Advancements and Future Perspectives of Human Papillomavirus (HPV) Vaccination in Latin America: Insights from Recent Decades
Abstract
1. Introduction
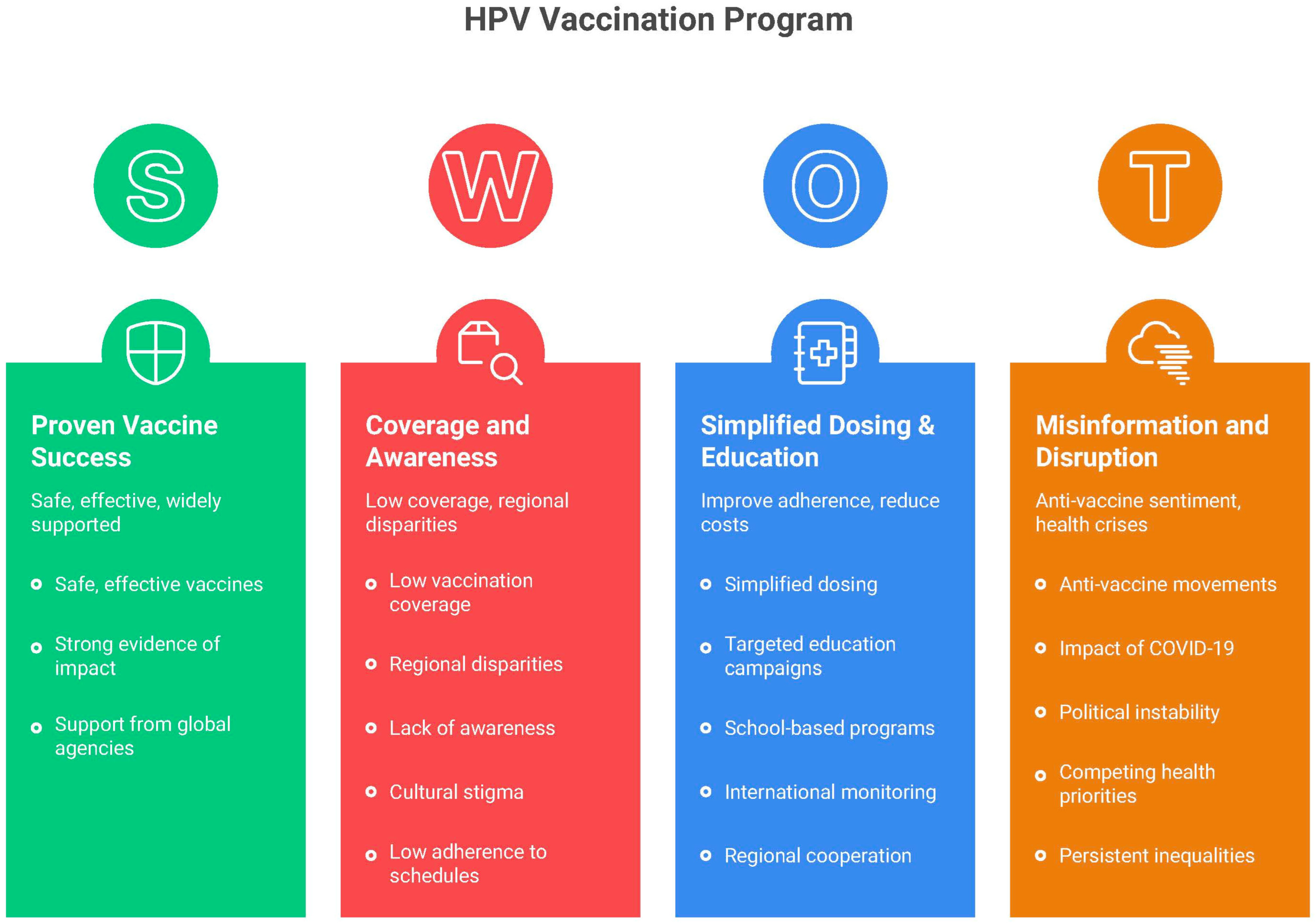
2. Epidemiology of HPV-Related Cancers in Latin America
3. Vaccine Types, Coverage and Efficacy
4. Vaccine Implementation in LATAM—Background, National Programs and Countries’ Target Populations
5. Current Landscape of HPV Vaccination in Latin America
6. Barriers and Challenges
7. Potential Solutions to Overcome Barriers and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Crosbie, E.J.; Einstein, M.H.; Franceschi, S.; Kitchener, H.C. Human papillomavirus and cervical cancer. Lancet 2013, 382, 889–899. [Google Scholar] [CrossRef]
- Wei, F.; Georges, D.; Man, I.; Baussano, I.; Clifford, G.M. Causal attribution of human papillomavirus genotypes to invasive cervical cancer worldwide: A systematic analysis of the global literature. Lancet 2024, 404, 435–444. [Google Scholar] [CrossRef]
- Paavonen, J.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.-N.; Apter, D.; Kitchener, H.; Castellsague, X.; Teixeira, J.C.; Skinner, S.R.; et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): Final analysis of a double-blind, randomised study in young women. Lancet 2009, 374, 301–314. [Google Scholar] [CrossRef]
- Future II Study Group. Quadrivalent vaccine against human papillomavirus to prevent high-grade cervical lesions. N. Engl. J. Med. 2007, 356, 1915–1927. [Google Scholar] [CrossRef]
- Huh, W.K.; Joura, E.A.; Giuliano, A.R.; Iversen, O.-E.; De Andrade, R.P.; Ault, K.A.; Bartholomew, D.; Cestero, R.M.; Fedrizzi, E.N.; Hirschberg, A.L.; et al. Final efficacy, immunogenicity, and safety analyses of a nine-valent human papillomavirus vaccine in women aged 16–26 years: A randomised, double-blind trial. Lancet 2017, 390, 2143–2159. [Google Scholar] [CrossRef]
- Saraiya, M.; Unger, E.R.; Thompson, T.D.; Lynch, C.F.; Hernandez, B.Y.; Lyu, C.W.; Steinau, M.; Watson, M.; Wilkinson, E.J.; Hopenhayn, C.; et al. US Assessment of HPV Types in Cancers: Implications for Current and 9-Valent HPV Vaccines. J. Natl. Cancer Inst. 2015, 107, djv086. [Google Scholar] [CrossRef]
- Gultekin, M.; Ramirez, P.T.; Broutet, N.; Hutubessy, R. World Health Organization call for action to eliminate cervical cancer globally. Int. J. Gynecol. Cancer 2020, 30, 426–427. [Google Scholar] [CrossRef]
- IARC Launches Website for the Center of Excellence for Monitoring HPV Vaccination Impact (CHRONOS). Available online: https://www.iarc.who.int/news-events/iarc-launches-website-for-the-center-of-excellence-for-monitoring-hpv-vaccination-impact-chronos (accessed on 31 August 2025).
- Drolet, M.; Bénard, É.; Pérez, N.; Brisson, M. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef] [PubMed]
- De Martel, C.; Plummer, M.; Vignat, J.; Franceschi, S. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int. J. Cancer 2017, 141, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Rositch, A.F. Global burden of cancer attributable to infections: The critical role of implementation science. Lancet Glob. Health 2020, 8, e153–e154. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Human Papillomavirus (HPV) Vaccination Coverage. Available online: https://immunizationdata.who.int/global/wiise-detail-page/human-papillomavirus-(hpv)-vaccination-coverage (accessed on 16 February 2025).
- Kirnbauer, R.; Booy, F.; Cheng, N.; Lowy, D.R.; Schiller, J.T. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 1992, 89, 12180–12184. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, L.; Fu, K.; Shen, Y.; Lu, Y.; Liao, Z.; Liu, Y.; Zha, W.; Wu, L.; Zhang, Y. Effects of different-valent vaccines against human papillomavirus (HPV) to prevent persistent HPV16/18 infections and CIN2+ in women: A systematic review and network meta-analysis. Int. J. Infect. Dis. 2024, 151, 107363. [Google Scholar] [CrossRef] [PubMed]
- Paavonen, J.; Jenkins, D.; Bosch, F.X.; Naud, P.; Salmerón, J.; Wheeler, C.M.; Chow, S.-N.; Apter, D.L.; Kitchener, H.C.; Castellsague, X.; et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: An interim analysis of a phase III double-blind, randomised controlled trial. Lancet 2007, 369, 2161–2170. [Google Scholar] [CrossRef]
- Garland, S.M.; Steben, M.; Sings, H.L.; James, M.; Lu, S.; Railkar, R.; Barr, E.; Haupt, R.M.; Joura, E.A. Natural history of genital warts: Analysis of the placebo arm of 2 randomized phase III trials of a quadrivalent human papillomavirus (types 6, 11, 16, and 18) vaccine. J. Infect. Dis. 2009, 199, 805–814. [Google Scholar] [CrossRef]
- Moreira, E.D.; Block, S.L.; Ferris, D.; Giuliano, A.R.; Iversen, O.-E.; Joura, E.A.; Kosalaraksa, P.; Schilling, A.; Van Damme, P.; Bornstein, J.; et al. Safety Profile of the 9-Valent HPV Vaccine: A Combined Analysis of 7 Phase III Clinical Trials. Pediatrics 2016, 138, e20154387. [Google Scholar] [CrossRef]
- Garland, S.M.; Kjaer, S.K.; Muñoz, N.; Block, S.L.; Brown, D.R.; DiNubile, M.J.; Lindsay, B.R.; Kuter, B.J.; Perez, G.; Dominiak-Felden, G.; et al. Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: A Systematic Review of 10 Years of Real-world Experience. Clin. Infect. Dis. 2016, 63, 519–527. [Google Scholar] [CrossRef]
- Markowitz, L.E.; Hariri, S.; Lin, C.; Dunne, E.F.; Steinau, M.; McQuillan, G.; Unger, E.R. Reduction in human papilloma-virus (HPV) prevalence among young women following HPV vaccine introduction in the United States, Nation-al Health and Nutrition Examination Surveys. J. Infect. Dis. 2013, 208, 385–393. [Google Scholar]
- Garland, S.; Brotherton, J.; Moscicki, A.; Kaufmann, A.; Stanley, M.; Bhatla, N.; Sankaranarayanan, R.; de Sanjosé, S.; Palefsky, J. HPV vaccination of immunocompromised hosts. Papillomavirus Res. 2017, 4, 35–38. [Google Scholar] [CrossRef]
- Zhai, L.; Tumban, E. Gardasil-9: A global survey of projected efficacy. Antivir. Res. 2016, 130, 101–109. [Google Scholar] [CrossRef]
- World Health Organization. Human Papillomavirus Vaccines: WHO Position Paper. March 2023. Available online: https://cdn.who.int/media/docs/default-source/immunization/position_paper_documents/human-papillomavirus-(hpv)/hpv-background-document--report-march-2022.pdf?sfvrsn=b600e252_1 (accessed on 18 February 2025).
- Nogueira-Rodrigues, A. HPV Vaccination in Latin America: Global Challenges and Feasible Solutions. Am. Soc. Clin. Oncol. Educ. Book 2019, 39, e45–e52. [Google Scholar] [CrossRef]
- Robles, C.; Hernández, M.d.l.L.; Almonte, M. Alternative HPV vaccination schedules in Latin America. Salud Publica De Mex. 2018, 60, 693–702. [Google Scholar] [CrossRef]
- Romero-Feregrino, R.; Romero-Cabello, R.; Romero-Feregrino, R.; Vilchis-Mora, P.; Muñoz-Cordero, B.; Rodríguez-León, M.A. Sixteen Years of HPV Vaccination in Mexico: Report of the Coverage, Procurement, and Program Performance (2008–2023). Int. J. Environ. Res. Public Health 2025, 22, 1028. [Google Scholar] [CrossRef]
- Soares, L.M.C.; de Medonça, A.E.O.; de Souza, D.L.B.; Carvalho, A.V.; Flores, M.G.; Neto, A.M.; de Andrade, D.A.P.; Rodrigues, A.N. Factors associated with HPV vaccine hesitancy: A nationally representative cross-sectional study. Vaccine 2025, 59, 127278. [Google Scholar] [CrossRef]
- Nogueira-Rodrigues, A.; Flores, M.G.; Neto, A.O.M.; Braga, L.A.C.; Vieira, C.M.; de Sousa-Lima, R.M.; de Andrade, D.A.P.; Machado, K.K.; Guimarães, A.P.G. HPV vaccination in Latin America: Coverage status, implementation challenges and strategies to overcome it. Front. Oncol. 2022, 12, 984449. [Google Scholar] [CrossRef]
- Stanley, M.; Schuind, A.; Muralidharan, K.K.; Guillaume, D.; Willens, V.; Borda, H.; Jurgensmeyer, M.; Limaye, R. Evidence for an HPV one-dose schedule. Vaccine 2024, 42, S16–S21. [Google Scholar] [CrossRef]
- Roteli-Martins, C.M.; Maranhão, A.G.K.; Fialho, S.C.A.V.; da Silva-Filho, A.L. The importance of the quadrivalent HPV vaccine in the elimination of cervical cancer in Brazil. Rev. Bras. Ginecol. Obstetrícia 2024, 46. [Google Scholar] [CrossRef] [PubMed]
- Han, J.; Zhang, L.; Chen, Y.; Zhang, Y.; Wang, L.; Cai, R.; Li, M.; Dai, Y.; Dang, L.; Chen, H.; et al. Global HPV vaccination programs and coverage rates: A systematic review. eClinicalMedicine 2025, 84, 103290. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fernández-Deaza, G.; Serrano, B.; Roura, E.; Castillo, J.S.; Caicedo-Martínez, M.; Bruni, L.; Murillo, R. Cervical cancer screening coverage in the Americas region: A synthetic analysis. Lancet Reg. Health Am. 2024, 30, 100689. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira-Silva, T.; Barral-Netto, M.; Boaventura, V.S. Effect of Brazil’s national human papillomavirus vaccination programme on the incidence of cervical cancer and cervical intraepithelial neoplasia grade 3 in women aged 20–24 years: A population-based study. Lancet Glob. Health 2025, 13, e1715–e1722. [Google Scholar] [CrossRef]
- Da Silva, T.M.R.; da Sá, A.C.M.G.; Carrato, B.A.; Siqueira Costa Schreck, R.; Prates, E.J.S.; Oliveira, S.R.D.; Malta, D.C. Lack of knowledge about the human papillomavirus vaccine among Brazilian adolescents: A cross-sectional study. Public Health Nurs. 2024, 41, 1453–1465. [Google Scholar] [CrossRef]
- Lobão, W.M.; Duarte, F.G.; Burns, J.D.; Santos, C.A.D.S.T.; De Almeida, M.C.C.; Reingold, A.; Moreira, E.D. Low coverage of HPV vaccination in the national immunization programme in Brazil: Parental vaccine refusal or barriers in health-service based vaccine delivery? PLoS ONE 2018, 13, e0206726. [Google Scholar] [CrossRef]
- McRee, A.-L.; Gilkey, M.B.; Dempsey, A.F. HPV vaccine hesitancy: Findings from a statewide survey of health care providers. J. Pediatr. Health Care 2014, 28, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Coleman, D.C.; Markham, C.; Guilamo-Ramos, V.; Maria, D.S. Relationship between religiosity and HPV vaccine initiation and intention in urban black and hispanic parents. BMC Public Health 2024, 24, 265. [Google Scholar] [CrossRef] [PubMed]
- Gilkey, M.B.; Malo, T.L.; Shah, P.D.; Hall, M.E.; Brewer, N.T. Quality of Physician Communication about Human Papillomavirus Vaccine: Findings from a National Survey. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1673–1679. [Google Scholar] [CrossRef]
- de Carvalho, N.S.; da Silva, R.J.d.C.; do Val, I.C.; Bazzo, M.L.; da Silveira, M.F. Protocolo Brasileiro para Infecções Sexualmente Transmissíveis 2020: Infecção pelo papilomavírus humano (HPV). Epidemiol Serv Saúde 2021, 30, e2020790. [Google Scholar] [CrossRef]
- Wagner, A.; Juvalta, S.; Speranza, C.; Suggs, L.S.; Drava, J.; COVIDisc Study Group. Let’s talk about COVID-19 vaccination: Relevance of conversations about COVID-19 vaccination and information sources on vaccination intention in Switzerland. Vaccine 2023, 41, 5313–5321. [Google Scholar] [CrossRef] [PubMed]
- Chao, C.; Velicer, C.; Slezak, J.M.; Jacobsen, S.J. Correlates for completion of 3-dose regimen of HPV vaccine in female members of a managed care organization. Mayo Clin Proc. 2009, 84, 864–870. [Google Scholar] [CrossRef]
- Liu, G.; Kong, L.; Du, P. HPV vaccine completion and dose adherence among commercially insured females aged 9 through 26 years in the US. Papillomavirus Res. 2015, 2, 1–8. [Google Scholar] [CrossRef]
- Santos, W.M.; Santos, D.M.; Fernandes, M.S. HPV immunization in Brazil and proposals to increase adherence to vaccination campaigns. Rev. Saude Publica 2023, 57, 79. [Google Scholar] [CrossRef]
- Puri, N.; Coomes, E.A.; Haghbayan, H.; Gunaratne, K. Social media and vaccine hesitancy: New updates for the era of COVID-19 and globalized infectious diseases. Hum. Vaccines Immunother. 2020, 16, 2586–2593. [Google Scholar] [CrossRef]
- Reimold, A.E.; D’ANgelo, H.; Rose, S.W.; Ribisl, K.M. Changes in retail tobacco product sales and market share among retail payroll establishments in the U.S. Economic Census between 1997 and 2017. Prev. Med. Rep. 2023, 35, 102294. [Google Scholar] [CrossRef]
- de Moura, I.A.; Silva, A.J.D.; de Macêdo, L.S.; Melo, K.M.T.B.d.; Leal, L.R.S.; Espinoza, B.C.F.; Invenção, M.d.C.V.; Pinho, S.S.d.; Freitas, A.C.d. Advances in the Functionalization of Vaccine Delivery Systems: Innovative Strategies and Translational Perspectives. Pharmaceutics 2025, 17, 640. [Google Scholar] [CrossRef]
- Rai, S.; Kornides, M.; Morgan, J.; Kumar, A.; Cappella, J.; Guntuku, S.C. Detecting and monitoring concerns against HPV vaccination on social media using large language models. Sci. Rep. 2024, 14, 14362. [Google Scholar] [CrossRef]
- Wight, A.J. Latin America is catching up on HPV vaccination and screening. BMJ 2024, 386, q1553. [Google Scholar] [CrossRef]
- Bahena, M.; Carvajal-Suarez, M.; Soliman, A.S.; Luo, J.; De Alba, A. The influence of medical providers on HPV vaccination among children of Mexican mothers: A comparison between Mexico and the Midwest region of the United States. BMC Public Health 2019, 19, 515. [Google Scholar] [CrossRef]
- Clavé Llavall, A.; de Wildt, G.; Meza, G.; Tattsbridge, J.; Jones, L. Nurses’ and teachers’ perceived barriers and facilitators to the uptake of the Human Papilloma Virus (HPV) vaccination program in Iquitos, Peru: A qualitative study. PLoS ONE 2021, 16, e0255218. [Google Scholar] [CrossRef]
- Leader, A.E.; Burke-Garcia, A.; Afanaseva, D.; Cutroneo, E.; Selvan, P.; Madden, K.; Banks, J.; Sustaita-Ruiz, A. Partnering with social media influencers to promote HPV vaccination in diverse communities. Vaccine 2025, 53, 127085. [Google Scholar] [CrossRef]
- Taumberger, N.A.; Joura, E.; Arbyn, M.; Kyrgiou, M.; Sehouli, J.; Gultekin, M. Myths and fake messages about human papillomavirus (HPV) vaccination: Answers from the ESGO Prevention Committee. Int. J. Gynecol. Cancer 2022, 32, 1316–1320. [Google Scholar] [CrossRef]
- Thilly, N.; Michel, M.; Simon, M.; Bocquier, A.; Gagneux-Brunon, A.; Gauchet, A.; Gilberg, S.; Le Duc-Banaszuk, A.-S.; Bruel, S.; Mueller, J.E.; et al. Effectiveness of a School- and Primary Care–Based HPV Vaccination Intervention. JAMA Netw. Open 2024, 7, e2411938. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.; Joshi, S.; Muwonge, R.; Esmy, P.O.; Basu, P.; Prabhu, P.; Bhatla, N.; Nene, B.M.; Shaw, J.; Poli, U.R.R.; et al. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine 2018, 36, 4783–4791. [Google Scholar] [CrossRef] [PubMed]
- Rockliffe, L.; McBride, E.; Heffernan, C.; Forster, A.S. Factors Affecting Delivery of the HPV Vaccination: A Focus Group Study With NHS School-Aged Vaccination Teams in London. J. Sch. Nurs. 2018, 36, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Vujovich-Dunn, C.; Wand, H.; Brotherton, J.M.L.; Gidding, H.; Sisnowski, J.; Lorch, R.; Veitch, M.; Sheppeard, V.; Effler, P.; Skinner, S.R.; et al. Measuring school level attributable risk to support school-based HPV vaccination programs. BMC Public Health 2022, 22, 822. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.T.; Simms, K.T.; Lew, J.-B.; Smith, M.A.; Saville, M.; Canfell, K. Projected future impact of HPV vaccination and primary HPV screening on cervical cancer rates from 2017–2035: Example from Australia. PLoS ONE 2018, 13, e0185332. [Google Scholar] [CrossRef]
- Brotherton, J.M.; Gertig, D.M.; May, C.; Chappell, G.; Saville, M. HPV vaccine impact in Australian women: Ready for an HPV-based screening program. Med. J. Aust. 2016, 204, 184. [Google Scholar] [CrossRef][Green Version]
- Lew, J.-B.; Simms, K.T.; Smith, M.A.; Hall, M.; Kang, Y.-J.; Xu, X.M.; Caruana, M.; Velentzis, L.S.; Bessell, T.; Saville, M.; et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: Effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health 2017, 2, e96–e107. [Google Scholar] [CrossRef]
- Australia on Track to Achieve Cervical Cancer Elimination by 2035—But There is Work to Done—ACPCC. 2025. Available online: https://acpcc.org.au/australia-on-track-to-achieve-cervical-cancer-elimination-by-2035-but-there-is-work-to-done/ (accessed on 18 September 2025).
- Luciani, S.; Bruni, L.; Agurto, I.; Ruiz-Matus, C. HPV vaccine implementation and monitoring in Latin America. Salud Publica Mex. 2018, 60, 683–692. [Google Scholar] [CrossRef]
- Gallagher, K.; LaMontagne, D.; Watson-Jones, D. Status of HPV vaccine introduction and barriers to country uptake. Vaccine 2018, 36, 4761–4767. [Google Scholar] [CrossRef]

| Country | Year of HPV Vaccine Implementation | Target Sex | Schedule (9–14 Years Old) | HPV Vaccination Program Coverage, First Dose, Females (2023) | HPV Vaccination Program Coverage, Last Dose, Females (2023) | HPV Vaccine Primary Delivery Strategy |
|---|---|---|---|---|---|---|
| Argentina | 2011 | F/M | 1 | 63% | 36% | MX |
| Bolivia | 2017 | F | 1 | 67% | 67% | SB |
| Brazil | 2014 | F/M | 1 | 87% | 73% | FB |
| Chile | 2014 | F/M | 2 | 88% | 83% | SB |
| Colombia | 2012 | F/M | 1 | 52% | 52% | SB |
| Costa Rica | 2019 | F | 2 | 77% | 66% | SB |
| Dominican Republic | 2017 | F | 1 | 66% | 66% | FB |
| Ecuador | 2014 | F/M | 2 | 79% | 50% | SB |
| El Salvador | 2020 | F/M | 1 | 76% | 36% | SB |
| Guatemala | 2018 | F/M | 1 | 48% | 48% | SB |
| Guyana | 2011 | F/M | 1 | 40% | 40% | SB |
| Honduras | 2016 | F | 1 | 81% | 61% | SB |
| Mexico | 2012 | F | 1 | 62% | 62% | SB |
| Nicaragua | 2023 | F | 2 | 78% | N/A | SB |
| Panama | 2008 | F/M | 2 | 79% | 66% | MB |
| Paraguay | 2013 | F/M | 1 | 44% | 22% | MB |
| Peru | 2015 | F/M | 1 | 74% | 74% | SB |
| Suriname | 2013 | F/M | 1 | N/A | N/A | SB |
| Uruguay | 2013 | F/M | 2 | 67% | 41% | FB |
| 2023 | 2022 | 2021 | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 | 2014 | 2013 | 2012 | 2011 | 2010 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| First dose—females | 68 | 69 | 53 | 57 | 60 | 66 | 69 | 62 | 60 | 64 | 38 | 32 | 17 | 11 |
| First dose—males | 43 | 39 | 39 | 38 | 39 | 38 | 27 | 15 | 14 | 11 | 9 | 5 | 3 | 0 |
| Last dose—females | 55 | 54 | 39 | 39 | 54 | 53 | 58 | 51 | 43 | 44 | 29 | 24 | 10 | 6 |
| Last dose—males | 33 | 29 | 28 | 26 | 26 | 28 | 13 | 9 | 7 | 4 | 3 | 2 | 1 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonalumi dos Santos, M.; Parenza Arenhardt, M.; Vieira Giannecchini, G.; Müller Gomes, L.; Lopes da Silva, J.; Alves Pinto de Andrade, D.; de Melo, A.C. Advancements and Future Perspectives of Human Papillomavirus (HPV) Vaccination in Latin America: Insights from Recent Decades. Healthcare 2025, 13, 2502. https://doi.org/10.3390/healthcare13192502
Bonalumi dos Santos M, Parenza Arenhardt M, Vieira Giannecchini G, Müller Gomes L, Lopes da Silva J, Alves Pinto de Andrade D, de Melo AC. Advancements and Future Perspectives of Human Papillomavirus (HPV) Vaccination in Latin America: Insights from Recent Decades. Healthcare. 2025; 13(19):2502. https://doi.org/10.3390/healthcare13192502
Chicago/Turabian StyleBonalumi dos Santos, Marcela, Martina Parenza Arenhardt, Giovanna Vieira Giannecchini, Larissa Müller Gomes, Jessé Lopes da Silva, Diocesio Alves Pinto de Andrade, and Andréia Cristina de Melo. 2025. "Advancements and Future Perspectives of Human Papillomavirus (HPV) Vaccination in Latin America: Insights from Recent Decades" Healthcare 13, no. 19: 2502. https://doi.org/10.3390/healthcare13192502
APA StyleBonalumi dos Santos, M., Parenza Arenhardt, M., Vieira Giannecchini, G., Müller Gomes, L., Lopes da Silva, J., Alves Pinto de Andrade, D., & de Melo, A. C. (2025). Advancements and Future Perspectives of Human Papillomavirus (HPV) Vaccination in Latin America: Insights from Recent Decades. Healthcare, 13(19), 2502. https://doi.org/10.3390/healthcare13192502






