Disability and Factors Associated with Disability in the Discharge Transition Phase After Acquired Brain Injury: An Observational Follow-Up Study
Abstract
1. Introduction
1.1. Disability in the Discharge Transition Phase
1.2. Factors Affecting Disability in the Transition Phase
2. Materials and Methods
2.1. Participants and Recruitment
2.2. Baseline Characteristics
2.3. Outcome Measures
2.3.1. Disability
2.3.2. Covariates of Disability
2.3.3. Secondary Outcome Measures
2.4. Data Analysis and Statistics
3. Results
3.1. Patient Characteristics
3.2. Disability in the Discharge Transition Phase for the Respondents
3.3. Other Aspects of Functioning in the Discharge Transition Phase
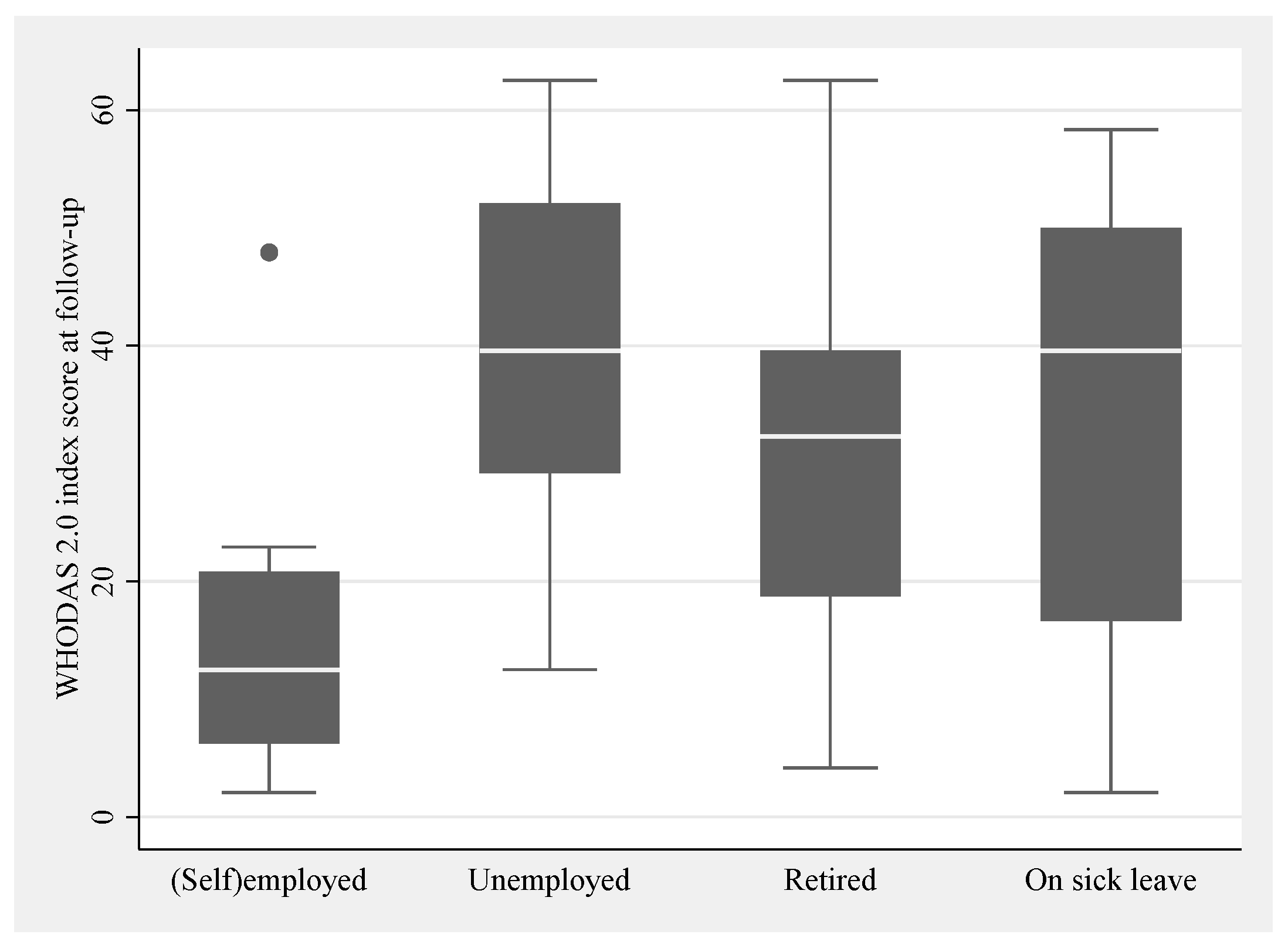
3.3.1. Work Status
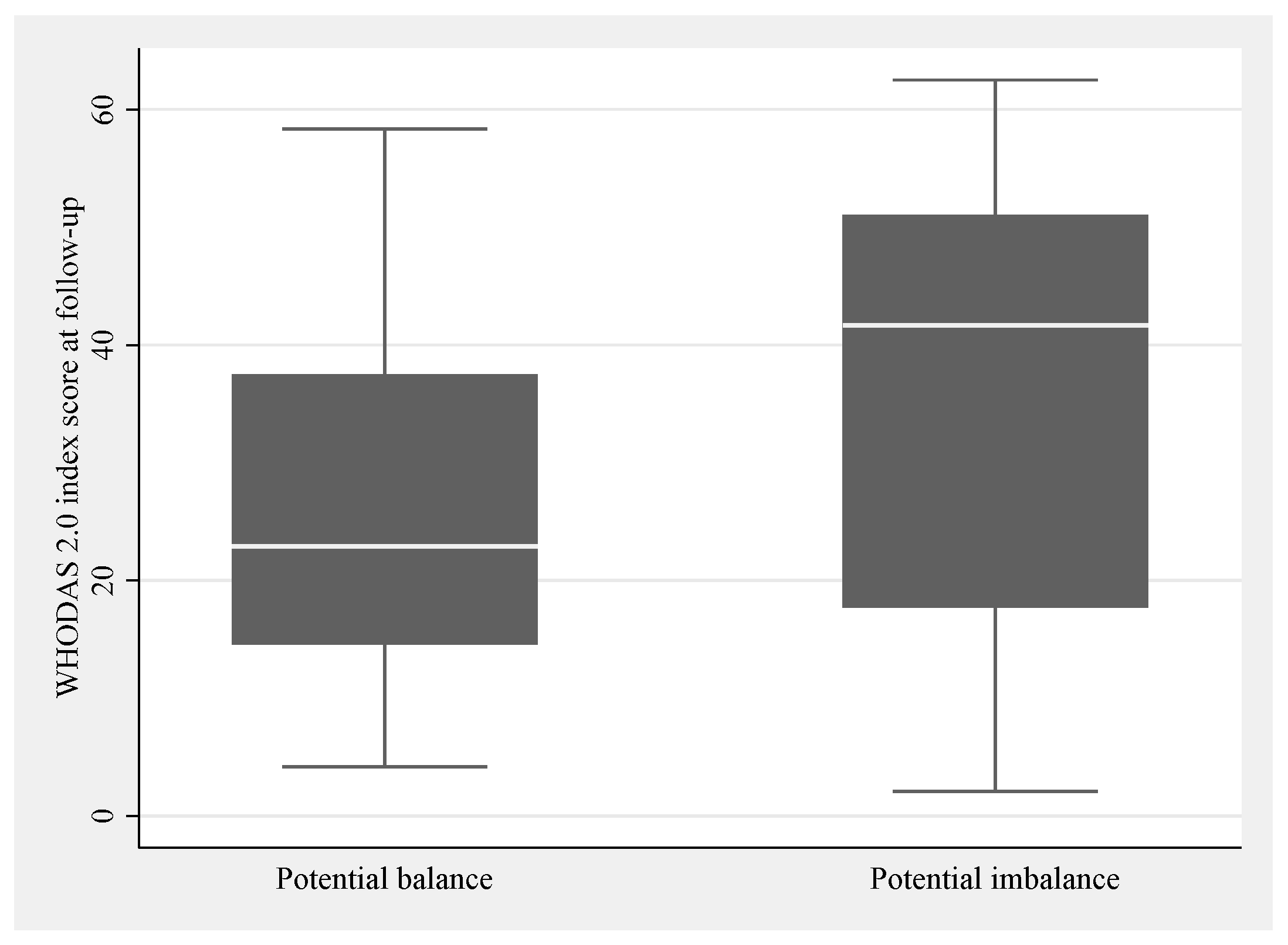
3.3.2. Occupational Balance
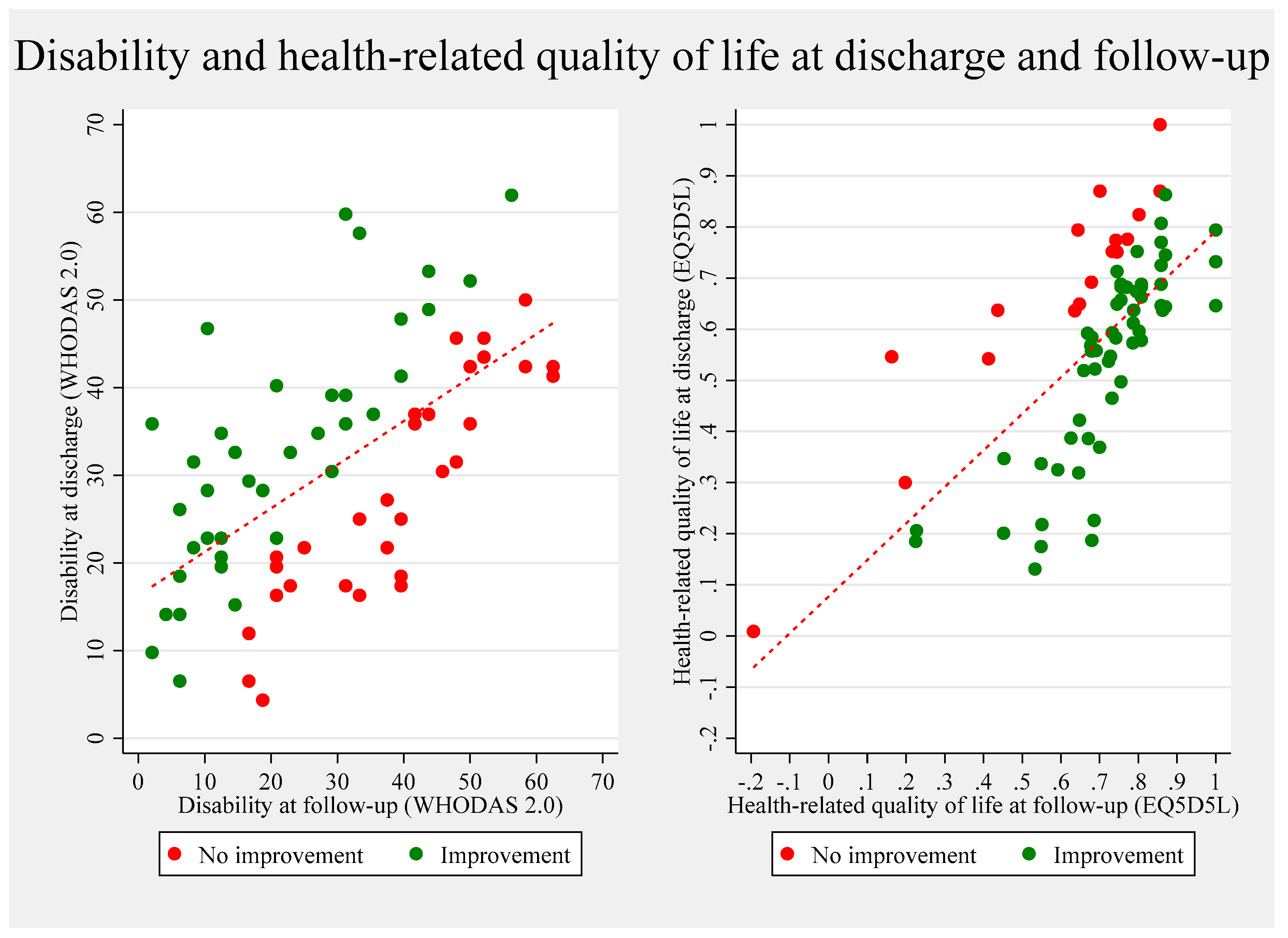
3.4. Patients at Follow-Up
4. Discussion
4.1. Comparison with Other Studies
4.1.1. Health-Related Quality of Life
4.1.2. Work
4.1.3. Occupational Balance
4.2. Clinical Implications—A Meaningful Life in the Discharge Transition Phase
4.3. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABI | Acquired Brain Injury |
| EQ-5D-5L | EuroQol Five-Dimensional Health-Related Quality of Life Assessment Tool |
| FIM | Functional Independence Measure |
| GSES | General Self-Efficacy Scale |
| HADS | Hospital Anxiety and Depression Scale |
| MFI | Multidimensional Fatigue Inventory |
| mRs | Modified Rankin Scale |
| OBQ-DK | Occupational Balance Questionnaire, Danish 11-item version |
| SG | Saltin-Grimsby Physical Activity Level Scale |
| WHODAS 2.0 | World Health Disability Assessment Schedule 2.0 |
Appendix A. Classification of Health
| Stroke | n = 106 | |
|---|---|---|
| NIHSS, mean (sd) | 96 | 7.12 (5.32) |
| Stroke subtype | 106 | |
| Ischaemic | 76 (72) | |
| Haemorrhage | 29 (27) | |
| Other | 1 (1) | |
| Stroke subtype, n (%) | 106 | |
| Lacunar | 0 (0) | |
| Large artery | 32 (30) | |
| Other | 65 (61) | |
| N/A | 9 (8) | |
| Stroke location, n (%) | 106 | |
| Cortical | 20 (19) | |
| Subcortical | 23 (22) | |
| Midbrain | 10 (9) | |
| Brainstem | 11 (10) | |
| Other | 39 (37) | |
| N/A | 3 (3) | |
| Affected area, n (%) | 106 | |
| Left | 46 (43) | |
| Right | 47 (44) | |
| Both | 10 (9) | |
| N/A | 3 (3) | |
| Thrombolysis/reperfusion therapy, yes n (%) | 106 | 26 (24) |
| Confirmed stroke on imaging, yes (%) | 106 (100) | |
| CT obtained | 63 (59) | |
| MRI obtained | 86 (81) |
Appendix A.1. Diagnosis
Appendix A.1.1. Stroke Severity
Appendix A.1.2. Other ABI Severity
Appendix A.1.3. Comorbidity
Appendix A.1.4. Functional Independence
Appendix B. Overview of PROM
| Construct | Patient Reported Outcome Measure (PROM) | Acronym | Baseline | Follow-Up |
|---|---|---|---|---|
| Disability | World Health Organisation Disability Assessment Schedule 36-items | WHODAS 2.0 | × | |
| General self-efficacy | General self-efficacy Scale | GSES | × | |
| Fatigue | Multi-dimensional Fatigue Inventory | MFI | × | |
| Physical activity level | Saltin-Grimsby Physical Activity Level Scale | SG | × | |
| Anxiety and Depression | The Hospital Anxiety and Depression Scale | HADS | × | |
| Health-related quality of life | EuroQol index value 5 Likert scale | EQ5D-5L | × | × |
| Work status | Customized questions | Work | × | × |
| Disability | World Health Organisation Disability Assessment Schedule 12-items | WHODAS 2.0 | × | |
| Occupational balance | Occupational Balance Questionnaire—Danish version | OBQ-DK | × | |
| Recovery | Two Simple Questions | × | ||
| Personal assistance requirements | Two Simple Questions | × | ||
| Hospital readmittance | Customized question (Yes/no) | × | ||
| Attending community-based rehabilitation | Customized question (Yes/no and type) | × |
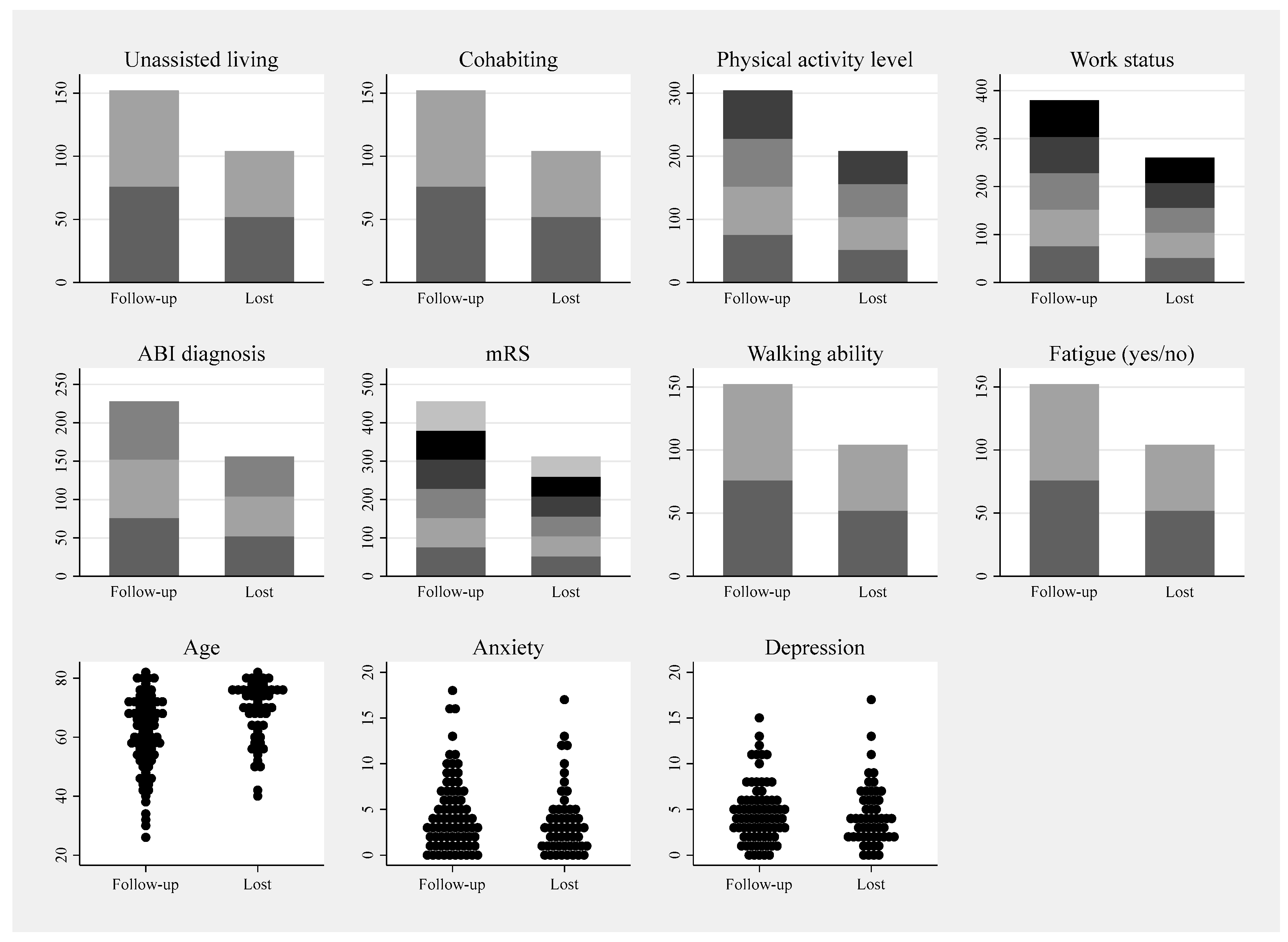
Appendix C. Plots of Variables from Table 2 for Visual Inspection of Drop-Out Selection

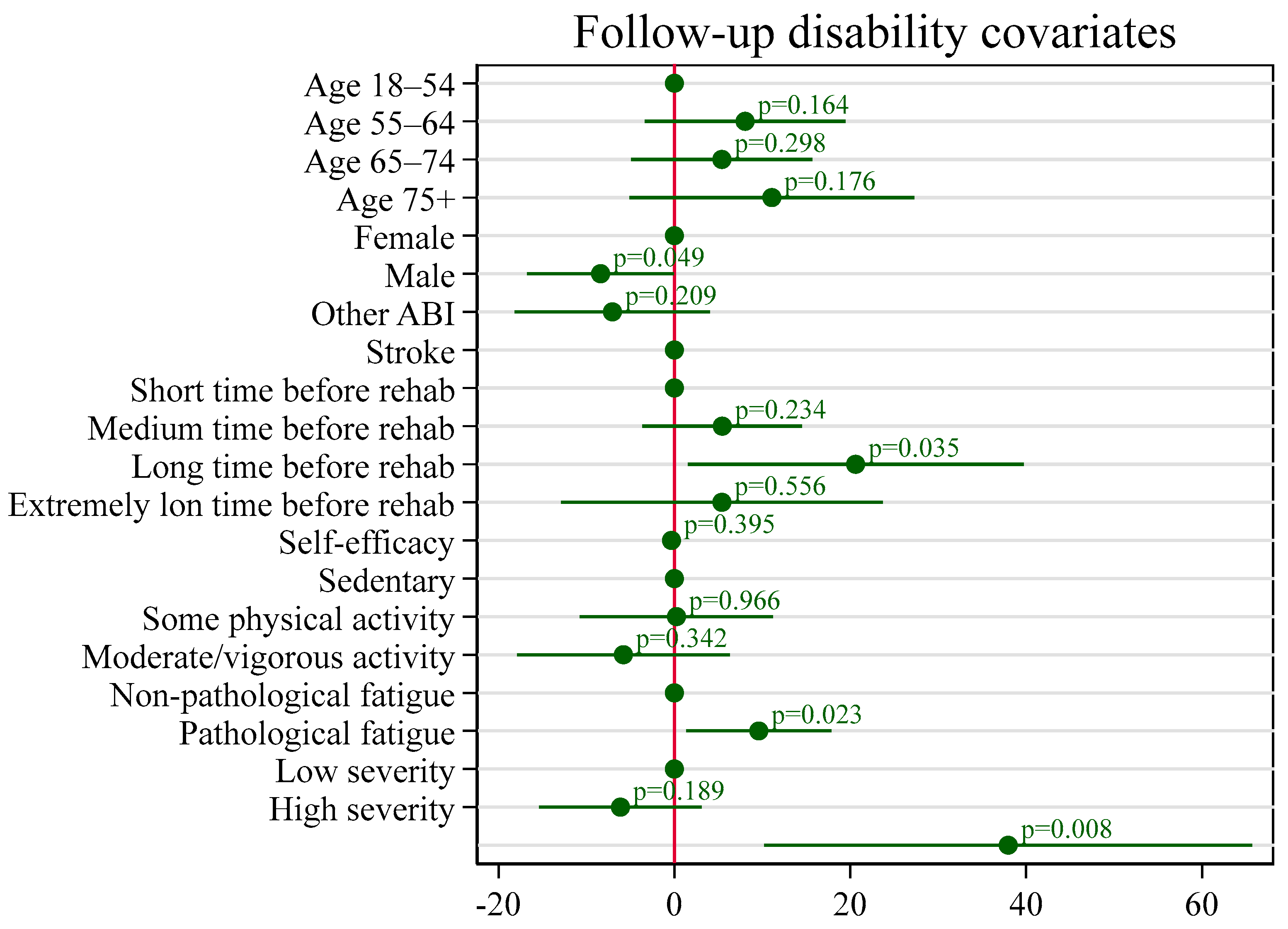
Appendix D. Covariates for Disability in the Discharge Transition Phase
| Disability at Follow-Up | ||||
|---|---|---|---|---|
| n = 62 | Estimate (β) | se | CI 2.5% | CI 97.5% |
| Reference value | 37.96 | 13.84 | 10.22 | 65.70 |
| Age | ||||
| 18–54 years old | Reference | |||
| 55–64 years old | 8.04 | 5.69 | −3.38 | 19.46 |
| 65–74 years old | 5.40 | 5.14 | −4.90 | 15.70 |
| 75 years and older | 11.08 | 8.08 | −5.11 | 27.28 |
| Sex | ||||
| Female | Reference | |||
| Male | −8.39 * | 4.17 | −16.75 | −0.04 |
| ABI type | ||||
| Stroke | Reference | |||
| Other | −7.05 | 5.54 | −18.16 | 4.06 |
| Duration from ABI to rehab | ||||
| Short 1–7 days | Reference | |||
| Medium 7–27 days | 5.45 | 4.53 | −3.63 | 14.54 |
| Long 28–44 days | 20.61 * | 9.52 | 1.53 | 39.70 |
| Very long +45 days | 5.41 | 9.13 | −12.89 | 23.71 |
| Self-efficacy (GSES) | 0.39 | −0.86 | −1.11 | 0.44 |
| Premorbid PA level | ||||
| Sedentary | Reference | |||
| Some PA | 0.24 | 5.49 | −10.77 | 11.24 |
| Moderate & vigorous PA | −5.79 | 6.04 | −17.90 | 6.31 |
| Pathological fatigue | ||||
| No | Reference | |||
| Yes | 9.60 * | 4.11 | 1.36 | 17.85 |
| FIM Cognitive subscore | ||||
| Low level (sum < 29) | Reference | |||
| High level (sum ≥ 30) | −6.13 | 4.61 | −15.38 | 3.11 |
References
- Maino, D.; Bartuccio, M.; Taub, M.B. Acquired Brain Injury; Wolters Kluwer: Riverwoods, IL, USA, 2012. [Google Scholar]
- Lucchesi, L.R.; Agrawal, S.; Ahmadi, A.; Aichour, A.N.; Altirkawi, K.; Ariani, F.; Awasthi, A.; Badali, H.; Banstola, A.; Bärnighausen, T.W.; et al. Global, regional, and national burden of traumatic brain injury and spinal cord injury, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Turner-Stokes, L.; Pick, A.; Nair, A.; Disler, P.B.; Wade, D.T.; Turner-Stokes, L. Multi-disciplinary rehabilitation for acquired brain injury in adults of working age. Cochrane Database Syst. Rev. 2015, 2015, CD004170. [Google Scholar] [CrossRef] [PubMed]
- Hartman-Maeir, A.; Soroker, N.; Ring, H.; Avni, N.; Katz, N. Activities, participation and satisfaction one-year post stroke. Disabil. Rehabil. 2007, 29, 559–566. [Google Scholar] [CrossRef]
- Kılınç, S.; Erdem, H.; Healey, R.; Cole, J. Finding meaning and purpose: A framework for the self-management of neurological conditions. Disabil. Rehabil. 2022, 44, 219–230. [Google Scholar] [CrossRef]
- Stucki, G.; Bickenbach, J. Functioning: The third health indicator in the health system and the key indicator for rehabilitation. Eur. J. Phys. Rehabil. Med. 2017, 53, 134–138. [Google Scholar] [CrossRef]
- ICF: International Classification of Functioning, Disability and Health; WHO: Geneva, Switzerland, 2001; p. 299.
- Carmo, J.F.D.; Morelato, R.L.; Pinto, H.P.; Oliveira, E.R.A.D. Disability after stroke: A systematic review. Fisioter. Em Mov. 2015, 28, 407–418. [Google Scholar] [CrossRef]
- World Health Organization; Kostanjsek, N.; Chatterji, S.; Rehm, J. Measuring Health and Disability: Manual for WHO Disability Assessment Schedule (WHODAS 2.0); World Health Organization: Albany, NY, USA, 2010. [Google Scholar]
- Pösl, M.; Cieza, A.; Stucki, G. Psychometric properties of the WHODASII in rehabilitation patients. Qual. Life Res. 2007, 16, 1521–1531. [Google Scholar] [CrossRef] [PubMed]
- Snell, D.L.; Silverberg, N.D. Derivation of minimal clinically important difference score for the WHODAS 2.0 in mild traumatic brain injury. NeuroRehabilitation 2022, 52, 249–257. [Google Scholar] [CrossRef]
- Young, C.A.; Rog, D.J.; Sharrack, B.; Constantinescu, C.; Kalra, S.; Harrower, T.; Langdon, D.; Tennant, A.; Mills, R.J.; the Trajectories of Outcome in Neurological Conditions Study Group. Measuring disability in multiple sclerosis: The WHODAS 2.0. Qual. Life Res. 2023, 32, 3235–3246. [Google Scholar] [CrossRef]
- Ciamarra, P.; Corbi, G.; Gimigliano, F.; Feola, A.; Campobasso, C.P. The World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) as a measure among elderly population: A review. Disabil. Rehabil. 2024, 47, 3773–3780. [Google Scholar] [CrossRef]
- Saltychev, M.; Katajapuu, N.; Bärlund, E.; Laimi, K. Psychometric properties of 12-item self-administered World Health Organization Disability Assessment Schedule 2.0 (WHODAS 2.0) among general population and people with non-acute physical causes of disability: A systematic review. Disabil. Rehabil. 2021, 43, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Sozańska, A.; Sozański, B.; Łagowska-Sado, A.; Wilmowska-Pietruszyńska, A.; Wiśniowska-Szurlej, A. Psychometric properties of Polish version of the 36-item WHODAS 2.0 in individuals with fibromyalgia. Sci. Rep. 2024, 14, 22192. [Google Scholar] [CrossRef]
- dos Santos, H.M.; Pereira, G.S.; de Oliveira, L.C.; da Silva, P.K.; Lima, M.G.; Feliz, V.H.A.d.A.; Silva, S.M. Diagnostic accuracy of the World Health Organization Disability Assessment Schedule (WHODAS 2.0) to estimate disability after stroke. Disabil. Rehabil. 2022, 45, 2169–2174. [Google Scholar] [CrossRef]
- Prodinger, B.; Kucukdeveci, A.A.; Kutlay, S.; Elhan, A.H.; Kreiner, S.; Tennant, A. Cross-diagnostic scale-banking using Rasch analysis: Developing a common reference metric for generic and health condition-specific scales in people with rheumatoid arthritis and stroke. J. Rehabil. Med. 2020, 52, jrm00107. [Google Scholar] [CrossRef]
- White, M.C.; Randall, K.; Alcorn, D.; Greenland, R.; Glasgo, C.; Shrime, M.G. Measurement of patient reported disability using WHODAS 2.0 before and after surgical intervention in Madagascar. BMC Health Serv. Res. 2018, 18, 305. [Google Scholar] [CrossRef]
- Maribo, T. Hvidbog om Rehabilitering, 1st ed.; Rehabiliteringsforum Danmark: Aarhus, Danmark, 2022. [Google Scholar]
- World Health Organization. Constitution of the World Health Organization; World Health Organization: Geneva, Switzerland, 1948. [Google Scholar]
- Aadal, L.; Hundborg, M.O.; Pallesen, H.; Steensgaard, R. A meaningful everyday life experienced by adults with acquired neurological impairments: A scoping review. PLoS ONE 2023, 18, e0286928. [Google Scholar] [CrossRef]
- Kjeldsen, S.S.; Søndergaard, S.; Mikkelsen, L.R.; Nielsen, J.F. A retrospective study of 251 patients admitted to a multidisciplinary, neurorehabilitation unit with intensive care unit capabilities. Disabil. Rehabil. 2018, 42, 528–535. [Google Scholar] [CrossRef]
- Turner, B.J.; Fleming, J.M.; Ownsworth, T.L.; Cornwell, P.L. The transition from hospital to home for individuals with acquired brain injury: A literature review and research recommendations. Disabil. Rehabil. 2008, 30, 1153–1176. [Google Scholar] [CrossRef] [PubMed]
- Maude, R.; Christopher, F.; Craig, B.; Jaber, F.G. The experience of time in the transition from hospital to home following stroke. J. Rehabil. Res. Dev. 2004, 41, 259–268. [Google Scholar]
- Fraser, C. The experience of transition for a daughter caregiver of a stroke survivor. J. Neurosci. Nurs. 1999, 31, 9–16. [Google Scholar] [CrossRef]
- Piccenna, L.; Lannin, N.A.; Gruen, R.; Pattuwage, L.; Bragge, P. The experience of discharge for patients with an acquired brain injury from the inpatient to the community setting: A qualitative review. Brain Inj. 2016, 30, 241–251. [Google Scholar] [CrossRef]
- Turner, B.; Fleming, J.; Cornwell, P.; Worrall, L.; Ownsworth, T.; Haines, T.; Kendall, M.; Chenoweth, L. A qualitative study of the transition from hospital to home for individuals with acquired brain injury and their family caregivers. Brain Inj. 2007, 21, 1119–1130. [Google Scholar] [CrossRef]
- Aas, R.W.; Haveraaen, L.A.; Brouwers, E.P.M.; Skarpaas, L.S. Who among patients with acquired brain injury returned to work after occupational rehabilitation? The rapid-return-to-work-cohort-study. Disabil. Rehabil. 2018, 40, 2561–2570. [Google Scholar] [CrossRef]
- Barker, R.N.; Gill, T.J.; Brauer, S.G. Factors contributing to upper limb recovery after stroke: A survey of stroke survivors in Queensland Australia. Disabil. Rehabil. 2007, 29, 981–989. [Google Scholar] [CrossRef]
- Mortensen, J.; Kjeldsen, S.S.; Honoré, H.; Pedersen, A.R. Using Routinely Gathered Clinical Data to Develop a Prognostic Online Tool for Decannulation in Subjects With Acquired Brain Injury. Respir. Care 2020, 65, 1678–1686. [Google Scholar] [CrossRef]
- Verdugo, M.A.; Fernández, M.; Gómez, L.E.; Amor, A.M.; Aza, A. Predictive factors of quality of life in acquired brain injury. Int. J. Clin. Health Psychol. 2019, 19, 189–197. [Google Scholar] [CrossRef]
- Maaijwee, N.A.M.M.; Arntz, R.M.; Rutten-Jacobs, L.C.A.; Schaapsmeerders, P.; Schoonderwaldt, H.C.; van Dijk, E.J.; de Leeuw, F.-E. Post-stroke fatigue and its association with poor functional outcome after stroke in young adults. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1120–1126. [Google Scholar] [CrossRef] [PubMed]
- Palm, S.; Rönnbäck, L.; Johansson, B. Long-term mental fatigue after traumatic brain injury and impact on employment status. J. Rehabil. Med. 2017, 49, 228–233. [Google Scholar] [CrossRef] [PubMed]
- van de Port, I.G.L.; Kwakkel, G.; Schepers, V.P.M.; Heinemans, C.T.I.; Lindeman, E. Is Fatigue an Independent Factor Associated with Activities of Daily Living, Instrumental Activities of Daily Living and Health-Related Quality of Life in Chronic Stroke? Cerebrovasc. Dis. 2007, 23, 40–45. [Google Scholar] [CrossRef]
- Korpershoek, C.; van der Bijl, J.; Hafsteinsdottir, T.B. Self-efficacy and its influence on recovery of patients with stroke: A systematic review. J. Adv. Nurs. 2011, 67, 1876–1894. [Google Scholar] [CrossRef] [PubMed]
- Allahverdipour, H.; Asgharijafarabadi, M.; Heshmati, R.; Hashemiparast, M. Functional status, anxiety, cardiac self-efficacy, and health beliefs of patients with coronary heart disease. Health Promot. Perspect. 2013, 3, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Worm, M.S.; Valentin, J.B.; Johnsen, S.P.; Nielsen, J.F.; Forchhammer, H.B.; Svendsen, S.W. Predictors of disability in adolescents and young adults with acquired brain injury after the acute phase. Brain Inj. 2021, 35, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Ruegsegger, G.N.; Booth, F.W. Health Benefits of Exercise. Cold Spring Harb. Perspect. Med. 2018, 8, a029694. [Google Scholar] [CrossRef]
- Gorgoraptis, N.; Zaw-Linn, J.; Feeney, C.; Tenorio-Jimenez, C.; Niemi, M.; Malik, A.; Ham, T.; Goldstone, A.P.; Sharp, D.J. Cognitive impairment and health-related quality of life following traumatic brain injury. NeuroRehabilitation 2019, 44, 321–331. [Google Scholar] [CrossRef]
- Saver, J.L.; Filip, B.; Hamilton, S.; Yanes, A.; Craig, S.; Cho, M.; Conwit, R.; Starkman, S. Improving the reliability of stroke disability grading in clinical trials and clinical practice: The Rankin Focused Assessment (RFA). Stroke 2010, 41, 992–995. [Google Scholar] [CrossRef]
- Snaith, R.P.; Zigmond, A.S. The hospital anxiety and depression scale. Br. Med. J. (Clin. Res. Ed.) 1986, 292, 344. [Google Scholar] [CrossRef] [PubMed]
- Ćwirlej-Sozańska, A.; Wilmowska-Pietruszyńska, A.; Sozański, B.; Wiśniowska-Szurlej, A. Analysis of Chronic Illnesses and Disability in a Community-Based Sample of Elderly People in South-Eastern Poland. Med. Sci. Monit. 2018, 24, 1387–1396. [Google Scholar] [CrossRef]
- Schwarzer, R.; Jerusalem, M. Measures in Health Psychology: A User’s Portfolio. Causal and Control Beliefs. In Causal and Control Beliefs; Weinman, S.W.J., Johnston, M., Eds.; NFER-NELSON: Windsor, UK, 1995; Volume 1, pp. 35–37. [Google Scholar]
- Rödjer, L.; Jonsdottir, I.H.; Rosengren, A.; Burell, G.; Karlsson, D.; Bengtsson, H.; Ahlborg, G., Jr. Self-reported leisure time physical activity: A useful assessment tool in everyday health care. BMC Public Health 2012, 12, 693. [Google Scholar] [CrossRef]
- Manoli, R.; Chartaux-Danjou, L.; Delecroix, H.; Daveluy, W.; Moroni, C. Is Multidimensional Fatigue Inventory (MFI-20) adequate to measure brain injury related fatigue? Disabil. Health J. 2020, 13, 100913. [Google Scholar] [CrossRef]
- Christensen, D.; Johnsen, S.P.; Watt, T.; Harder, I.; Kirkevold, M.; Andersen, G. Dimensions of Post-Stroke Fatigue: A Two-Year Follow-Up Study. Cerebrovasc. Dis. 2008, 26, 134–141. [Google Scholar] [CrossRef]
- Stubbs, P.W.; Pallesen, H.; Pedersen, A.R.; Nielsen, J.F. Using EFA and FIM rating scales could provide a more complete assessment of patients with acquired brain injury. Disabil. Rehabil. 2014, 36, 2278–2281. [Google Scholar] [CrossRef] [PubMed]
- Cathrine Elgaard, J.; Sabrina Storgaard, S.; Claire, G.; Morten Berg, J.; Kjeld Møller, P.; Lars Holger, E. The Danish EQ-5D-5L Value Set: A Hybrid Model Using cTTO and DCE Data. Appl. Health Econ. Health Policy 2021, 19, 579–591. [Google Scholar]
- Wagman, P.; Håkansson, C. Introducing the Occupational Balance Questionnaire (OBQ). Scand. J. Occup. Ther. 2014, 21, 227–231. [Google Scholar] [CrossRef]
- Håkansson, C.; Wagman, P.; Hagell, P. Construct validity of a revised version of the Occupational Balance Questionnaire. Scand. J. Occup. Ther. 2019, 27, 441–449. [Google Scholar] [CrossRef]
- Lindley, R.I.; Waddell, F.; Livingstone, M.; Sandercock, P.; Dennis, M.S.; Slattery, J.; Smith, B.; Warlow, C. Can Simple Questions Assess Outcome after Stroke? Cerebrovasc. Dis. 1994, 4, 314–324. [Google Scholar] [CrossRef]
- McKevitt, C.; Dundas, R.; Wolfe, C. Two simple questions to assess outcome after stroke: A European study. Stroke 2001, 32, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Appelros, P.; Stegmayr, B.; Terent, A. A review on sex differences in stroke treatment and outcome. Acta Neurol. Scand. 2010, 121, 359–369. [Google Scholar] [CrossRef]
- Sandset, E.C.; Ferretti, M.T. Sex and gender differences in stroke—The need for individualised follow-up. Eur. J. Neurol. 2021, 28, 365–366. [Google Scholar] [CrossRef]
- Svendsen, H.A.; Teasdale, T.W. The influence of neuropsychological rehabilitation on symptomatology and quality of life following brain injury: A controlled long-term follow-up. Brain Inj. 2006, 20, 1295–1306. [Google Scholar] [CrossRef]
- Gage, M.; Cook, J.V.; Fryday-Field, K. Understanding the transition to community living after discharge from an acute care hospital: An exploratory study. Am. J. Occup. Ther. 1997, 51, 96–103. [Google Scholar] [CrossRef]
- Coretti, S.; Ruggeri, M.; McNamee, P. The minimum clinically important difference for EQ-5D index: A critical review. Expert. Rev. Pharmacoecon. Outcomes Res. 2014, 14, 221–233. [Google Scholar] [CrossRef]
- Walters, S.J.; Brazier, J.E. Comparison of the Minimally Important Difference for Two Health State Utility Measures: EQ-5D and SF-6D. Qual. Life Res. 2005, 14, 1523–1532. [Google Scholar] [CrossRef]
- Oyewole, O.O.; Ogunlana, M.O.; Gbiri, C.A.O.; Oritogun, K.S.; Osalusi, B.S. Impact of post-stroke disability and disability-perception on health-related quality of life of stroke survivors: The moderating effect of disability-severity. Neurol. Res. 2020, 42, 835–843. [Google Scholar] [CrossRef] [PubMed]
- Tengs, T.O.; Yu, M.; Luistro, E. Health-related quality of life after stroke a comprehensive review. Stroke 2001, 32, 964–972. [Google Scholar] [CrossRef]
- Barclay-Goddard, R.P.; King, J.P.; Dubouloz, C.-J.P.; Schwartz, C.E.S. Building on Transformative Learning and Response Shift Theory to Investigate Health-Related Quality of Life Changes Over Time in Individuals With Chronic Health Conditions and Disability. Arch. Phys. Med. Rehabil. 2012, 93, 214–220. [Google Scholar] [CrossRef]
- Barclay, R.; Tate, R.B. Response shift recalibration and reprioritization in health-related quality of life was identified prospectively in older men with and without stroke. J. Clin. Epidemiol. 2014, 67, 500–507. [Google Scholar] [CrossRef]
- Wade, D.T. What is rehabilitation? An empirical investigation leading to an evidence-based description. Clin. Rehabil. 2020, 34, 571–583. [Google Scholar] [CrossRef]
- World Health Organization. World Report on Disability 2011; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Buse, E.R.; Grunow, J.J.; Spies, C.D.; Weiss, B.; Paul, N. Health-related quality of life correlates with patient- and proxy-reported disability in critical illness survivors: A secondary analysis of the ERIC trial. Crit. Care 2025, 29, 158. [Google Scholar] [CrossRef] [PubMed]
- van Velzen, J.M.; van Bennekom, C.A.M.; Edelaar, M.J.A.; Sluiter, J.K.; Frings-Dresen, M.H.W. How many people return to work after acquired brain injury?: A systematic review. Brain Inj. 2009, 23, 473–488. [Google Scholar] [CrossRef]
- Burke, V.; O’Rourke, L.; Duffy, E. Returning to work after acquired brain injury: A mixed method case study. J. Vocat. Rehabil. 2021, 55, 297–312. [Google Scholar] [CrossRef]
- Kassberg, A.-C.; Nyman, A.; Larsson Lund, M. Perceived occupational balance in people with stroke. Disabil. Rehabil. 2021, 43, 553–558. [Google Scholar] [CrossRef]
- Ortiz-Rubio, A.; Cabrera-Martos, I.; Haro-Piedra, E.; López-López, L.; Rodríguez-Torres, J.; Granados-Santiago, M.; Valenza, M.C. Exploring perceived occupational balance in women with fibromyalgia. A descriptive study. Scand. J. Occup. Ther. 2022, 29, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Wagman, P.; Hjärthag, F.; Håkansson, C.; Hedin, K.; Gunnarsson, A.B. Factors associated with higher occupational balance in people with anxiety and/or depression who require occupational therapy treatment. Scand. J. Occup. Ther. 2021, 28, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Honoré, H.; Boll, M.; Hansen, A.Ø.; Kristensen, H.K. Putting occupational balance on the radar—Content validity of the 11-item Danish occupational balance questionnaire. Br. J. Occup. Ther. 2023, 87, 169–180. [Google Scholar] [CrossRef]
- Gasquoine, P.G. Blissfully unaware: Anosognosia and anosodiaphoria after acquired brain injury. Neuropsychol. Rehabil. 2016, 26, 261–285. [Google Scholar] [CrossRef]
- Moro, V.; Besharati, S.; Scandola, M.; Bertagnoli, S.; Gobbetto, V.; Ponzo, S.; Bulgarelli, C.; Jenkinson, P.M. The Motor Unawareness Assessment (MUNA): A new tool for the assessment of Anosognosia for hemiplegia. J. Clin. Exp. Neuropsychol. 2021, 43, 91–104. [Google Scholar] [CrossRef]
- Berti, A.; Làdavas, E.; Della Corte, M. Anosognosia for hemiplegia, neglect dyslexia, and drawing neglect: Clinical findings and theoretical considerations. J. Int. Neuropsychol. Soc. 1996, 2, 426–440. [Google Scholar] [CrossRef] [PubMed]
- Bisiach, E.; Vallar, G.; Perani, D.; Papagno, C.; Berti, A. Unawareness of disease following lesions of the right hemisphere: Anosognosia for hemiplegia and anosognosia for hemianopia. Neuropsychologia 1986, 24, 471–482. [Google Scholar] [CrossRef]
- Wade, D. Rehabilitation—A new approach. Part four: A new paradigm, and its implications. Clin. Rehabil. 2016, 30, 109–118. [Google Scholar] [CrossRef]
- Tarvonen-Schröder, S.; Hurme, S.; Laimi, K. The World Health Organization Disability Assessment Schedule (WHODAS 2.0) and the WHO Minimal Generic Set of Domains of Functioning and Health versus Conventional Instruments in subacute stroke. J. Rehabil. Med. 2019, 51, 675–682. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, Q.; Yu, Y.; Xiao, S.; Chen, L.; Khoshnood, K.; Zheng, S. Proxy reliability of the 12-item world health organization disability assessment schedule II among adult patients with mental disorders. Qual. Life Res. 2020, 29, 2219–2229. [Google Scholar] [CrossRef] [PubMed]
- Maher, C.A.; Olds, T.; Williams, M.T.; Lane, A.E. Self-Reported Quality of Life in Adolescents with Cerebral Palsy. Phys. Occup. Ther. Pediatr. 2008, 28, 41–57. [Google Scholar] [CrossRef]
- Synne Garder, P.; Oddgeir, F.; Guri Anita, H.; Cathrine, A.; Henriette Holm, S.; Gyrd, T.; Jørgen Feldbæk, N.; Audny, A. Stroke-Specific Quality of life one-year post-stroke in two Scandinavian country-regions with different organisation of rehabilitation services: A prospective study. Disabil. Rehabil. 2021, 43, 3810–3820. [Google Scholar]
- Cerniauskaite, M.; Quintas, R.; Koutsogeorgou, E.; Meucci, P.; Sattin, D.; Leonardi, M.; Raggi, A. Quality-of-life and disability in patients with stroke. Am. J. Phys. Med. Rehabil. 2012, 91, S39–S47. [Google Scholar] [CrossRef] [PubMed]
- Sundhedsstyrelsen. Anbefalinger for Tværsektorielle Forløb for Voksne med Erhvervet Hjerneskade [Cross-Sectoral Recommendations for Adults with Acquired Brain Damage in Denmark]; Sundhedsstyrelsen: København, Denmark, 2020. [Google Scholar]
- Kwakkel, G.; Lannin, N.A.; Borschmann, K.; English, C.; Ali, M.; Churilov, L.; Saposnik, G.; Winstein, C.; Van Wegen, E.E.; Wolf, S.L.; et al. Standardized measurement of sensorimotor recovery in stroke trials: Consensus-based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Int. J. Stroke 2017, 12, 451–461. [Google Scholar] [CrossRef]
- Askim, T.; Bernhardt, J.; Churilov, L.; Indredavik, B. The Scandinavian Stroke Scale is equally as good as The National Institutes of Health Stroke Scale in identifying 3-month outcome. J. Rehabil. Med. 2016, 48, 909–912. [Google Scholar] [CrossRef] [PubMed]
- Gray, L.J.; Ali, M.; Lyden, P.D.; Bath, P.M.; Virtual International Stroke Trials Archive Collaboration. Interconversion of the National Institutes of Health Stroke Scale and Scandinavian Stroke Scale in Acute Stroke. J. Stroke Cerebrovasc. Dis. 2009, 18, 466–468. [Google Scholar] [CrossRef]
- Teasdale, G.; Jennett, B. Assessment of coma and impaired consciousness. A practical scale. Lancet 1974, 2, 81–84. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
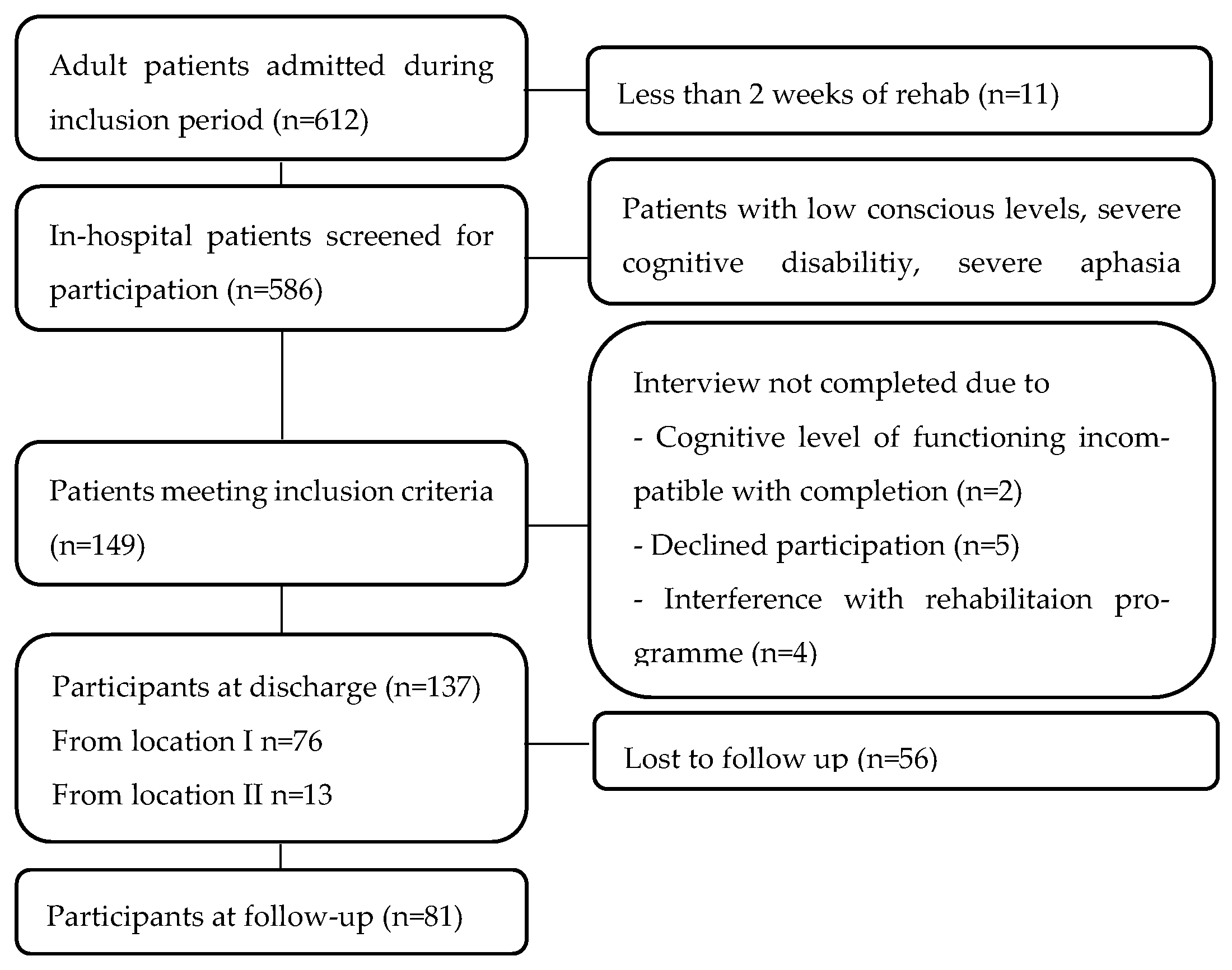
| Characteristics | Included n = 137 | Excluded n = 449 | p-Value | |
|---|---|---|---|---|
| Age in years a | 63 (13) | 62 (14) | 0.72 1 | |
| Sex male c | 86 (62) | 268 (60) | 0.69 2 | |
| Diagnosis and severity at onset | ||||
| ABI diagnosis c | Stroke | 106 (77) | 334 (74) | 0.98 2 |
| SAH | 6 (4) | 26 (6) | ||
| Trauma | 8 (6) | 27 (6) | ||
| Encephalopathy | 6 (4) | 23 (5) | ||
| Other | 11 (8) | 39 (9) | ||
| Charlson Comorbidity Index b | 5.13 (4.12) | 5.97 (6.52) | 0.56 2 | |
| Days from ABI to rehab admission b | 10 (15) | 13 (18) | 0.85 3 | |
| Rehab days b | 51 (29) | 51 (30) | 0.97 3 | |
| Functional independence measure b | Cognitive | 27.5 (20) | 26 (22) | <0.01 3 |
| Motor | 81 (59) | 81 (78) | 0.87 3 | |
| Baseline (n = 137) | Participants at Follow-Up (n = 81) | Lost to Follow-Up (n = 56) | |||
|---|---|---|---|---|---|
| Pre-morbid characteristics | Possible range | ||||
| Age in years a | - | 63 (13) | 60 (13) | 68 (13) | |
| Sex male c | Yes/No | 86 (62) | 50 (62) | 35 (63) | |
| Unassisted living c | Yes/No | 131 (96) | 78 (96) | 53 (95) | |
| Cohabiting c | Yes/No | 98 (72) | 61 (75) | 37 (66) | |
| Physical activity level (SG) c | Sedentary | Yes/No | 37 (27) | 19 (23) | 18 (32) |
| Some physical activity | Yes/No | 59 (43) | 37 (46) | 22 (39) | |
| Moderate physical activity | Yes/No | 39 (29) | 23 (28) | 16 (29) | |
| Vigorous physical activity | Yes/No | 2 (1) | 2 (2) | 0 (0) | |
| Work status c | (Self-)employed | Yes/No | 56 (41) | 38 (47) | 18 (32) |
| Unemployed | Yes/No | 5 (4) | 4 (5) | 1 (2) | |
| Retired | Yes/No | 68 (50) | 32 (40) | 36 (64) | |
| Student | Yes/No | 1 (1) | 1 (1) | 0 (0) | |
| On sick leave | Yes/No | 7 (5) | 6 (7) | 1 (0) | |
| Diagnosis, severity and duration | |||||
| ABI diagnosis c | Stroke | Yes/No | 106 (77) | 62 (77) | 44 (79) |
| SAH | Yes/No | 6 (4) | 5 (6) | 1 (2) | |
| Trauma | Yes/No | 8 (6) | 6 (7) | 2 (4) | |
| Encephalopathy | Yes/No | 6 (4) | 2 (2) | 4 (7) | |
| Other | Yes/No | 11 (8) | 6 (7) | 5 (9) | |
| ABI severity c | Mild | Yes/No | 69 (50) | 43 (53) | 26 (47) |
| Moderate | Yes/No | 35 (26) | 21 (26) | 14 (25) | |
| Severe | Yes/No | 33 (24) | 17 (21) | 16 (29) | |
| Days from ABI to rehab admission b | - | 10 (15) | 10 (13) | 10 (22) | |
| Rehab days b | - | 43 (34) | 42 (44) | 46 (28) | |
| Health and functioning | |||||
| Charlson Comorbidity Index b | 0–100 | 3 (3) | 3 (3) | 3 (3) | |
| Functional independence measure b | Cognitive | 5–13 | 28 (7) | 29 (7) | 26 (9) |
| Motor | 13–91 | 81 (15) | 83 (13) | 80 (17) | |
| Modified Rankin Scale score (mRs) c | No symptoms | Yes/No | 2 (1) | 1 (1) | 1 (2) |
| No significant disability | Yes/No | 44 (32) | 24 (30) | 20 (36) | |
| Slight disability | Yes/No | 35 (26) | 27 (33) | 8 (14) | |
| Moderate disability | Yes/No | 29 (21) | 14 (17) | 15 (27) | |
| Moderate-severe disability | Yes/No | 26 (19) | 14 (17) | 12 (21) | |
| Severe disability | Yes/No | 1 (1) | 1 (1) | 0 (0) | |
| Walking ability c | Able to walk 10 m | Yes/No | 107 (78) | 65 (80) | 42 (75) |
| Anxiety (HADS) | Sum score b | 0–21 | 3 (5) | 3 (5) | 3 (4) |
| Moderate-high level c | Yes/No | 23 (16) | 15 (18) | 8 (14) | |
| Depression (HADS) | Sum score b | 0–21 | 4 (4) | 4 (4) | 4 (4) |
| Moderate-high level c | Yes/No | 22 (16) | 14 (18) | 8 (14) | |
| Fatigue (MFI) | General fatigue b | 4–20 | 12 (8) | 12 (9) | 12 (9) |
| Physical fatigue b | 4–20 | 12 (8) | 12 (9) | 12 (7) | |
| Reduced activity b | 4–20 | 12 (9) | 12 (9) | 13 (7) | |
| Reduced motivation b | 4–20 | 7 (5) | 7 (5) | 6 (5) | |
| Mental fatigue b | 4–20 | 8 (9) | 8 (7) | 8 (11) | |
| Pathological fatigue c | Yes/No | 72 (53) | 44 (54) | 28 (50) | |
| General self-efficacy (GSES) b | 0–40 | 33 (9) | 33 (8) | 33 (10) | |
| Health-related quality of life (EQ5D-5L) b | −0.757–1 | 0.63 (0.35) | 0.67 (0.32) | 0.55 (0.34) | |
| Disability (36-item WHODAS 2.0) b | Cognition | 0–100 | 10 (30) | 5 (25) | 15 (40) |
| Mobility | 0–100 | 31 (56) | 31 (56) | 38 (56) | |
| Self-care | 0–100 | 20 (40) | 20 (50) | 30 (50) | |
| Getting along | 0–100 | 17 (25) | 17 (25) | 17 (25) | |
| Life activities | 0–100 | 60 (60) | 60 (60) | 65 (60) | |
| Social participation | 0–100 | 38 (25) | 38 (21) | 38 (21) | |
| Summary score | 0–100 | 34 (25) | 33 (22) | 35 (27) | |
| Self-Reported Functioning at Follow-Up, n = 81 | Possible Range | Estimate | Missing | |
|---|---|---|---|---|
| Complete recovery c | Yes/No | 9 (11) | 0 | |
| Any assistance required c | Yes/No | 31 (38) | 0 | |
| Community-based rehab c | Yes/No | 55 (71) | 4 | |
| Hospital readmission c | Yes/No | 8 (11) | 6 | |
| Work status c | (Self-)employed | Yes/No | 10 (13) | 5 |
| Unemployed | Yes/No | 8 (11) | 5 | |
| Retired | Yes/No | 38 (50) | 5 | |
| On sick leave | Yes/No | 20 (26) | 5 | |
| Occupational balance | OBQ-DK sum b | 0–33 | 23 (9) | 9 |
| Potential good balance c | 43 (60) | 9 | ||
| Health-related quality of life (EQ5D-5L) b | −0.757–1 | 0.83 (0.21) | 6 | |
| Disability (12-item WHODAS 2.0) b | 0–100 | 30 (25) | 9 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Honoré, H.; Eggertsen, P.P.; Pedersen, A.R.; Nielsen, J.F. Disability and Factors Associated with Disability in the Discharge Transition Phase After Acquired Brain Injury: An Observational Follow-Up Study. Healthcare 2025, 13, 1989. https://doi.org/10.3390/healthcare13161989
Honoré H, Eggertsen PP, Pedersen AR, Nielsen JF. Disability and Factors Associated with Disability in the Discharge Transition Phase After Acquired Brain Injury: An Observational Follow-Up Study. Healthcare. 2025; 13(16):1989. https://doi.org/10.3390/healthcare13161989
Chicago/Turabian StyleHonoré, Helene, Peter Preben Eggertsen, Asger Roer Pedersen, and Jørgen Feldbæk Nielsen. 2025. "Disability and Factors Associated with Disability in the Discharge Transition Phase After Acquired Brain Injury: An Observational Follow-Up Study" Healthcare 13, no. 16: 1989. https://doi.org/10.3390/healthcare13161989
APA StyleHonoré, H., Eggertsen, P. P., Pedersen, A. R., & Nielsen, J. F. (2025). Disability and Factors Associated with Disability in the Discharge Transition Phase After Acquired Brain Injury: An Observational Follow-Up Study. Healthcare, 13(16), 1989. https://doi.org/10.3390/healthcare13161989






