Evaluation of the Effectiveness of a Cardiac Telerehabilitation Program in Chronic Heart Failure: Design and Rationale of the TELEREHAB-HF Study
Abstract
1. Background
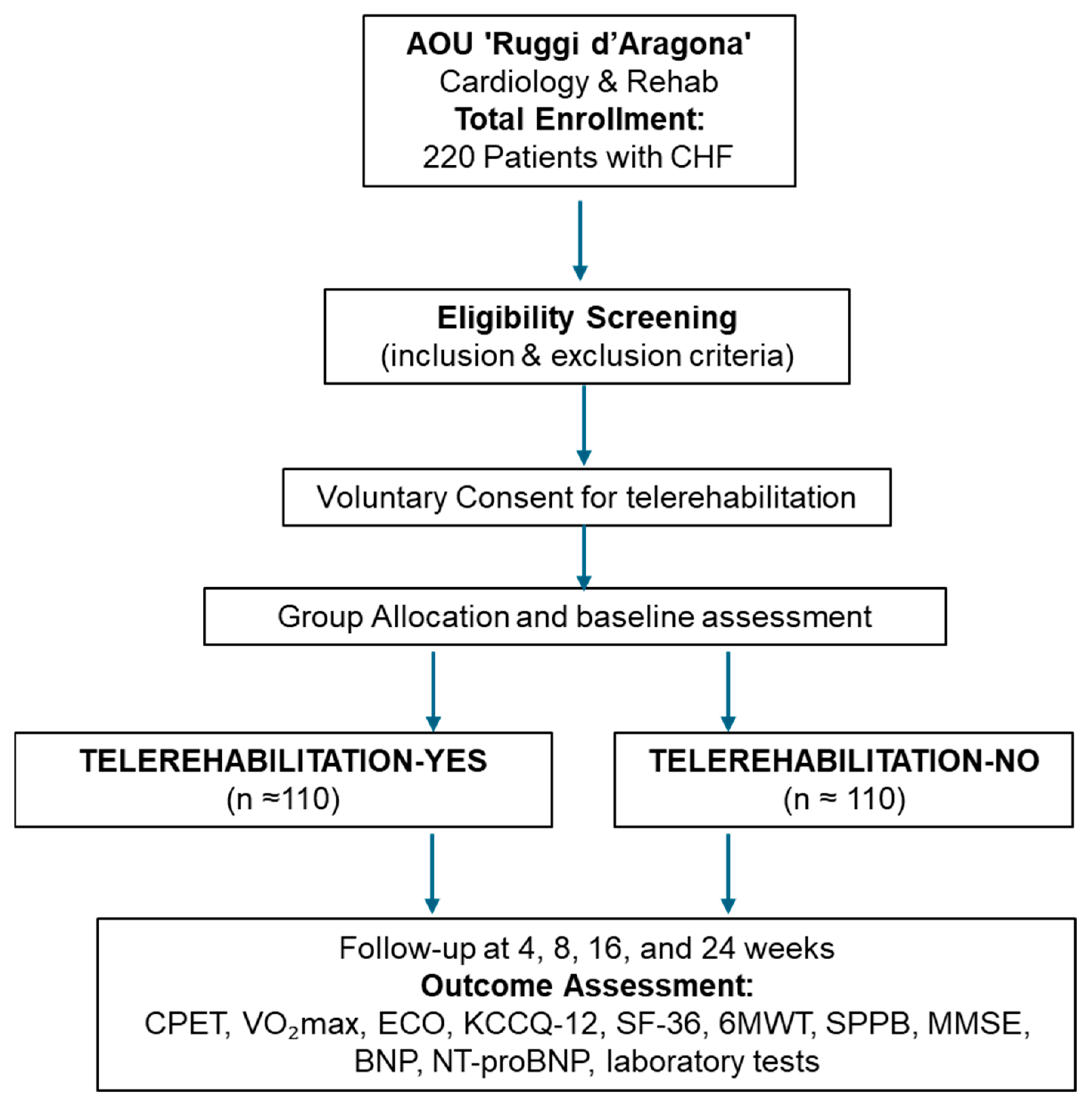
2. Methods
Study Design and Population
3. Groups and Interventions
- 1.
- TELEREHABILITATION—YES (Remote Group)
- 2.
- TELEREHABILITATION—NO (In-Person Group)
- 10 min of interval training (warm-up) including flexibility, breathing, and coordination exercises;
- 10 min of cool-down exercises with stretching and breathing techniques.
4. In-Person Group (TELEREHABILITATION—NO)
5. Remote Group (TELEREHABILITATION—YES)
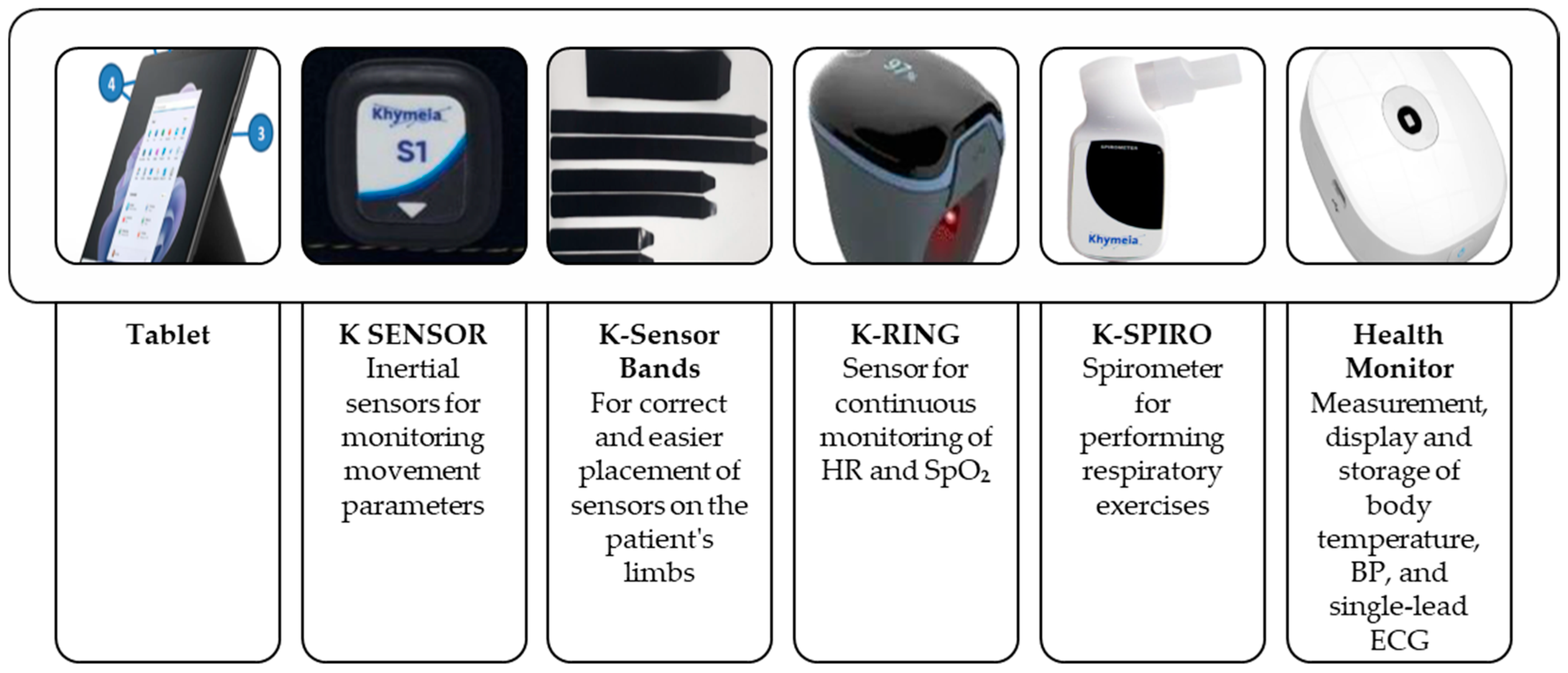
6. Technological Solution Used for the TELEREHABILITATION—YES Group
- A processing unit enclosed in a dedicated cabinet, a capacitive touchscreen LCD monitor, and a low-intensity electromagnetic field generator
- Wireless 3D passive sensors worn by the patient, detecting position and orientation (6 degrees of freedom) through the electromagnetic field
- The number of sensors is adapted to specific rehabilitation needs
- K-SENSOR: inertial sensors for monitoring movement parameters
- K-Sensor bands: to support accurate and easy placement of sensors on the limbs during exercises
- K-RING: a wearable sensor for continuous monitoring of HR and SpO2
- K-SPIRO: a spirometer for guided respiratory exercises
- Tablet device
- K-SENSOR inertial sensors
- K-Sensor bands for proper placement on limbs
- K-RING for continuous HR and SpO2
- K-SPIRO spirometer for breathing exercises
- Health Monitor for measuring and storing body temperature, BP, and ECG (single lead)
- Continuous online updates of connected devices within the hub and spoke network
- Centralized sharing of clinical protocols and activities across all devices
- Real-time sharing and visualization of patient rehabilitation results
- Remote device allocation and control
- Assignment of personalized treatment programs and monitoring of adherence
7. Risk Management and Contingency Planning
- Borg Dyspnea Scale ≥ 8/10
- Rate of Perceived Exertion ≥ 18/20
- Resting HR > 120 bpm or <50 bpm
- Systolic BP > 180 mmHg or <70 mmHg
- Diastolic BP > 100 mmHg or <50 mmHg
- SpO2 < 88%
- Sudden fall, acute chest pain, neurological symptoms (e.g., diplopia, motor/sensory deficits, aphasia), altered consciousness
8. Outcomes
- QoL: Assessed using the KCCQ-12 and SF-36 at each timepoint.
- Biochemical parameters: Changes in BNP, NT-proBNP, creatinine, eGFR, serum electrolytes (sodium, potassium, chloride), glucose, and lipid profile.
- Functional assessments: Performance on the 6MWT and SPPB.
- Cognitive status: Assessed with the MMSE.
- Echocardiographic parameters: Left ventricular ejection fraction (LVEF), diastolic function indices (E/A—Ratio of early E to late A ventricular filling velocities; E/e’—Ratio of early transmitral flow velocity to early diastolic mitral annular velocity), and right ventricular function (TAPSE—Tricuspid Annular Plane Systolic Excursion, RVs’—Right Ventricular Systolic Velocity).
- Adverse events: Monitoring of any complications related to training or telemonitoring (e.g., hypotension, arrhythmias, device issues).
9. Statistical Analysis
9.1. Primary Endpoint Analysis
9.2. Secondary Endpoint Analysis
- For repeated measurements (e.g., QoL scores, biochemical markers, functional parameters), comparisons across timepoints (T0, T1, T2, T3, T4) will be conducted using one-way repeated measures ANOVA for normally distributed data, or Friedman test for non-parametric data.
- For post hoc comparisons, Bonferroni correction will be applied to control for type I error.
- Categorical variables (e.g., proportion of patients with adverse events) will be compared using the Pearson chi-square test or Fisher’s exact test where appropriate.
9.3. Sample Size Calculation
10. Expected Results
11. Discussion
12. Study Limitations and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef] [PubMed]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032. [Google Scholar] [CrossRef]
- Keteyian, S.J.; Jackson, S.L.; Chang, A.; Brawner, C.A.; Wall, H.K.; Forman, D.E.; Sukul, D.; Ritchey, M.D.; Sperling, L.S. Tracking Cardiac Rehabilitation Utilization in Medicare Beneficiaries: 2017 UPDATE. J. Cardiopulm. Rehabil. Prev. 2022, 42, 235–245. [Google Scholar] [CrossRef] [PubMed]
- Taylor, R.S.; Dalal, H.M.; Zwisler, A.D. Cardiac rehabilitation for heart failure: ‘Cinderella’ or evidence-based pillar of care? Eur. Heart J. 2023, 44, 1511–1518. [Google Scholar] [CrossRef]
- Jansen-Kosterink, S.; In ‘t Veld, R.H.; Hermens, H.; Vollenbroek-Hutten, M. A Telemedicine Service as Partial Replacement of Face-to-Face Physical Rehabilitation: The Relevance of Use. Telemed. J. E-Health 2015, 21, 808–813. [Google Scholar] [CrossRef]
- Frederix, I.; Solmi, F.; Piepoli, M.F.; Dendale, P. Cardiac telerehabilitation: A novel cost-efficient care delivery strategy that can induce long-term health benefits. Eur. J. Prev. Cardiol. 2017, 24, 1708–1717. [Google Scholar] [CrossRef] [PubMed]
- Garofano, M.; Vecchione, C.; Calabrese, M.; Rusciano, M.R.; Visco, V.; Granata, G.; Carrizzo, A.; Galasso, G.; Bramanti, P.; Corallo, F.; et al. Technological Developments, Exercise Training Programs, and Clinical Outcomes in Cardiac Telerehabilitation in the Last Ten Years: A Systematic Review. Healthcare 2024, 12, 1534. [Google Scholar] [CrossRef]
- Seron, P.; Oliveros, M.J.; Gutierrez-Arias, R.; Fuentes-Aspe, R.; Torres-Castro, R.C.; Merino-Osorio, C.; Nahuelhual, P.; Inostroza, J.; Jalil, Y.; Solano, R.; et al. Effectiveness of Telerehabilitation in Physical Therapy: A Rapid Overview. Phys. Ther. 2021, 101, pzab053. [Google Scholar] [CrossRef]
- Zhong, W.; Liu, R.; Cheng, H.; Xu, L.; Wang, L.; He, C.; Wei, Q. Longer-Term Effects of Cardiac Telerehabilitation on Patients With Coronary Artery Disease: Systematic Review and Meta-Analysis. JMIR Mhealth Uhealth 2023, 11, e46359. [Google Scholar] [CrossRef]
- Kraal, J.J.; Van den Akker-Van Marle, M.E.; Abu-Hanna, A.; Stut, W.; Peek, N.; Kemps, H.M. Clinical and cost-effectiveness of home-based cardiac rehabilitation compared to conventional, centre-based cardiac rehabilitation: Results of the FIT@Home study. Eur. J. Prev. Cardiol. 2017, 24, 1260–1273. [Google Scholar] [CrossRef]
- Scherrenberg, M.; Falter, M.; Abreu, A.; Aktaa, S.; Busnatu, S.; Casado-Arroyo, R.; Dendale, P.; Dilaveris, P.; Locati, E.T.; Marques-Sule, E.; et al. Standards for cardiac telerehabilitation: A scientific statement of the European Association of Preventive Cardiology (EAPC) and the Association of Cardiovascular Nursing & Allied Professions (ACNAP) of the ESC, and the ESC Working Group on e-Cardiology. Eur. Heart J. 2025, ehaf408. [Google Scholar] [CrossRef]
- Fang, J.; Huang, B.; Xu, D.; Li, J.; Au, W.W. Innovative Application of a Home-Based and Remote Sensing Cardiac Rehabilitation Protocol in Chinese Patients After Percutaneous Coronary Intervention. Telemed. J. E-Health 2019, 25, 288–293. [Google Scholar] [CrossRef]
- Alemanno, F.; Houdayer, E.; Emedoli, D.; Locatelli, M.; Mortini, P.; Mandelli, C.; Raggi, A.; Iannaccone, S. Efficacy of virtual reality to reduce chronic low back pain: Proof-of-concept of a non-pharmacological approach on pain, quality of life, neuropsychological and functional outcome. PLoS ONE 2019, 14, e0216858. [Google Scholar] [CrossRef] [PubMed]
- Macchitella, L.; Amendola, S.; Barraco, G.; Scoditti, S.; Gallo, I.; Oliva, M.C.; Trabacca, A. A narrative review of the use of a cutting-edge virtual reality rehabilitation technology in neurological and neuropsychological rehabilitation. NeuroRehabilitation 2023, 53, 439–457. [Google Scholar] [CrossRef] [PubMed]
- Contrada, M.; Arcuri, F.; Tonin, P.; Pignolo, L.; Mazza, T.; Nudo, G.; Pignataro, M.L.; Quintieri, M.; Iozzi, A.; Cerasa, A. Stroke Telerehabilitation in Calabria: A Health Technology Assessment. Front. Neurol. 2021, 12, 777608. [Google Scholar] [CrossRef]
- Galasso, O.; Calabrese, M.; Scanniello, G.; Garofano, M.; Pepe, L.; Budaci, L.; Ungaro, G.; Fimiani, G.; Bramanti, P.; Schiavo, L.; et al. Accelerating Recovery: A Case Report on Telerehabilitation for a Triathlete’s Post-Meniscus Surgery Comeback. Healthcare 2025, 13, 406. [Google Scholar] [CrossRef]
- Wagner, A.K. Rehabilomics: A conceptual framework to drive biologics research. PM R J. Inj. Funct. Rehabil. 2011, 3, S28–S30. [Google Scholar] [CrossRef]
- Wagner, A.K. A Rehabilomics framework for personalized and translational rehabilitation research and care for individuals with disabilities: Perspectives and considerations for spinal cord injury. J. Spinal Cord. Med. 2014, 37, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Badianyama, M.; Mpanya, D.; Adamu, U.; Sigauke, F.; Nel, S.; Tsabedze, N. New Biomarkers and Their Potential Role in Heart Failure Treatment Optimisation-An African Perspective. J. Cardiovasc. Dev. Dis. 2022, 9, 335. [Google Scholar] [CrossRef]
- Mariappan, V.; Srinivasan, R.; Pratheesh, R.; Jujjuvarapu, M.R.; Pillai, A.B. Predictive biomarkers for the early detection and management of heart failure. Heart Fail. Rev. 2024, 29, 331–353. [Google Scholar] [CrossRef]
- Malandish, A.; Ghadamyari, N.; Karimi, A.; Naderi, M. The role of exercise training on cardiovascular peptides in patients with heart failure: A systematic review and meta-analysis. Curr. Res. Physiol. 2022, 5, 270–286. [Google Scholar] [CrossRef]
- Verdicchio, C.; Freene, N.; Hollings, M.; Maiorana, A.; Briffa, T.; Gallagher, R.; Hendriks, J.M.; Abell, B.; Brown, A.; Colquhoun, D.; et al. A Clinical Guide for Assessment and Prescription of Exercise and Physical Activity in Cardiac Rehabilitation. A CSANZ Position Statement. Heart Lung Circ. 2023, 32, 1035–1048. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Pack, Q.R.; Aberegg, E.; Brewer, L.C.; Ford, Y.R.; Forman, D.E.; Gathright, E.C.; Khadanga, S.; Ozemek, C.; Thomas, R.J. Core Components of Cardiac Rehabilitation Programs: 2024 Update: A Scientific Statement From the American Heart Association and the American Association of Cardiovascular and Pulmonary Rehabilitation. Circulation 2024, 150, e328–e347. [Google Scholar] [CrossRef] [PubMed]
- Giannitsi, S.; Bougiakli, M.; Bechlioulis, A.; Kotsia, A.; Michalis, L.K.; Naka, K.K. 6-minute walking test: A useful tool in the management of heart failure patients. Ther. Adv. Cardiovasc. Dis. 2019, 13, 1753944719870084. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Yamaga, T.; Nishie, K.; Sakai, Y.; Ishida, T.; Oka, K.; Ikegami, S.; Horiuchi, H. Impact of physical performance on prognosis among patients with heart failure: Systematic review and meta-analysis. J. Cardiol. 2020, 76, 139–146. [Google Scholar] [CrossRef]
- Brown, K. A review to examine the use of SF-36 in cardiac rehabilitation. Br. J. Nurs. 2003, 12, 904–909. [Google Scholar] [CrossRef] [PubMed]
- Sukosd, I.E.; Pescariu, S.A.; Faur, C.; Danila, A.I.; Prodan-Barbulescu, C.; Fira-Mladinescu, O. Utility of Kansas City Cardiomyopathy Questionnaire (KCCQ) in Assessing Quality of Life among Patients with Heart Failure Undergoing Exercise Training Rehabilitation: A Systematic Review. Diseases 2024, 12, 64. [Google Scholar] [CrossRef]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; Bittner, V.; Brewer, L.C.; Demeter, S.H.; Dixon, D.L.; Fearon, W.F.; Hess, B.; Johnson, H.M.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef]
- Beatty, A.L.; Beckie, T.M.; Dodson, J.; Goldstein, C.M.; Hughes, J.W.; Kraus, W.E.; Martin, S.S.; Olson, T.P.; Pack, Q.R.; Stolp, H.; et al. A New Era in Cardiac Rehabilitation Delivery: Research Gaps, Questions, Strategies, and Priorities. Circulation 2023, 147, 254–266. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Bäck, M.; Börjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Conraads, V.; Corrà, U.; Dickstein, K.; Francis, D.P.; Jaarsma, T.; McMurray, J.; Pieske, B.; Piotrowicz, E.; Schmid, J.P.; et al. Exercise training in heart failure: From theory to practice. A consensus document of the Heart Failure Association and the European Association for Cardiovascular Prevention and Rehabilitation. Eur. J. Heart Fail. 2011, 13, 347–357. [Google Scholar] [CrossRef] [PubMed]
- Corrà, U.; Piepoli, M.F.; Carré, F.; Heuschmann, P.; Hoffmann, U.; Verschuren, M.; Halcox, J.; Giannuzzi, P.; Saner, H.; Wood, D.; et al. Secondary prevention through cardiac rehabilitation: Physical activity counselling and exercise training: Key components of the position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. Eur. Heart J. 2010, 31, 1967–1974. [Google Scholar] [CrossRef] [PubMed]
- Sachdev, V.; Sharma, K.; Keteyian, S.J.; Alcain, C.F.; Desvigne-Nickens, P.; Fleg, J.L.; Florea, V.G.; Franklin, B.A.; Guglin, M.; Halle, M. Supervised exercise training for chronic heart failure with preserved ejection fraction: A scientific statement from the American Heart Association and American College of Cardiology. Circulation 2023, 147, e699–e715. [Google Scholar] [CrossRef] [PubMed]
- Gayda, M.; Ribeiro, P.A.B.; Juneau, M.; Nigam, A. Comparison of Different Forms of Exercise Training in Patients With Cardiac Disease: Where Does High-Intensity Interval Training Fit? Can. J. Cardiol. 2016, 32, 485–494. [Google Scholar] [CrossRef]
- Borg, G.A. Psychophysical bases of perceived exertion. Med. Sci. Sports Exerc. 1982, 14, 377–381. [Google Scholar] [CrossRef]
- McGregor, G.; Powell, R.; Begg, B.; Birkett, S.T.; Nichols, S.; Ennis, S.; McGuire, S.; Prosser, J.; Fiassam, O.; Hee, S.W.; et al. High-intensity interval training in cardiac rehabilitation: A multi-centre randomized controlled trial. Eur. J. Prev. Cardiol. 2023, 30, 745–755. [Google Scholar] [CrossRef]
- Keteyian, S.J.; Brawner, C.A.; Savage, P.D.; Ehrman, J.K.; Schairer, J.; Divine, G.; Aldred, H.; Ophaug, K.; Ades, P.A. Peak aerobic capacity predicts prognosis in patients with coronary heart disease. Am. Heart J. 2008, 156, 292–300. [Google Scholar] [CrossRef]
- Baccanelli, G.; Tomaselli, M.; Ferri, U.; Giglio, A.; Munforti, C.; Parati, G.; Facchini, M.; Crotti, L.; Malfatto, G. Effects of cardiac rehabilitation on cardiopulmonary test parameters in heart failure: A real world experience. Int. J. Cardiol. Cardiovasc. Risk Prev. 2023, 17, 200178. [Google Scholar] [CrossRef]
- Scherrenberg, M.; Zeymer, U.; Schneider, S.; Van der Velde, A.E.; Wilhelm, M.; Van’t Hof, A.W.J.; Kolkman, E.; Prins, L.F.; Prescott, E.; Iliou, M.C.; et al. EU-CaRE study: Could exercise-based cardiac telerehabilitation also be cost-effective in elderly? Int. J. Cardiol. 2021, 340, 1–6. [Google Scholar] [CrossRef]
- Albarqi, M.N. Exploring the Effectiveness of Technology-Assisted Interventions for Promoting Independence in Elderly Patients: A Systematic Review. Healthcare 2024, 12, 2105. [Google Scholar] [CrossRef] [PubMed]
- Areias, A.C.; Molinos, M.; Moulder, R.G.; Janela, D.; Scheer, J.K.; Bento, V.; Yanamadala, V.; Cohen, S.P.; Correia, F.D.; Costa, F. The potential of a multimodal digital care program in addressing healthcare inequities in musculoskeletal pain management. NPJ Digit. Med. 2023, 6, 188. [Google Scholar] [CrossRef]
- Brouwers, R.W.M.; van Exel, H.J.; van Hal, J.M.C.; Jorstad, H.T.; de Kluiver, E.P.; Kraaijenhagen, R.A.; Kuijpers, P.; van der Linde, M.R.; Spee, R.F.; Sunamura, M.; et al. Cardiac telerehabilitation as an alternative to centre-based cardiac rehabilitation. Neth. Heart J. 2020, 28, 443–451. [Google Scholar] [CrossRef]
- Ezeamii, V.C.; Okobi, O.E.; Wambai-Sani, H.; Perera, G.S.; Zaynieva, S.; Okonkwo, C.C.; Ohaiba, M.M.; William-Enemali, P.C.; Obodo, O.R.; Obiefuna, N.G. Revolutionizing Healthcare: How Telemedicine Is Improving Patient Outcomes and Expanding Access to Care. Cureus 2024, 16, e63881. [Google Scholar] [CrossRef]
- Jack, K.; McLean, S.M.; Moffett, J.K.; Gardiner, E. Barriers to treatment adherence in physiotherapy outpatient clinics: A systematic review. Man. Ther. 2010, 15, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Rawstorn, J.C.; Gant, N.; Rolleston, A.; Whittaker, R.; Stewart, R.; Benatar, J.; Warren, I.; Meads, A.; Jiang, Y.; Maddison, R. End Users Want Alternative Intervention Delivery Models: Usability and Acceptability of the REMOTE-CR Exercise-Based Cardiac Telerehabilitation Program. Arch. Phys. Med. Rehabil. 2018, 99, 2373–2377. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.B.; Mentz, R.J.; Sun, J.L.; Schulte, P.J.; Fleg, J.L.; Cooper, L.S.; Piña, I.L.; Leifer, E.S.; Kraus, W.E.; Whellan, D.J.; et al. Psychosocial Factors, Exercise Adherence, and Outcomes in Heart Failure Patients: Insights From Heart Failure: A Controlled Trial Investigating Outcomes of Exercise Training (HF-ACTION). Circ. Heart Fail. 2015, 8, 1044–1051. [Google Scholar] [CrossRef] [PubMed]



| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Age ≥ 18 years | Age < 18 years |
| NYHA class IV |
|
|
| Able to perform physical exercise | Inability to perform exercise due to medical conditions |
| Able to provide written informed consent | Severe cognitive impairment (e.g., advanced dementia) |
| Basic digital literacy (patient or caregiver, for tele-group) | No digital literacy and no caregiver (for tele-group) |
| Referred to cardiac rehabilitation | Participation in another clinical trial interfering with outcomes |
| — | Pregnancy or breastfeeding |
| Exercise Component | TELEREHABILITATION—YES (Remote) | TELEREHABILITATION—NO (In-Person) |
|---|---|---|
| Warm-up (10 min) |
|
|
| Endurance Training (40 min) | stationary cycling | stationary cycling |
| Cool-down (10 min) |
|
|
| Outcome Type | Outcome Measure | Unit of Measure | Domain | Clinical Relevance/Rationale | T0 (Baseline) | T1 (4 wks) | T2 (8 wks) | T3 (12 wks) | T4 (24 wks) |
|---|---|---|---|---|---|---|---|---|---|
| Primary | ≥10% improvement in VO2max (CPET) | mL/kg/min | Functional Capacity | Gold standard for evaluating aerobic capacity and predicting prognosis in CHF | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in body weight | Kg | Anthropometric | Important for monitoring nutritional status; weight fluctuations are clinically relevant predictors of CHF decompensation. | ✔ | ✔ | ✔ | ✔ | ✔ |
| Secondary | Change in BMI | kg/m2 | Anthropometric | Static measure to stratify overweight, obesity, which may influence exercise response and prognosis in CHF | ✔ | ✔ | ✔ | ✔ | ✔ |
| Secondary | Change in KCCQ-12 Score | Points (0–100) | QoL | CHF-specific QoL instrument sensitive to clinical changes | ✔ | ✔ | ✔ | ✔ | ✔ |
| Secondary | Change in SF-36 Score | Points (0–100) | QoL | Generic QoL questionnaire for broader health-related QoL | ✔ | ✔ | ✔ | ✔ | ✔ |
| Secondary | Change in 6MWT | Meters | Functional | Test of submaximal exercise capacity; reflects improvements in daily functional status | ✔ | ✔ | ✔ | ✔ | ✔ |
| Secondary | Change in SPPB Score | Points (0–12) | Functional | Assesses lower extremity strength, balance, and mobility—predictive of disability and frailty | ✔ | ✔ | ✔ | ✔ | ✔ |
| Secondary | Change in MMSE | points (0–30) | Cognitive | Screens for cognitive impairment, which can affect adherence and prognosis in CHF patients. | ✔ | ✔ | ✔ | ✔ | ✔ |
| Secondary | Change in BNP | pg/mL | Biochemical | Established biomarkers of myocardial stress; reflect CHF severity and response to therapy. | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in NT-proBNP | pg/mL | Biochemical | Established biomarkers of myocardial stress; reflect CHF severity and response to therapy. | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in Serum Creatinine | mg/dL | Biochemical | Monitor renal function, critical in CHF management due to cardiorenal interactions and therapy impact | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in eGFR | mL/min/1.73 m2 | Biochemical | Monitor renal function, critical in CHF management due to cardiorenal interactions and therapy impact | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in Serum Sodium | mmol/L | Biochemical | Important for assessing treatment safety (e.g., diuretics, ACE inhibitors) and arrhythmic risk | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in Serum Potassium | mmol/L | Biochemical | Important for assessing treatment safety (e.g., diuretics, ACE inhibitors) and arrhythmic risk | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in Serum Chloride | mmol/L | Biochemical | Important for assessing treatment safety (e.g., diuretics, ACE inhibitors) and arrhythmic risk | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in Glucose | mg/dL | Biochemical | Cardiovascular risk profile monitoring and potential metabolic benefits from exercise | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in Lipids | mg/dL | Biochemical | Cardiovascular risk profile monitoring and potential metabolic benefits from exercise | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in LVEF | % | Echocardiographic | Quantifies systolic and diastolic function; allows tracking of cardiac remodeling and hemodynamic response to rehabilitation | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in E/A and E/e’ | Ratio | Echocardiographic | Quantifies systolic and diastolic function; allows tracking of cardiac remodeling and hemodynamic response to rehabilitation | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Change in TAPSE, RVs’ | mm/cm/s | Echocardiographic | Quantifies systolic and diastolic function; allows tracking of cardiac remodeling and hemodynamic response to rehabilitation | ✔ | ✔ | ✔ | ✔ | |
| Secondary | Adverse Events | Number of events over the total number of treatments administered | Safety | Monitors safety and tolerability of both telerehabilitation and standard rehabilitation interventions | ✔ | ✔ | ✔ | ✔ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garofano, M.; Vecchione, C.; Calabrese, M.; Rusciano, M.R.; Visco, V.; Granata, G.; Carrizzo, A.; Galasso, G.; Bramanti, P.; Corallo, F.; et al. Evaluation of the Effectiveness of a Cardiac Telerehabilitation Program in Chronic Heart Failure: Design and Rationale of the TELEREHAB-HF Study. Healthcare 2025, 13, 2074. https://doi.org/10.3390/healthcare13162074
Garofano M, Vecchione C, Calabrese M, Rusciano MR, Visco V, Granata G, Carrizzo A, Galasso G, Bramanti P, Corallo F, et al. Evaluation of the Effectiveness of a Cardiac Telerehabilitation Program in Chronic Heart Failure: Design and Rationale of the TELEREHAB-HF Study. Healthcare. 2025; 13(16):2074. https://doi.org/10.3390/healthcare13162074
Chicago/Turabian StyleGarofano, Marina, Carmine Vecchione, Mariaconsiglia Calabrese, Maria Rosaria Rusciano, Valeria Visco, Giovanni Granata, Albino Carrizzo, Gennaro Galasso, Placido Bramanti, Francesco Corallo, and et al. 2025. "Evaluation of the Effectiveness of a Cardiac Telerehabilitation Program in Chronic Heart Failure: Design and Rationale of the TELEREHAB-HF Study" Healthcare 13, no. 16: 2074. https://doi.org/10.3390/healthcare13162074
APA StyleGarofano, M., Vecchione, C., Calabrese, M., Rusciano, M. R., Visco, V., Granata, G., Carrizzo, A., Galasso, G., Bramanti, P., Corallo, F., Pepe, L., Budaci, L., Ciccarelli, M., & Bramanti, A. (2025). Evaluation of the Effectiveness of a Cardiac Telerehabilitation Program in Chronic Heart Failure: Design and Rationale of the TELEREHAB-HF Study. Healthcare, 13(16), 2074. https://doi.org/10.3390/healthcare13162074


_MD__MPH_PhD.png)






