamada, K.; Morioka, I. Relationship between explanation from guardians and preventive behaviors against uterine cervical cancer at the time of inoculation of HPV vaccine: A case involving junior high school girls. J. Jpn. Soc. Hyg. 2016, 71, 69–75. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Newman, P.A.; Logie, C.H.; Lacombe-Duncan, A.; Baiden, P.; Tepjan, S.; Rubincam, C.; Doukas, N.; Asey, F. Parents’ uptake of human papillomavirus vaccines for their children: A systematic review and meta-analysis of observational studies. BMJ Open 2018, 8, e019206. [Google Scholar] [CrossRef]
- Hanley, S.J.; Yoshioka, E.; Ito, Y.; Konno, R.; Hayashi, Y.; Kishi, R.; Sakuragi, N. Acceptance of and attitudes towards human papillomavirus vaccination in Japanese mothers of adolescent girls. Vaccine 2012, 30, 5740–5747. [Google Scholar] [CrossRef]
- Egawa-Takata, T.; Nakae, R.; Shindo, M.; Miyoshi, A.; Takiuchi, T.; Miyatake, T.; Kimura, T. Fathers. Fathers’ participation in the HPV vaccination decision-making process doesn’t increase parents’ intention to make daughters get the vaccine. Hum. Vaccin. Immunother. 2020, 16, 1653–1658. [Google Scholar] [CrossRef]
- Spleen, A.M.; Kluhsman, B.C.; Clark, A.D.; Dignan, M.B.; Lengerich, E.J.; ACTION Health Cancer Task Force. An increase in HPV-related knowledge and vaccination intent among parental and non-parental caregivers of adolescent girls, age 9–17 years, in Appalachian Pennsylvania. J. Cancer Educ. 2012, 27, 312–319. [Google Scholar] [CrossRef]
- Smolarczyk, K.; Duszewska, A.; Drozd, S.; Majewski, S. Parents’ knowledge and attitude towards HPV and HPV vaccination in Poland. Vaccines 2022, 10, 228. [Google Scholar] [CrossRef]
- Thomas, T.L.; Caldera, M.; Maurer, J. A short report: Parents HPV vaccine knowledge in rural South Florida. Hum. Vaccin. Immunother. 2019, 15, 1666–1671. [Google Scholar] [CrossRef]
- Bwanali, A.N.; Liundi, P.; Lubanga, A.F.; Mpinganjira, S.L.; Gadama, L.A. Caregiver acceptance of human papillomavirus vaccine for their female children in Chileka, Blantyre, Malawi. Vaccin. X 2024, 20, 100557. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.; Taylor, L.; Kooker, P.; Frank, I. Parent and adolescent knowledge of HPV and subsequent vaccination. Pediatrics 2014, 134, e1049–e1056. [Google Scholar] [CrossRef] [PubMed]
- Ogunbajo, A.; Hansen, C.E.; North, A.L.; Okoloko, E.; Niccolai, L.M. ‘I think they’re all basically the same’: Parents’ perceptions of human papilloma virus (HPV) vaccine compared with other adolescent vaccines. Child Care Health Dev. 2016, 42, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Cunningham-Erves, J.; Talbott, L.L.; O’Neal, M.R.; Ivankova, N.V.; Wallston, K.A. Development of a theory-based, sociocultural instrument to assess Black maternal intentions to vaccinate their daughters aged 9 to 12 against HPV. J. Cancer Educ. 2016, 31, 514–521. [Google Scholar] [CrossRef]
- Perez, S.; Tatar, O.; Ostini, R.; Shapiro, G.K.; Waller, J.; Zimet, G.; Rosberger, Z. Extending and validating a human papillomavirus (HPV) knowledge measure in a national sample of Canadian parents of boys. Prev. Med. 2016, 91, 43–49. [Google Scholar] [CrossRef]
- Thomas, T.L.; Strickland, O.L.; DiClemente, R.; Higgins, M.; Williams, B.; Hickey, K. Parental Human Papillomavirus Vaccine Survey (PHPVS): Nurse-led instrument development and psychometric testing for use in research and primary care screening. J. Nurs. Meas. 2013, 21, 96–109. [Google Scholar] [CrossRef]
- Waller, J.; Ostini, R.; Marlow, L.A.; McCaffery, K.; Zimet, G. Validation of a measure of knowledge about human papillomavirus (HPV) using item response theory and classical test theory. Prev. Med. 2013, 56, 35–40. [Google Scholar] [CrossRef]
- Harrison, S.E.; Yelverton, V.; Wang, Y.; Ostermann, J.; Fish, L.J.; Williams, C.L.; Vasudevan, L.; Walter, E.B. Examining associations between knowledge and vaccine uptake using the human papillomavirus knowledge questionnaire (HPV-KQ). Am. J. Health Behav. 2021, 45, 810–827. [Google Scholar] [CrossRef]
- Japan Society of Obstetrics and Gynecology. Japan Society of Obstetrics and Gynecology’s Stance on Introducing HPV Testing into Cervical Cancer Screening. Available online: https://www.jsog.or.jp/news_m/2163/ (accessed on 12 September 2025). (In Japanese).
- Demir Bozkurt, F.; Özdemir, S. Validity and reliability of a Turkish version of the human papillomavirus knowledge scale: A methodological study. J. Turk. Ger. Gynecol. Assoc. 2023, 24, 177–186. [Google Scholar] [CrossRef]
- Crimson Interactive Japan, Inc. Professional Translation Company. Available online: https://www.crimsonjapan.co.jp/en/ (accessed on 12 September 2025). (In Japanese).
- Google. Google Forms. Available online: https://www.google.com/forms/about/ (accessed on 12 September 2025). (In Japanese).
- Nakamura, T. How to use factor analysis in developing psychological scales. Annu. Rep. Educ. Psychol. Jpn. 2007, 46, 42–45. (In Japanese) [Google Scholar] [CrossRef]
- Chihu-Amparan, L.; Pedroza-Saavedra, A.; Gutierrez-Xicotencatl, L. The immune response generated against HPV infection in men and its implications in the diagnosis of cancer. Microorganisms 2023, 11, 1609. [Google Scholar] [CrossRef]
- Maldonado, I.; Plata, M.; Gonzalez, M.; Correa, A.; Nossa, C.; Giuliano, A.R.; Joura, E.A.; Ferenczy, A.; Ronnett, B.M.; Stoler, M.H.; et al. Effectiveness, immunogenicity, and safety of the quadrivalent HPV vaccine in women and men aged 27–45 years. Hum. Vaccin. Immunother. 2022, 18, 2078626. [Google Scholar] [CrossRef]
- Olsson, S.E.; Villa, L.L.; Costa, R.L.; Petta, C.A.; Andrade, R.P.; Malm, C.; Iversen, O.E.; Høye, J.; Steinwall, M.; Riis-Johannessen, G.; et al. Induction of immune memory following administration of a prophylactic quadrivalent human papillomavirus (HPV) types 6/11/16/18 L1 virus-like particle (VLP) vaccine. Vaccine 2007, 25, 4931–4939. [Google Scholar] [CrossRef]
- Giuliano, A.R.; Isaacs-Soriano, K.; Torres, B.N.; Abrahamsen, M.; Ingles, D.J.; Sirak, B.A.; Quiterio, M.; Lazcano-Ponce, E. Immunogenicity and safety of Gardasil among mid-adult aged men (27–45 years)—The MAM Study. Vaccine 2015, 33, 5640–5646. [Google Scholar] [CrossRef]
- Naidoo, D.; Govender, K.; Mantell, J.E. Breaking barriers: Why including boys and men is key to HPV prevention. BMC Med. 2024, 22, 525. [Google Scholar] [CrossRef]
| Item No. | General HPV Knowledge |
|---|---|
| 1 | HPV can cause cervical cancer |
| 2 | A person could have HPV for many years without knowing it |
| 3 | Having many sexual partners increases the risk of getting HPV |
| 4 | HPV is very rare (F) |
| 5 | HPV can be passed on during sexual intercourse |
| 6 | HPV always has visible signs or symptoms (F) |
| 7 | Using condoms reduces the risk of getting HPV |
| 8 | HPV can cause HIV/AIDS (F) |
| 9 | HPV can be passed on by genital skin-to-skin contact |
| 10 | Men cannot get HPV (F) |
| 11 | Having sex at an early age increases the risk of getting HPV |
| 12 | There are many types of HPV |
| 13 | HPV can cause genital warts |
| 14 | HPV can be cured with antibiotics |
| 15 | Most sexually active people will get HPV at some point in their lives |
| 16 | HPV usually doesn’t need any treatment |
| 17 | HPV can cause anal cancer |
| 18 | HPV can cause penile cancer |
| 19 | HPV can cause pharyngeal cancer |
| HPV Vaccination Knowledge | |
| 20 | Girls who have the HPV vaccine do not need [Pap test/Smear test/Pap smear test] when they are older (F) |
| 21 | One of the HPV vaccines offers protection against genital warts |
| 22 | Th The HPV vaccines offer protection against all sexually transmitted infections (F) |
| 23 | Someone who has had HPV vaccine cannot develop cervical cancers (F) |
| 24 | The HPV vaccines offer protection against most cervical cancers |
| 25 | HPV vaccines require three doses |
| 26 | The HPV vaccines are most effective if given to people who have never had sex |
| 27 | The incidence of various symptoms (widespread pain, difficulty moving arms and legs, etc.) observed after HPV vaccination is less than 10 per 10,000 people |
| 28 | The causal relationship between the various symptoms observed after HPV vaccination and HPV vaccination has not been proven |
| 29 | Scientific verification has confirmed that the benefits of HPV vaccination outweigh the risk of side effects |
 Original item;
Original item;  Added item; (F): False.
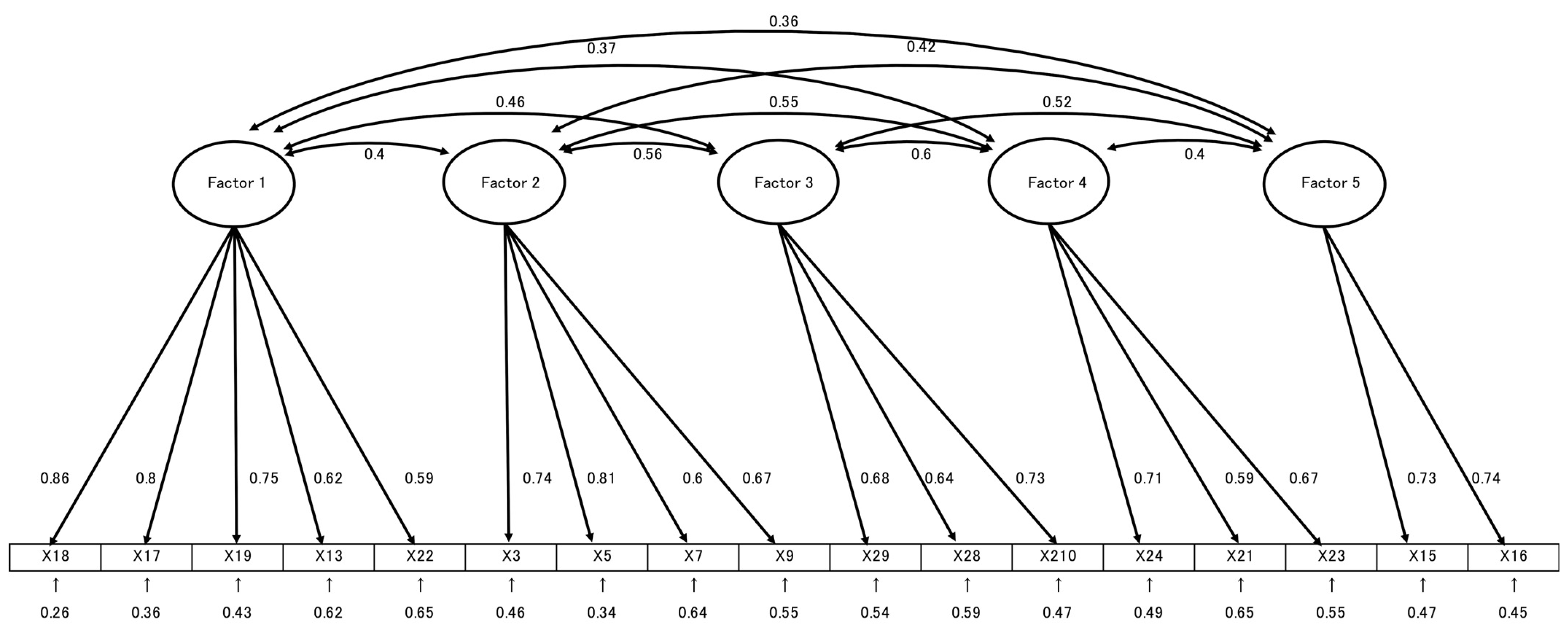
Added item; (F): False.| Items | Factor Loading | Communality | ||||
|---|---|---|---|---|---|---|
| Factor 1 | Factor 2 | Factor 3 | Factor 4 | Factor 5 | ||
| Factor 1: Risk of HPV-related diseases and prevention (α: 0.845) | ||||||
| Item 18 | 0.896 | 0.759 | ||||
| Item 17 | 0.895 | 0.696 | ||||
| Item 19 | 0.748 | 0.571 | ||||
| Item 13 | 0.485 | 0.410 | ||||
| Item 21 | 0.424 | 0.397 | ||||
| Item 18 | 0.896 | 0.759 | ||||
| Factor 2: Pathways of HPV transmission and prevention (α: 0.784) | ||||||
| Item 3 | 0.821 | 0.594 | ||||
| Item 5 | 0.788 | 0.656 | ||||
| Item 7 | 0.643 | 0.377 | ||||
| Item 9 | 0.530 | 0.432 | ||||
| Factor 3: Safety and effectiveness of the HPV vaccine (α: 0.724) | ||||||
| Item 28 | 0.737 | 0.488 | ||||
| Item 27 | 0.729 | 0.458 | ||||
| Item 29 | 0.576 | 0.458 | ||||
| Factor 4: Limitations of HPV vaccine efficacy (α: 0.688) | ||||||
| Item 23 | 0.813 | 0.585 | ||||
| Item 20 | 0.588 | 0.361 | ||||
| Item 22 | 0.565 | 0.407 | ||||
| Factor 5: HPV susceptibility and natural history (α: 0.702) | ||||||
| Item 15 | 0.790 | 0.577 | ||||
| Item 16 | 0.710 | 0.509 | ||||
| Factor 1 | Factor2 | Factor 3 | Factor 4 | Factor 5 | ||
| Factor 1 | ||||||
| Factor 2 | 0.386 | |||||
| Factor 3 | 0.472 | 0.537 | ||||
| Factor 4 | 0.361 | 0.542 | 0.558 | |||
| Factor 5 | 0.385 | 0.416 | 0.541 | 0.407 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tokuda, A.; Shiota, A.; Wangmo, P.; Kawata, K. Development and Validation of the Knowledge of Human Papillomavirus Scale in Japan. Healthcare 2025, 13, 2536. https://doi.org/10.3390/healthcare13192536
Tokuda A, Shiota A, Wangmo P, Kawata K. Development and Validation of the Knowledge of Human Papillomavirus Scale in Japan. Healthcare. 2025; 13(19):2536. https://doi.org/10.3390/healthcare13192536
Chicago/Turabian StyleTokuda, Ayano, Atsuko Shiota, Pasang Wangmo, and Kimiko Kawata. 2025. "Development and Validation of the Knowledge of Human Papillomavirus Scale in Japan" Healthcare 13, no. 19: 2536. https://doi.org/10.3390/healthcare13192536
APA StyleTokuda, A., Shiota, A., Wangmo, P., & Kawata, K. (2025). Development and Validation of the Knowledge of Human Papillomavirus Scale in Japan. Healthcare, 13(19), 2536. https://doi.org/10.3390/healthcare13192536






