Differential Impacts of Extreme Weather Events on Vector-Borne Disease Transmission Across Urban and Rural Settings: A Scoping Review
Abstract
1. Introduction
2. Methodology
2.1. Study Design and Research Questions
2.2. Eligibility Criteria and Search Strategy

2.3. Data Charting, Extraction, Synthesis and Analysis
3. Results
3.1. Search Results and Study Characteristics
3.2. Quality Assessment
3.3. Climate Change and Extreme Weather Impacts on VBD Transmission
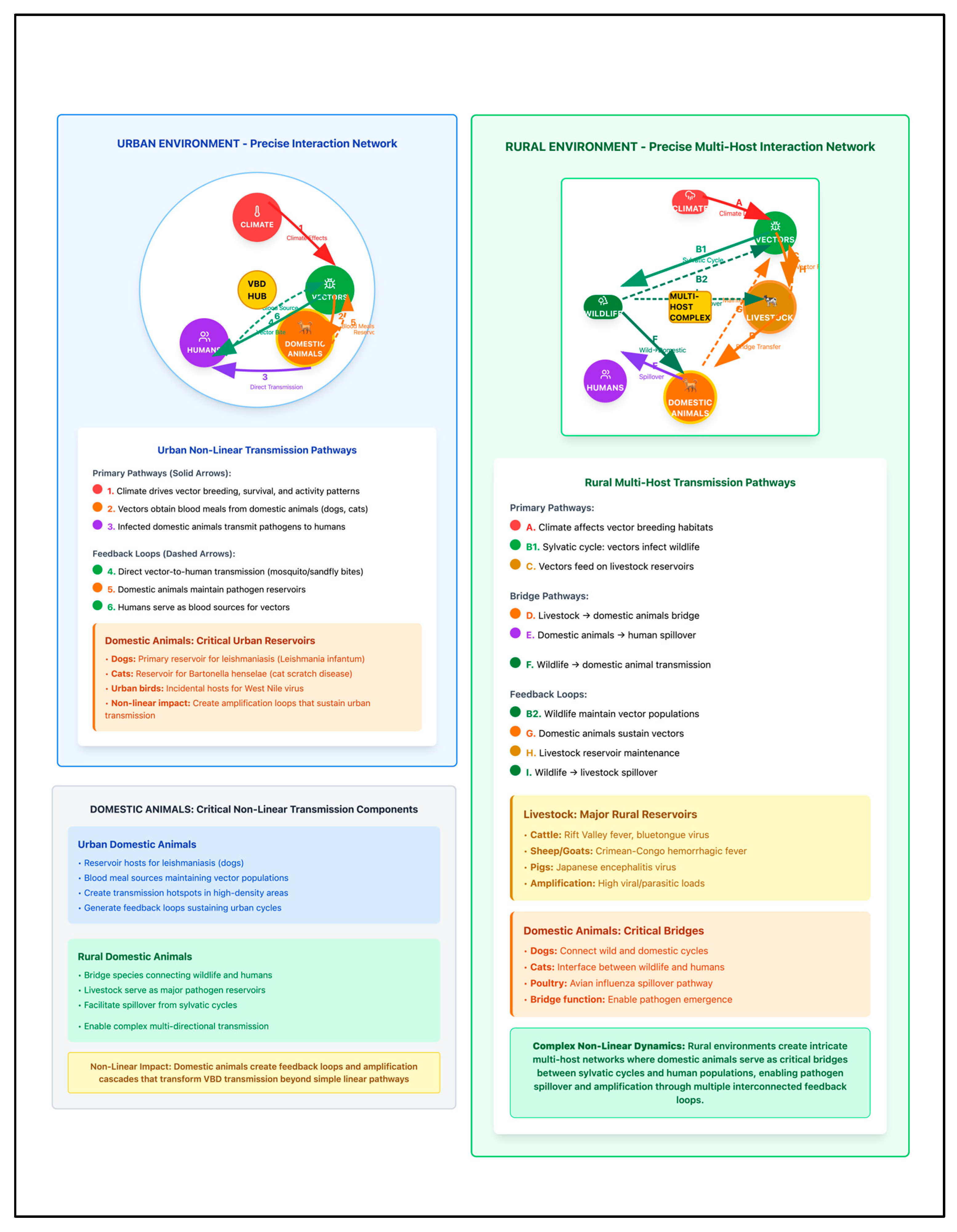
3.4. Urban Settings: Infrastructure-Mediated Transmission Dynamics and Vulnerabilities
3.5. Rural Settings: Ecosystem-Mediated Transmission Dynamics and Vulnerabilities
3.6. Comparative Analysis: Urban vs. Rural Differences
3.7. Adaptation and Response Strategies
4. Evidence Gaps and Research Needs
5. Discussion
5.1. Key Findings: Differential Climate–VBD Dynamics Across Settlement Types
5.2. Vulnerability Patterns and Adaptive Capacity Analysis
5.3. Settlement-Specific Implementation Requirements and Policy Implications
5.4. Future Directions and Recommendations
5.5. Study Limitations
6. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. Climate Change. 12 October 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/climate-change-and-health (accessed on 4 March 2025).
- Rocklöv, J.; Dubrow, R. Climate change: An enduring challenge for vector-borne disease prevention and control. Nat. Immunol. 2020, 21, 479–483. [Google Scholar] [CrossRef]
- WHO. Vector-Borne Diseases; 26 September 2024. Available online: https://www.who.int/news-room/fact-sheets/detail/vector-borne-diseases (accessed on 4 March 2025).
- Watts, N.; Amann, M.; Arnell, N.; Ayeb-Karlsson, S.; Beagley, J.; Belesova, K.; Boykoff, M.; Byass, P.; Cai, W.; Campbell-Lendrum, D.; et al. The 2020 report of The Lancet Countdown on health and climate change: Responding to converging crises. Lancet 2021, 397, 129–170. [Google Scholar] [CrossRef] [PubMed]
- WHO. WHO Global Strategy on Health, Environment and Climate Change: The Transformation Needed to Improve Lives and Wellbeing Sustainably Through Healthy Environments; Meeting Report; WHO: Geneva, Switzerland, 2020. [Google Scholar]
- Kraemer, M.U.G.; Sinka, M.E.; Duda, K.A.; Mylne, A.Q.N.; Shearer, F.M.; Barker, C.M.; Moore, C.G.; Carvalho, R.G.; Coelho, G.E.; Van Bortel, W.; et al. The global distribution of the arbovirus vectors Aedes aegypti and Ae. albopictus. eLife 2015, 4, e08347. [Google Scholar] [CrossRef]
- Colón-González, F.J.; Harris, I.; Osborn, T.J.; Steiner São Bernardo, C.; Peres, C.A.; Hunter, P.R.; Warren, R.; van Vuurene, D.; Lake, I.R. Limiting global-mean temperature increase to 1.5–2 °C could reduce the incidence and spatial spread of dengue fever in Latin America. Proc. Natl. Acad. Sci. USA 2018, 115, 6243–6248. [Google Scholar] [CrossRef]
- Tesla, B.; Demakovsky, L.R.; Mordecai, E.A.; Ryan, S.J.; Bonds, M.H.; Ngonghala, C.N.; Brindley, M.A.; Murdock, C.C. Temperature drives Zika virus transmission: Evidence from empirical and mathematical models. Proc. R. Soc. B Biol. Sci. 2018, 285, 20180795. [Google Scholar] [CrossRef] [PubMed]
- WHO. World Malaria Report 2023; World Health Organization: Geneva, Switzerland, 2023; p. 283. [Google Scholar]
- Caminade, C.; McIntyre, K.M.; Jones, A.E. Impact of recent and future climate change on vector-borne diseases. Ann. N. Y. Acad. Sci. 2019, 1436, 157–173. [Google Scholar] [CrossRef] [PubMed]
- Alvar, J.; Vélez, I.D.; Bern, C.; Herrero, M.; Desjeux, P.; Cano, J.; Jannin, J.; Boer, M.d.; WHO Leishmaniasis Control Team. Leishmaniasis Worldwide and Global Estimates of Its Incidence. PLoS ONE 2012, 7, e35671. [Google Scholar] [CrossRef]
- United Nations Department of Economic and Social Affairs. World Urbanization Prospects: The 2018 Revision; United Nations: New York, NY, USA, 2019. [Google Scholar]
- Georgeson, L.; Maslin, M.; Poessinouw, M. The global green economy: A review of concepts, definitions, measurement methodologies and their interactions. Geo Geogr. Environ. 2017, 4, e00036. [Google Scholar] [CrossRef]
- Mordecai, E.A.; Ryan, S.J.; Caldwell, J.M.; Shah, M.M.; LaBeaud, A.D. Climate change could shift disease burden from malaria to arboviruses in Africa. Lancet Planet. Health 2020, 4, e416–e423. [Google Scholar] [CrossRef]
- Li, R.; Xu, L.; Bjørnstad, O.N.; Liu, K.; Song, T.; Chen, A.; Xu, B.; Liu, Q.; Stenseth, N.C. Climate-driven variation in mosquito density predicts the spatiotemporal dynamics of dengue. Proc. Natl. Acad. Sci. USA 2019, 116, 3624–3629. [Google Scholar] [CrossRef]
- Ryan, S.J.; Carlson, C.J.; Mordecai, E.A.; Johnson, L.R. Global expansion and redistribution of Aedes-borne virus transmission risk with climate change. PLoS Negl. Trop. Dis. 2019, 13, e0007213. [Google Scholar] [CrossRef]
- Fornace, K.; Conn, J.; Anice Mureb, M.; Suveges Moreira Chaves, L.; Logan, J.; Finch, E.; Lotto Batista, M.; Alcayna, T.; Lee, S.A.; Fletcher, I.K.; et al. Planetary Health Approaches to Understand and Control Vector-Borne Diseases; Wageningen Academic: Wageningen, The Netherlands, 2023; pp. 347–386. [Google Scholar]
- Githeko, A.K.; Lindsay, S.W.; Confalonieri, U.E.; Patz, J.A. Climate change and vector-borne diseases: A regional analysis. Bull. World Health Organ. 2000, 78, 1136–1147. [Google Scholar]
- González, C.; Wang, O.; Strutz, S.E.; González-Salazar, C.; Sánchez-Cordero, V.; Sarkar, S. Climate Change and Risk of Leishmaniasis in North America: Predictions from Ecological Niche Models of Vector and Reservoir Species. PLoS Negl. Trop. Dis. 2010, 4, e585. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Mak, S.; Thomas, A. Steps for Conducting a Scoping Review. J. Grad. Med. Educ. 2022, 14, 565–567. [Google Scholar] [CrossRef]
- Rijal, K.R.; Adhikari, B.; Ghimire, B.; Dhungel, B.; Pyakurel, U.R.; Shah, P.; Bastola, A.; Lekhak, B.; Banjara, M.R.; Pandey, B.D.; et al. Epidemiology of dengue virus infections in Nepal, 2006–2019. Infect. Dis. Poverty 2021, 10, 52. [Google Scholar] [CrossRef] [PubMed]
- Siraj, A.S.; Santos-Vega, M.; Bouma, M.J.; Yadeta, D.; Carrascal, D.R.; Pascual, M. Altitudinal Changes in Malaria Incidence in Highlands of Ethiopia and Colombia. Science 2014, 343, 1154–1158. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.; Shea, B.; O’Connell, D.; Pereson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 3 February 2025).
- Shaman, J.; Day, J.F.; Stieglitz, M. Drought-Induced Amplification and Epidemic Transmission of West Nile Virus in Southern Florida. J. Med. Entomol. 2005, 42, 134–141. [Google Scholar] [CrossRef]
- Islam, N.; Winkel, J. Climate Change and Social Inequality; DESA Working Paper No. 152 ST/ESA/2017/DWP/152; Department of Economic & Social Affairs: New York, NY, USA, 2017. [Google Scholar]
- Hales, S.; de Wet, N.; Maindonald, J.; Woodward, A. Potential effect of population and climate changes on global distribution of dengue fever: An empirical model. Lancet 2002, 360, 830–834. [Google Scholar] [CrossRef]
- Brady, O.J.; Golding, N.; Pigott, D.M.; Kraemer, M.U.G.; Messina, J.P.; Reiner, R.C., Jr.; Scott, T.W.; Smith, D.L.; Gething, P.W.; Hay, S.I. Global temperature constraints on Aedes aegypti and Ae. albopictus persistence and competence for dengue virus transmission. Parasites Vectors 2014, 7, 338. [Google Scholar] [CrossRef]
- Agyekum, T.P.; Arko-Mensah, J.; Botwe, P.K.; Hogarh, J.N.; Issah, I.; Dwomoh, D.; Billah, M.K.; Dadzie, S.K.; Robins, T.G.; Fobil, J.N. Effects of Elevated Temperatures on the Growth and Development of Adult Anopheles gambiae (s.l.) (Diptera: Culicidae) Mosquitoes. J. Med. Entomol. 2022, 59, 1413–1420. [Google Scholar] [CrossRef]
- Ciota, A.T.; Matacchiero, A.C.; Kilpatrick, A.M.; Kramer, L.D. The Effect of Temperature on Life History Traits of Culex Mosquitoes. J. Med. Entomol. 2014, 51, 55–62. [Google Scholar] [CrossRef]
- Caillouët, K.A.; Michaels, S.R.; Xiong, X.; Foppa, I.; Wesson, D.M. Increase in West Nile neuroinvasive disease after Hurricane Katrina. Emerg. Infect. Dis. 2008, 14, 804–807. [Google Scholar] [CrossRef]
- Danforth, M.E.; Reisen, W.K.; Barker, C.M. The Impact of Cycling Temperature on the Transmission of West Nile Virus. J. Med. Entomol. 2016, 53, 681–686. [Google Scholar] [CrossRef]
- Mourelatos, S.; Charizani, E.; Kalaitzopoulou, S.; Tseni, X.; Lazos, N.; Tsioka, K.; Papa, A.; Dafka, S.; Rocklöv, J.; Gewehr, S. Extreme flood and WNV transmission in Thessaly, Greece, 2023. Sci. Rep. 2025, 15, 22433. [Google Scholar] [CrossRef] [PubMed]
- Gachoki, S.; Groen, T.; Vrieling, A.; Okal, M.; Skidmore, A.; Masiga, D. Satellite-based modelling of potential tsetse (Glossina pallidipes) breeding and foraging sites using teneral and non-teneral fly occurrence data. Parasites Vectors 2021, 14, 506. [Google Scholar] [CrossRef] [PubMed]
- Konan, Y.J.R.; Berté, D.; Ta, B.T.D.; Demoncheaux, J.-P.; Sauzet, S.; Watier-Grillot, S.; Kouadio, K.A.D.M.; N’dri, L.; Coulibaly, B.; Solano, P.; et al. Tsetse fly ecology and risk of transmission of African trypanosomes related to a protected forest area at a military base in the city of Abidjan, Côte d’Ivoire. Parasite 2023, 30, 36. [Google Scholar] [CrossRef] [PubMed]
- Lindström, A.; Eklöf, D.; Lilja, T. Different Hatching Rates of Floodwater Mosquitoes Aedes sticticus, Aedes rossicus and Aedes cinereus from Different Flooded Environments. Insects 2021, 12, 279. [Google Scholar] [CrossRef]
- Ma, J.; Guo, Y.; Gao, J.; Tang, H.; Xu, K.; Liu, Q.; Xu, L. Climate Change Drives the Transmission and Spread of Vector-Borne Diseases: An Ecological Perspective. Biology 2022, 11, 1628. [Google Scholar] [CrossRef]
- Ready, P.D. Leishmaniasis emergence in Europe. Eurosurveillance 2010, 15, 19505. [Google Scholar] [CrossRef]
- Randolph, S.E. Tick ecology: Processes and patterns behind the epidemiological risk posed by ixodid ticks as vectors. Parasitology 2004, 129, S37–S65. [Google Scholar] [CrossRef] [PubMed]
- Heidecke, J.; Wallin, J.; Fransson, P.; Singh, P.; Sjödin, H.; Stiles, P.C.; Treskova, M.; Rocklöv, J. Uncovering temperature sensitivity of West Nile virus transmission: Novel computational approaches to mosquito-pathogen trait responses. PLoS Comput. Biol. 2025, 21, e1012866. [Google Scholar] [CrossRef]
- Moser, S.K.; Barnard, M.; Frantz, R.M.; Spencer, J.A.; Rodarte, K.A.; Crooker, I.K.; Bartlow, A.W.; Romero-Severson, E.; Manore, C.A. Scoping review of Culex mosquito life history trait heterogeneity in response to temperature. Parasites Vectors 2023, 16, 200. [Google Scholar] [CrossRef]
- Abeku, T.; Alam, M.; Beggs, P.; Clot, B.; Furgal, C.; Hales, S.; Hutton, G.; Islam, S.; Kjellstrom, T.; Lewis, N.; et al. Climate Change 2007: Impacts, Adaptation and Vulnerability; Contribution of Working Group II to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change; Parry, M., Canziani, O., Palutikof, J., van der Linden, P., Hanson, C., Eds.; Cambridge University Press: Cambridge, UK, 2007; pp. 391–431. [Google Scholar]
- McMichael, C.; Barnett, J.; McMichael, A.J. An Ill Wind? Climate Change, Migration, and Health. Environ. Health Perspect. 2012, 120, 646–654. [Google Scholar] [CrossRef]
- Hunter, L.M.; Luna, J.K.; Norton, R.M. Environmental Dimensions of Migration. Annu. Rev. Sociol. 2015, 41, 377–397. [Google Scholar] [CrossRef] [PubMed]
- Zscheischler, J.; Westra, S.; van den Hurk, B.J.J.M.; Seneviratne, S.I.; Ward, P.J.; Pitman, A.; AghaKouchak, A.; Bresch, D.N.; Leonard, M.; Wahl, T.; et al. Future climate risk from compound events. Nat. Clim. Change 2018, 8, 469–477. [Google Scholar] [CrossRef]
- Pescaroli, G.; Alexander, D. Understanding Compound, Interconnected, Interacting, and Cascading Risks: A Holistic Framework. Risk Anal. 2018, 38, 2245–2257. [Google Scholar] [CrossRef]
- Ebi, K.L.; Vanos, J.; Baldwin, J.W.; Bell, J.E.; Hondula, D.M.; Errett, N.A.; Hayes, K.; Reid, C.E.; Saha, S.; Spector, J.; et al. Extreme Weather and Climate Change: Population Health and Health System Implications. Annu. Rev. Public Health 2021, 42, 293–315. [Google Scholar] [CrossRef]
- Cianconi, P.; Betrò, S.; Janiri, L. The Impact of Climate Change on Mental Health: A Systematic Descriptive Review. Front. Psychiatry 2020, 11, 490206. [Google Scholar] [CrossRef] [PubMed]
- McMichael, A.J.; Woodruff, R.E.; Hales, S. Climate change and human health: Present and future risks. Lancet 2006, 367, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Hansford, K.M.; Gillingham, E.L.; Vaux, A.G.C.; Cull, B.; McGinley, L.; Catton, M.; Wheeler, B.W.; Tschirren, B.; Medlock, J.M. Impact of green space connectivity on urban tick presence, density and Borrelia infected ticks in different habitats and seasons in three cities in southern England. Ticks Tick-Borne Dis. 2023, 14, 102103. [Google Scholar] [CrossRef] [PubMed]
- Hassell, J.M.; Begon, M.; Ward, M.J.; Fèvre, E.M. Urbanization and Disease Emergence: Dynamics at the Wildlife&—Livestock—Human Interface. Trends Ecol. Evol. 2017, 32, 55–67. [Google Scholar] [CrossRef]
- Dhiman, R.C.; Pahwa, S.; Dhillon, G.P.S.; Dash, A.P. Climate change and threat of vector-borne diseases in India: Are we prepared? Parasitol. Res. 2010, 106, 763–773. [Google Scholar] [CrossRef]
- Benedict, M.Q.; Levine, R.S.; Hawley, W.A.; Lounibos, L.P. Spread of The Tiger: Global Risk of Invasion by The Mosquito Aedes albopictus. Vector-Borne Zoonotic Dis. 2007, 7, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Emilsson, T.; Ode Sang, Å. Impacts of Climate Change on Urban Areas and Nature-Based Solutions for Adaptation. In Nature-Based Solutions to Climate Change Adaptation in Urban Areas: Linkages Between Science, Policy and Practice; Kabisch, N., Korn, H., Stadler, J., Bonn, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 15–27. [Google Scholar]
- Fournet, F.; Simard, F.; Fontenille, D. Green cities and vector-borne diseases: Emerging concerns and opportunities. Eurosurveillance 2024, 29, 2300548. [Google Scholar] [CrossRef]
- Costello, A.; Abbas, M.; Allen, A.; Ball, S.; Bell, S.; Bellamy, R.; Friel, S.; Groce, N.; Johnson, A.; Kett, M.; et al. Managing the health effects of climate change: Lancet and University College London Institute for Global Health Commission. Lancet 2009, 373, 1693–1733. [Google Scholar] [CrossRef]
- Gould, E.A.; Higgs, S. Impact of climate change and other factors on emerging arbovirus diseases. Trans. R. Soc. Trop. Med. Hyg. 2009, 103, 109–121. [Google Scholar] [CrossRef]
- Ferraguti, M.; Magallanes, S.; Suarez-Rubio, M.; Bates, P.J.J.; Marzal, A.; Renner, S.C. Does land-use and land cover affect vector-borne diseases? A systematic review and meta-analysis. Landsc. Ecol. 2023, 38, 2433–2451. [Google Scholar] [CrossRef]
- Abbasi, E. Climate Change and Vector-Borne Disease Transmission: The Role of Insect Behavioral and Physiological Adaptations. Integr. Org. Biol. 2025, 7, obaf011. [Google Scholar] [CrossRef] [PubMed]
- Wijerathna, T.; Gunathilaka, N. Diurnal adult resting sites and breeding habitats of phlebotomine sand flies in cutaneous leishmaniasis endemic areas of Kurunegala District, Sri Lanka. Parasites Vectors 2020, 13, 284. [Google Scholar] [CrossRef]
- Heffernan, C. Climate change and multiple emerging infectious diseases. Vet. J. 2018, 234, 43–47. [Google Scholar] [CrossRef]
- LoGiudice, K.; Ostfeld, R.S.; Schmidt, K.A.; Keesing, F. The ecology of infectious disease: Effects of host diversity and community composition on Lyme disease risk. Proc. Natl. Acad. Sci. USA 2003, 100, 567–571. [Google Scholar] [CrossRef]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible Effects of Climate Change on Ixodid Ticks and the Pathogens They Transmit: Predictions and Observations. J. Med. Entomol. 2020, 58, 1536–1545. [Google Scholar] [CrossRef]
- Tchouassi, D.P.; Milugo, T.K.; Torto, B. Feasibility of sand fly control based on knowledge of sensory ecology. Curr. Opin. Insect Sci. 2024, 66, 101274. [Google Scholar] [CrossRef]
- Ostfeld, R.S. Climate change and the distribution and intensity of infectious diseases. Ecology 2009, 90, 903–905. [Google Scholar] [CrossRef]
- Hamer, G.L.; Kitron, U.D.; Goldberg, T.L.; Brawn, J.D.; Loss, S.R.; Ruiz, M.O.; Hayes, D.B.; Walker, E.D. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. Am. J. Trop. Med. Hyg. 2009, 80, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Komar, N.; Langevin, S.; Hinten, S.; Nemeth, N.; Edwards, E.; Hettler, D.; Davis, B.; Bowen, R.; Bunning, M. Experimental infection of North American birds with the New York 1999 strain of West Nile virus. Emerg. Infect. Dis. 2003, 9, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Altizer, S.; Ostfeld, R.S.; Johnson, P.T.J.; Kutz, S.; Harvell, C.D. Climate Change and Infectious Diseases: From Evidence to a Predictive Framework. Science 2013, 341, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Kabisch, N.; Korn, H.; Stadler, J.; Bonn, A. Nature-Based Solutions to Climate Change Adaptation in Urban Areas—Linkages Between Science, Policy and Practice. In Nature-Based Solutions to Climate Change Adaptation in Urban Areas: Linkages Between Science, Policy and Practice; Kabisch, N., Korn, H., Stadler, J., Bonn, A., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–11. [Google Scholar]
- Checchi, F.; Gayer, M.; Grais, R.F.; Mills, E.J. Public Health in Crisis-Affected Populations; A practical guide for decision-makers; Humanitarian Practice Network: London, UK, 2007. [Google Scholar]
- Rezvani, S.M.H.S.; de Almeida, N.M.; Falcão, M.J. Climate Adaptation Measures for Enhancing Urban Resilience. Buildings 2023, 13, 2163. [Google Scholar] [CrossRef]
- Janzén, T.; Choudhury, F.; Hammer, M.; Petersson, M.; Dinnétz, P. Ticks—Public health risks in urban green spaces. BMC Public Health 2024, 24, 1031. [Google Scholar] [CrossRef]
- Ghatee, M.A.; Haghdoost, A.A.; Kooreshnia, F.; Kanannejad, Z.; Parisaie, Z.; Karamian, M.; Moshfe, A. Role of environmental, climatic risk factors and livestock animals on the occurrence of cutaneous leishmaniasis in newly emerging focus in Iran. J. Infect. Public Health 2018, 11, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Tirivangasi, H.M.; Nyahunda, L. Determinants of transformative climate change adaptation in rural Zimbabwe: An afrocentric analysis. Clim. Policy 2025, 1–15. [Google Scholar] [CrossRef]
- Makwarela, T.G.; Seoraj-Pillai, N.; Nangammbi, T.C. Tick Control Strategies: Critical Insights into Chemical, Biological, Physical, and Integrated Approaches for Effective Hard Tick Management. Vet. Sci. 2025, 12, 114. [Google Scholar] [CrossRef] [PubMed]
- Ryan, S.J. Mapping Thermal Physiology of Vector-Borne Diseases in a Changing Climate: Shifts in Geographic and Demographic Risk of Suitability. Curr. Environ. Health Rep. 2020, 7, 415–423. [Google Scholar] [CrossRef]
- Patz, J.A.; Graczyk, T.K.; Geller, N.; Vittor, A.Y. Effects of environmental change on emerging parasitic diseases. Int. J. Parasitol. 2000, 30, 1395–1405. [Google Scholar] [CrossRef]
- WHO. Atlas of Health and Climate; WHO: Geneva, Switzerland, 2012; p. 64. [Google Scholar]
- Budimir, M.; Šakić Trogrlić, R.; Almeida, C.; Arestegui, M.; Chuquisengo Vásquez, O.; Cisneros, A.; Cuba Iriarte, M.; Dia, A.; Lizon, L.; Madueño, G.; et al. Opportunities and challenges for people-centered multi-hazard early warning systems: Perspectives from the Global South. iScience 2025, 28, 112353. [Google Scholar] [CrossRef]
- Hussainzad, E.A.; Gou, Z. Climate Risk and Vulnerability Assessment in Informal Settlements of the Global South: A Critical Review. Land 2024, 13, 1357. [Google Scholar] [CrossRef]
- WHO; UNICEF. Global Vector Control Response 2017–2030; WHO: Geneva, Switzerland; UNICEF: New York, NY, USA, 2017. [Google Scholar]
- United Nations Office for Disaster Risk Reduction. Sendai Framework for Disaster Risk Reduction 2015–2030; United Nations Office for Disaster Risk Reduction: Geneva, Switzerland, 2015; p. 37. [Google Scholar]
- United Nations Office for Disaster Risk Reduction. Transforming Our World: The 2030 Agenda for Sustainable Development; United Nations Office for Disaster Risk Reduction: Geneva, Switzerland, 2015. [Google Scholar]
| No. | Study | Year | Geographic Location | Study Type | Climate Factor | Vector/ Disease | Key Findings |
|---|---|---|---|---|---|---|---|
| Temperature and Vector Dynamics | |||||||
| 1. | Brady et al. [29] | 2014 | Global | Modeling/Laboratory | Temperature | Aedes spp./Dengue | Ae. aegypti: 14–34 °C range; Ae. albopictus: 13–29.4 °C; 42× higher suitability for albopictus |
| 2. | Agyekum et al. [30] | 2022 | Ghana | Laboratory | Elevated temperature | Anopheles gambiae/Malaria | Temperature effects on mosquito development and survival |
| 3. | Ciota et al. [31] | 2014 | USA | Laboratory | Temperature | Culex spp./West Nile | Temperature-dependent life history trait variations |
| 4. | Tesla et al. [8] | 2018 | Global | Empirical/Modeling | Temperature | Ae. spp./Zika | Optimal transmission at 29 °C; unimodal temperature response |
| Extreme Weather Events and Disease Outbreaks | |||||||
| 5. | Caillouët et al. [32] | 2008 | Louisiana, Mississippi, USA | Surveillance | Hurricane | West Nile virus | 0→11 cases (Louisiana), 0→10 cases (Mississippi) post-Katrina; 2-fold increase in 2006 |
| 6. | Shaman et al. [26] | 2005 | Southern Florida, USA | Surveillance | Drought | West Nile virus | Drought amplification of WNV transmission |
| 7. | Danforth et al. [33] | 2016 | California, USA | Laboratory/Modeling | Temperature cycling | West Nile virus | Impact of diurnal temperature variation on transmission |
| 8. | Mourelatos et al. [34] | 2025 | Thessaly, Greece | Surveillance | Extreme flooding | West Nile virus | Flood-associated WNV transmission increase |
| Geographic Range Expansion and Altitude Shifts | |||||||
| 9. | Siraj et al. [23] | 2014 | Ethiopia, Colombia | Surveillance | Temperature increase | Malaria | 200 m upward shift (Ethiopia), 180 m (Colombia); 2.3 °C temperature correlation |
| 10. | Colón-González et al. [7] | 2018 | Latin America | Modeling | Temperature scenarios | Dengue | 1.5–2 °C limit could reduce dengue incidence and spread |
| 11. | González et al. [19] | 2010 | North America | Ecological modeling | Climate change | Leishmaniasis | Northward expansion predictions for vector and reservoir species |
| Disease Burden and Geographic Distribution | |||||||
| 12. | Alvar et al. [11] | 2012 | Global | Surveillance/Review | Climate factors | Leishmaniasis | 0.2–0.4 M VL cases, 0.7–1.2 M CL cases annually; 90% VL in 6 countries |
| 13. | Li et al. [15] | 2019 | China | Surveillance | Climate variation | Dengue | Mosquito density predicts spatiotemporal dengue dynamics |
| Vector Ecology and Environmental Factors | |||||||
| 14. | Gachoki et al. [35] | 2021 | Kenya | Satellite modeling | Environmental factors | Tsetse flies/Trypanosomiasis | Satellite-based breeding site identification |
| 15. | Konan et al. [36] | 2023 | Côte d’Ivoire | Field study | Environmental factors | Tsetse flies/Trypanosomiasis | Urban forest ecology and transmission risk |
| 16. | Lindström et al. [37] | 2021 | Sweden | Field study | Flooding | Floodwater mosquitoes | Species-specific hatching responses to flood environments |
| Vector Species | Development Temperature Range (°C) | Critical Lower Threshold (°C) | Critical Upper Threshold (°C) | Pathogen | Optimal Transmission Temperature (°C) | Data Source | Reference |
| Ae. aegypti | Immatures: 16–35 °C | ~16 °C | >35 °C (reproductive failure), >40 °C (death point) | Zika virus | 28.9 °C | Lab | (Tesla et al., 2018) [8] |
| Ae. aegypti | Variable by trait | Variable by trait | >35 °C | Dengue, Chikungunya | 29.1 °C | Lab/Model | (Mordecai et al., 2020) [14] |
| Ae. albopictus | 10.4–35 °C | 10.4 °C | >35 | Dengue, Chikungunya | 26.4 °C | Lab/Model | (Mordecai et al., 2020) [14] |
| Anopheles gambiae | 25–34 °C (study range) | Not specified | >32 °C (reproductive failure), >38 °C (larval mortality), >40 °C (egg hatching failure) | Malaria | 25–28 °C | Lab | (Agyekum et al., 2022) [30] |
| Phlebotomine sandflies | Variable by species | Variable by species | >30 | Leishmaniasis | 20–30 | Field review | (Ready 2010) [39] |
| Ixodes ticks | Variable by species/stage | >7 °C (activity threshold) | Variable by species | Lyme disease | >7 | Field/Model | (Randolph 2004) [40] |
| Glossina tsetse flies | Not clearly specified | Not clearly specified | >32 °C (mortality risk) | Trypanosomiasis | 22–26 °C | Lab/Field | (Konan et al., 2025; Gachoki et al., 2021) [35,36] |
| Culex mosquitoes | Species-specific variation | Variable by species | Species-specific: 32–38 °C range | West Nile virus | ~24 °C | Lab/Model | (Heidecke et al., 2025; Moser et al., 2023) [41,42] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqassim, A.Y. Differential Impacts of Extreme Weather Events on Vector-Borne Disease Transmission Across Urban and Rural Settings: A Scoping Review. Healthcare 2025, 13, 2425. https://doi.org/10.3390/healthcare13192425
Alqassim AY. Differential Impacts of Extreme Weather Events on Vector-Borne Disease Transmission Across Urban and Rural Settings: A Scoping Review. Healthcare. 2025; 13(19):2425. https://doi.org/10.3390/healthcare13192425
Chicago/Turabian StyleAlqassim, Ahmad Y. 2025. "Differential Impacts of Extreme Weather Events on Vector-Borne Disease Transmission Across Urban and Rural Settings: A Scoping Review" Healthcare 13, no. 19: 2425. https://doi.org/10.3390/healthcare13192425
APA StyleAlqassim, A. Y. (2025). Differential Impacts of Extreme Weather Events on Vector-Borne Disease Transmission Across Urban and Rural Settings: A Scoping Review. Healthcare, 13(19), 2425. https://doi.org/10.3390/healthcare13192425







