Diagnostic Yield of Fusion-Guided and Randomized Biopsies in Prostate Cancer: Evidence for an Integrated Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
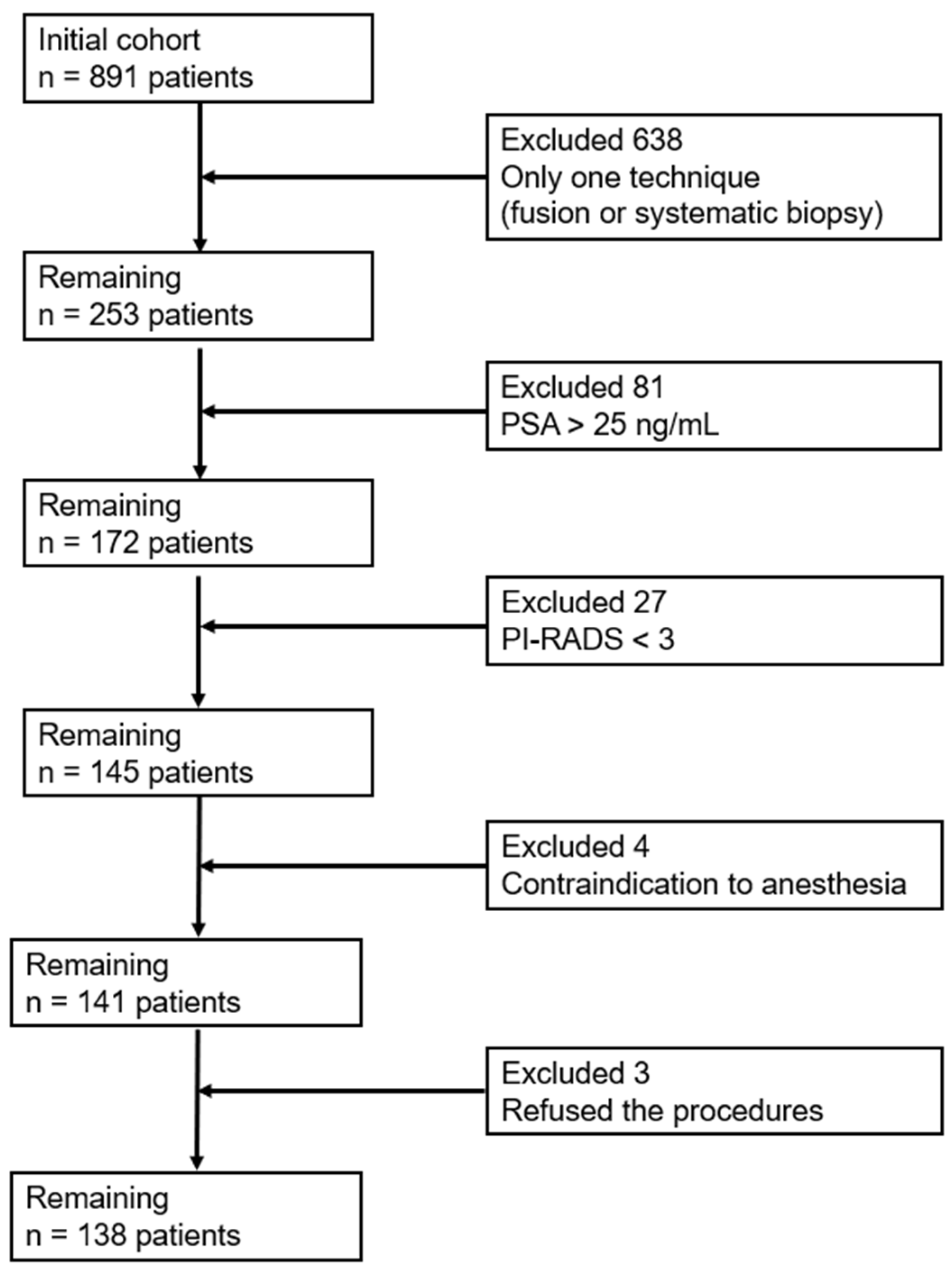
2.2. Participants and Sampling
2.3. Patient Preparation
2.4. Imaging and PI-RADS Scoring
2.5. Biopsy Procedure
2.6. Histopathological Analysis
2.7. Data Collection
2.8. Statistical Analysis
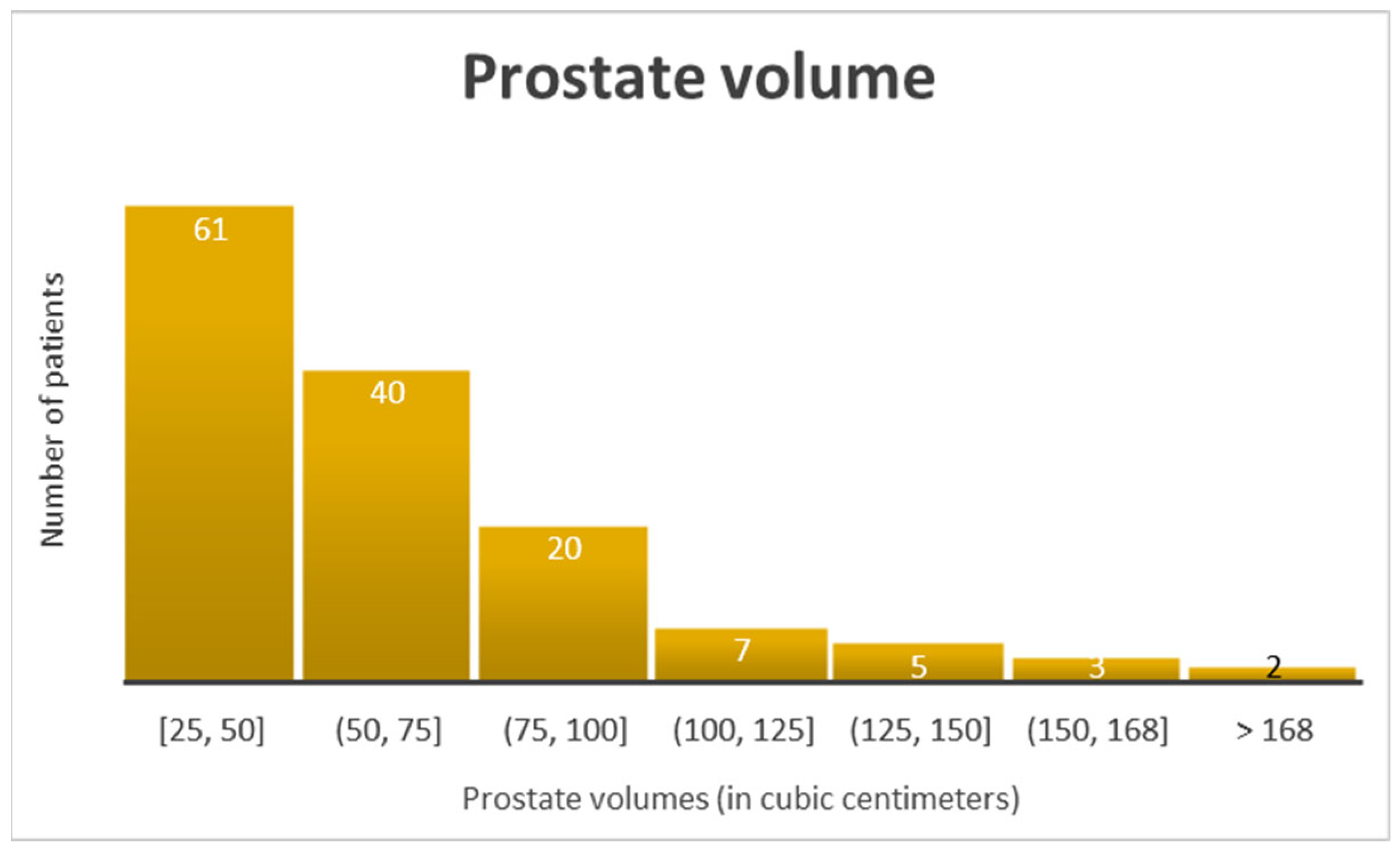
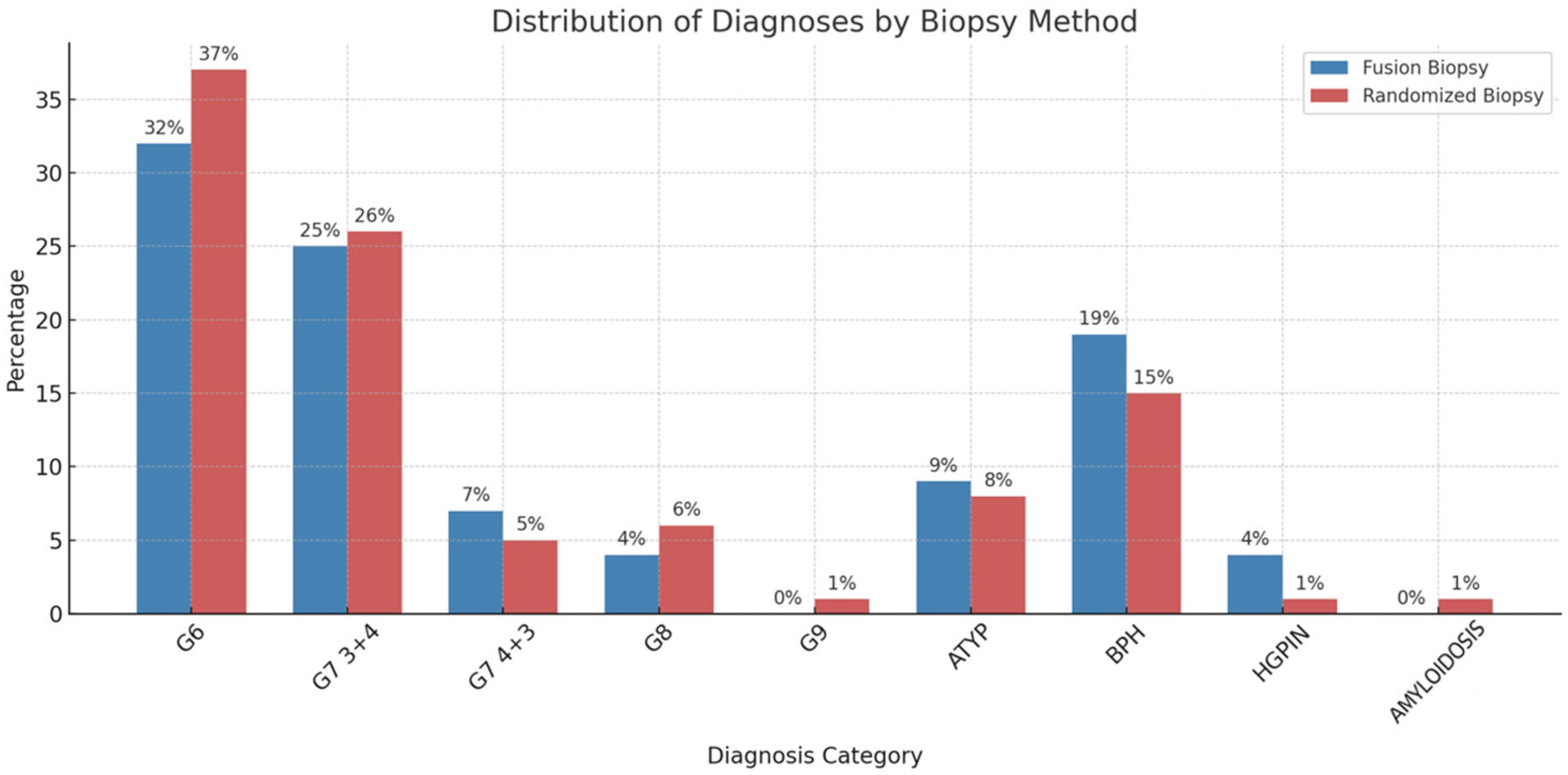
3. Results
4. Discussion
4.1. General Discussion
4.2. Future Directions
4.3. Other Observations
4.4. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATYP | Atypical glands suspicious for cancer |
| ADC | Apparent diffusion coefficient |
| BPH | Benign prostate hyperplasia |
| DCE | Dynamic contrast-enhanced |
| HGPIN | High-grade prostatic intraepithelial neoplasia |
| mp-MRI | Multiparametric magnetic resonance imaging |
| PCa | Prostate cancer |
| PI-RADS | Prostate Imaging Reporting and Data System |
| PSA | Prostate-specific antigen |
| T2W | T2-weighted |
| VIF | Variance inflation factor |
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer statistics, 2022. CA Cancer J. Clin. 2022, 72, 7–33. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of Prostate Cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef]
- Osama, S.; Serboiu, C.; Taciuc, I.-A.; Angelescu, E.; Petcu, C.; Priporeanu, T.A.; Marinescu, A.; Costache, A. Current Approach to Complications and Difficulties during Transrectal Ultrasound-Guided Prostate Biopsies. J. Clin. Med. 2024, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Mesko, S.B.; Marks, L.; Ragab, O.; Patel, S.; Margolis, D.A.; Demanes, D.J.; Kamrava, M. Targeted Prostate Biopsy Gleason Score Heterogeneity and Implications for Risk Stratification. Am. J. Clin. Oncol. 2018, 41, 497–501. [Google Scholar] [CrossRef]
- Leitão, T.P.; Alfarelos, J.; Rodrigues, T.; e Silva, R.P.; Garcia, R.M.; Martinho, D.; Sandul, A.; Mendonça, T.; Pereira, S.; Lopes, T.M. A Prospective Randomized Trial Comparing the Vienna Nomogram and a Ten-Core Prostate Biopsy Protocol: Effect on Cancer Detection Rate. Clin. Genitourin. Cancer 2017, 15, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Stone, B.V.; Shoag, J.; Halpern, J.A.; Mittal, S.; Lewicki, P.; Golombos, D.M.; Bedretdinova, D.; Chughtai, B.; Barbieri, C.E.; Lee, R.K. Prostate size, nocturia and the digital rectal examination: A cohort study of 30 500 men. BJU Int. 2017, 119, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Albers, P. PSA screening—For whom and when? Asian J. Androl. 2019, 21, 3–5. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marks, L.; Young, S.; Natarajan, S. MRI–ultrasound fusion for guidance of targeted prostate biopsy. Curr. Opin. Urol. 2013, 23, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Sherrer, R.L.; Lai, W.S.; Thomas, J.V.; Nix, J.W.; Rais-Bahrami, S. Incidental findings on multiparametric MRI performed for evaluation of prostate cancer. Abdom. Radiol. 2018, 43, 696–701. [Google Scholar] [CrossRef]
- Shin, T.; Smyth, T.B.; Ukimura, O.; Ahmadi, N.; Abreu, A.L.d.C.; Ohe, C.; Oishi, M.; Mimata, H.; Gill, I.S. Diagnostic accuracy of a five-point Likert scoring system for magnetic resonance imaging (MRI) evaluated according to results of MRI/ultrasonography image-fusion targeted biopsy of the prostate. BJU Int. 2018, 121, 77–83. [Google Scholar] [CrossRef]
- Mariotti, G.C.; Falsarella, P.M.; Garcia, R.G.; Queiroz, M.R.G.; Lemos, G.C.; Baroni, R.H. Incremental diagnostic value of targeted biopsy using mpMRI-TRUS fusion versus 14-fragments prostatic biopsy: A prospective controlled study. Eur. Radiol. 2018, 28, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Barentsz, J.O.; Richenberg, J.; Clements, R.; Choyke, P.; Verma, S.; Villeirs, G.; Rouviere, O.; Logager, V.; Fütterer, J.J. ESUR prostate MR guidelines 2012. Eur. Radiol. 2012, 22, 746–757. [Google Scholar] [CrossRef] [PubMed]
- Turkbey, B.; Rosenkrantz, A.B.; Haider, M.A.; Padhani, A.R.; Villeirs, G.; Macura, K.J.; Tempany, C.M.; Choyke, P.L.; Cornud, F.; Margolis, D.J.; et al. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur. Urol. 2019, 76, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Gold, S.A.; Hale, G.R.; Bloom, J.B.; Smith, C.P.; Rayn, K.N.; Valera, V.; Wood, B.J.; Choyke, P.L.; Turkbey, B.; Pinto, P.A. Follow-up of negative MRI-targeted prostate biopsies: When are we missing cancer? World J. Urol. 2019, 37, 235–241. [Google Scholar] [CrossRef]
- He, H.; Magi-Galluzzi, C.; Li, J.; Carver, P.; Falzarano, S.; Smith, K.M.; Rubin, M.A.; Zhou, M. The diagnostic utility of novel immunohistochemical marker ERG in the workup of prostate biopsies with “atypical glands suspicious for cancer”. Am. J. Surg. Pathol. 2011, 35, 608–614. [Google Scholar] [CrossRef] [PubMed]
- Lassed, S.; Deus, C.M.; Lourenço, N.; Dahdouh, A.; Rizvanov, A.A.; Oliveira, P.J.; Zama, D. Diet, Lifestyles, Family History, and Prostate Cancer Incidence in an East Algerian Patient Group. BioMed Res. Int. 2016, 2016, 5730569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Campi, R.; Brookman-May, S.D.; Henríquez, J.D.S.; Akdoğan, B.; Brausi, M.; Klatte, T.; Langenhuijsen, J.F.; Linares-Espinos, E.; Marszalek, M.; Roupret, M.; et al. Impact of Metabolic Diseases, Drugs, and Dietary Factors on Prostate Cancer Risk, Recurrence, and Survival: A Systematic Review by the European Association of Urology Section of Oncological Urology. Eur. Urol. Focus 2019, 5, 1029–1057. [Google Scholar] [CrossRef] [PubMed]
- Kamrava, M.; Hegde, J.V.; Abgaryan, N.; Chang, E.; Le, J.D.; Wang, J.; Kupelian, P.A.; Marks, L.S. Does the addition of targeted prostate biopsies to standard systemic biopsies influence treatment management for radiation oncologists? BJU Int. 2016, 117, 584–591. [Google Scholar] [CrossRef]
- Rosenkrantz, A.B.; Taneja, S.S. Radiologist, Be Aware: Ten Pitfalls That Confound the Interpretation of Multiparametric Prostate MRI. Am. J. Roentgenol. 2014, 202, 109–120. [Google Scholar] [CrossRef]
- Tang, Y.; Liu, Z.; Tang, L.; Zhang, R.; Lu, Y.; Liang, J.; Zou, Z.; Zhou, C.; Wang, Y. Significance of MRI/Transrectal Ultrasound Fusion Three-Dimensional Model-Guided, Targeted Biopsy Based on Transrectal Ultrasound-Guided Systematic Biopsy in Prostate Cancer Detection: A Systematic Review and Meta-Analysis. Urol. Int. 2018, 100, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Kayano, P.P.; Carneiro, A.; Castilho, T.M.L.; Sivaraman, A.; Claros, O.R.; Baroni, R.H.; Garcia, R.G.; Mariotti, G.C.; Smaletz, O.; Filippi, R.Z.; et al. Comparison of Gleason upgrading rates in transrectal ultrasound systematic random biopsies versus US-MRI fusion biopsies for prostate cancer. Int. Braz. J. Urol. 2018, 44, 1106–1113. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Churukanti, G.; Siddiqui, M.M. MRI–TRUS fusion biopsy versus 12-core systematic biopsy. Nat. Rev. Urol. 2015, 12, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Ukimura, O.; Marien, A.; Palmer, S.; Villers, A.; Aron, M.; Abreu, A.L.d.C.; Leslie, S.; Shoji, S.; Matsugasumi, T.; Gross, M.; et al. Trans-rectal ultrasound visibility of prostate lesions identified by magnetic resonance imaging increases accuracy of image-fusion targeted biopsies. World J. Urol. 2015, 33, 1669–1676. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Drudi, F.M.; Cantisani, V.; Angelini, F.; Ciccariello, M.; Messineo, D.; Ettorre, E.; Liberatore, M.; Scialpi, M. Multiparametric MRI Versus Multiparametric US in the Detection of Prostate Cancer. Anticancer Res. 2019, 39, 3101–3110. [Google Scholar] [CrossRef] [PubMed]
- Spena, G.; Moretti, T.B.; Dávila, F.S.; DOS Anjos, G.; Khan, I.; Calace, F.P.; Aveta, A.; Pandolfo, S.D.; Tufano, A.; Izzo, A.; et al. Ga68-PSMA PET for lymph node staging in intermediate and high-risk prostate cancer patients undergoing robotic assisted radical prostatectomy. Minerva Urol. Nephrol. 2024, 76, 467–473. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef]
- Quaresma, M.; Coleman, M.P.; Rachet, B. 40-year trends in an index of survival for all cancers combined and survival adjusted for age and sex for each cancer in England and Wales, 1971–2011: A population-based study. Lancet 2015, 385, 1206–1218. [Google Scholar] [CrossRef]
- Joung, J.Y.; Lim, J.; Oh, C.-M.; Jung, K.-W.; Cho, H.; Kim, S.H.; Seo, H.K.; Park, W.S.; Chung, J.; Lee, K.H.; et al. Current trends in the incidence and survival rate of urological cancers in Korea. Cancer Res. Treat. 2017, 49, 607–615. [Google Scholar] [CrossRef]
- Shin, D.W.; Park, H.S.; Lee, S.H.; Jeon, S.H.; Cho, S.; Kang, S.H.; Park, S.C.; Park, J.H.; Park, J. Health-Related Quality of Life, Perceived Social Support, and Depression in Disease-Free Survivors Who Underwent Curative Surgery Only for Prostate, Kidney and Bladder Cancer: Comparison among Survivors and with the General Population. Cancer Res. Treat. 2019, 51, 289–299. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chattopadhyay, S.; Hemminki, O.; Försti, A.; Sundquist, K.; Sundquist, J.; Hemminki, K. Impact of family history of cancer on risk and mortality of second cancers in patients with prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Foix, M.P.; Calvo, D.F.; i Mundó, E.C.; Novo, J.F.S.; Serra, E.M.; Benett, J.R.G.; Gàllego, M.G.; Viladomat, S.Y.; Julià, F.V.; i Bel, A.V. Clinical relevance of amyloid in prostate samples: A report on 40 patients. Histopathology 2022, 81, 363–370. [Google Scholar] [CrossRef] [PubMed]
- De Luca, L.; Crocetto, F.; Barone, B.; Creta, M.; Pesce, S.; Aveta, A.; Campanino, M.R.; Imbimbo, C.; Longo, N. Granulomatous prostatitis mimicking prostate cancer in a patient with psoriatic arthritis: A case report. Futur. Sci. OA 2020, 6, FSO591. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Technique | Positive Cases |
|---|---|
| Fusion positive | 94 |
| Randomized positive | 105 |
| Fusion positive, randomized negative (F+/R−) | 16 |
| Randomized biopsy (F−/R+) | 28 |
| Both positive (F+ ∩ R+) | 78 |
| Positive via any method (F+ ∪ R+) | 122 |
| Fusion negative, randomized negative (F−/R−) | 16 |
| Total patients | 138 |
| Fusion vs. Combined | ||
|---|---|---|
| Combined+ | Combined− | |
| Fusion+ | 94 | 0 |
| Fusion− | 28 | 16 |
| Randomized vs. Combined | ||
| Combined+ | Combined− | |
| Randomized+ | 105 | 0 |
| Randomized− | 17 | 16 |
| Parameter | Tolerance | VIF |
|---|---|---|
| AGE | 0.907 | 1.102 |
| PSA | 0.877 | 1.140 |
| PI-RADS | 0.873 | 1.146 |
| PROSTATIC VOLUME | 0.782 | 1.278 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salloum, O.; Taciuc, I.-A.; Dick, A.; Petcu, C.; Gingu, C.; Sanda, N.; Marinescu, A.N.; Serboiu, C.; Costache, A. Diagnostic Yield of Fusion-Guided and Randomized Biopsies in Prostate Cancer: Evidence for an Integrated Approach. Healthcare 2025, 13, 2214. https://doi.org/10.3390/healthcare13172214
Salloum O, Taciuc I-A, Dick A, Petcu C, Gingu C, Sanda N, Marinescu AN, Serboiu C, Costache A. Diagnostic Yield of Fusion-Guided and Randomized Biopsies in Prostate Cancer: Evidence for an Integrated Approach. Healthcare. 2025; 13(17):2214. https://doi.org/10.3390/healthcare13172214
Chicago/Turabian StyleSalloum, Osama, Iulian-Alexandru Taciuc, Alexandru Dick, Costin Petcu, Costin Gingu, Nicoleta Sanda, Andreea Nicoleta Marinescu, Crenguta Serboiu, and Adrian Costache. 2025. "Diagnostic Yield of Fusion-Guided and Randomized Biopsies in Prostate Cancer: Evidence for an Integrated Approach" Healthcare 13, no. 17: 2214. https://doi.org/10.3390/healthcare13172214
APA StyleSalloum, O., Taciuc, I.-A., Dick, A., Petcu, C., Gingu, C., Sanda, N., Marinescu, A. N., Serboiu, C., & Costache, A. (2025). Diagnostic Yield of Fusion-Guided and Randomized Biopsies in Prostate Cancer: Evidence for an Integrated Approach. Healthcare, 13(17), 2214. https://doi.org/10.3390/healthcare13172214







