Building Lay Society Knowledge and Education for Health Technology Assessment and Policy Engagement: Case of CFTR Modulator Access in Brazil
Abstract
1. Introduction
2. Methods
3. CF Diagnosis and Access to Modulator Therapies: From Global Cases to Brazil
4. HTA in Brazil
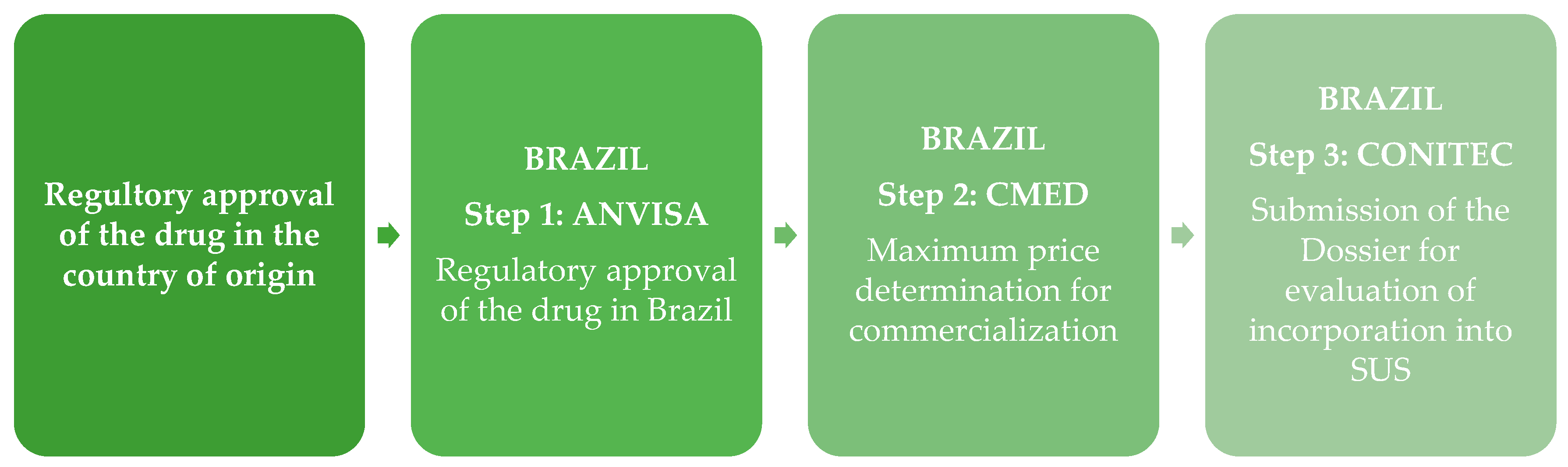
4.1. The Regulatory Approval and Price Setting for Commercialization: The Role of the ANVISA
4.2. An Overview of the Brazilian Unified Healthcare System (SUS)
4.3. The Incorporation of a New Health Technology into the SUS
4.3.1. The Role of the CONITEC
4.3.2. The Structure of the CONITEC
4.4. HTA for Rare Diseases
5. Lay Society Participation in HTA
5.1. Patient Organizations as Advocates and Educators
5.2. Patient Experience and Knowledge Production
5.3. The CF Case: Building Capacity and Advocating for Access
5.4. Establishing the Path to CFTR Modulator Access in Brazil
6. Ethics in HTA: A Subject That Needs Further Attention
7. Outlook and Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ABRAM | Association for Assistance to Mucoviscidosis |
| ANMAT | Argentinian National Administration of Drugs, Food and Medical Devices |
| ANVISA | Brazilian Health Regulatory Agency |
| CF | Cystic Fibrosis |
| CFTR | Cystic Fibrosis Transmembrane Conductance Regulator |
| CMED | Chamber for the Regulation of the Drug Market |
| CONITEC | Brazilian National Commission for the Incorporation of Technologies into SUS |
| dIVA | Deutivacaftor |
| ELX | Elexacaftor |
| EMA | European Medicines Agency |
| ETI | Elexacaftor/Tezacaftor/Ivacaftor |
| FDA | U.S. Food and Drug Administration |
| GBEFC | Brazilian Cystic Fibrosis Study Group |
| HTA | Health Technology Assessment |
| IVA | Ivacaftor |
| MHRA | Medicines and Healthcare Products Regulatory Agency |
| PwCF | People with Cystic Fibrosis |
| SAHPRA | South African Health Products Regulatory Authority |
| SUS | Brazilian Unified Health Care System |
| TEZ | Tezacaftor |
| TGA | Australian Therapeutic Goods Administration |
| UPV | United for Life Institute |
| VNZ | Vanzacaftor |
References
- O’Rourke, B.; Oortwijn, W.; Schuller, T.; International Joint Task Group. The new definition of health technology assessment: A milestone in international collaboration. Int. J. Technol. Assess. Health Care 2020, 36, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Alkhaldi, M.; Al Basuoni, A.; Matos, M.; Tanner, M.; Ahmed, S. Health Technology Assessment in High, Middle, and Low-income Countries: New Systematic and Interdisciplinary Approach For Sound Informed-policy Making: Research Protocole. Risk Manag. Healthc. Policy 2021, 14, 2757–2770. [Google Scholar] [CrossRef]
- Haendel, M.; Vasilevsky, N.; Unni, D.; Bologa, C.; Harris, N.; Rehm, H.; Hamosh, A.; Baynam, G.; Groza, T.; McMurry, J.; et al. How many rare diseases are there? Nat. Rev. Drug Discov. 2020, 19, 77–78. [Google Scholar] [CrossRef]
- Amaral, M.B.; Rego, S. Doenças raras na agenda da inovação em saúde: Avanços e desafios na fibrose cística. Cad. Saude Publica 2020, 36, e00115720. [Google Scholar] [CrossRef]
- Rommens, J.M.; Iannuzzi, M.C.; Kerem, B.; Drumm, M.L.; Melmer, G.; Dean, M.; Rozmahel, R.; Cole, J.L.; Kennedy, D.; Hidaka, N.; et al. Identification of the cystic fibrosis gene: Chromosome walking and jumping. Science 1989, 245, 1059–1065. [Google Scholar] [CrossRef]
- Riordan, J.R.; Rommens, J.M.; Kerem, B.S.; Alon, N.O.A.; Rozmahel, R.; Grzelczak, Z.; Zielenski, J.; Lok, S.I.; Plavsic, N.; Chou, J.L.; et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science 1989, 245, 1066–1073. [Google Scholar] [CrossRef]
- Kerem, B.; Rommens, J.M.; Buchanan, J.A.; Markiewicz, D.; Cox, T.K.; Chakravarti, A.; Buchwald, M.; Tsui, L. Identification of the cystic fibrosis gene: Genetic analysis. Science 1989, 245, 1073–1080. [Google Scholar] [CrossRef]
- Andersen, D.H. Cystic fibrosis of the pancreas and its relation to celiac disease. Am. J. Dis. Child. 1938, 56, 344–399. [Google Scholar] [CrossRef]
- Narayanan, S.; Mainz, J.G.; Gala, S.; Tabori, H.; Grossoehme, D. Adherence to therapies in cystic fibrosis: A targeted literature review. Expert Rev. Respir. Med. 2017, 11, 129–145. [Google Scholar] [CrossRef] [PubMed]
- Southern, K.W.; Addy, C.; Bell, S.C.; Bevan, A.; Borawska, U.; Brown, C.; Burgel, P.-R.; Button, B.; Castellani, C.; Chansard, A.; et al. Standards for the care of people with cystic fibrosis; establishing and maintaining health. J. Cyst. Fibros. 2024, 23, 12–28. [Google Scholar] [CrossRef] [PubMed]
- Silva, I.A.L.; Laselva, O.; Lopes-Pacheco, M. Advances in Preclinical In Vitro Models for the Translation of Precision Medicine for Cystic Fibrosis. J. Pers. Med. 2022, 12, 1321. [Google Scholar] [CrossRef]
- Bacalhau, M.; Camargo, M.; Lopes-Pacheco, M. Laboratory Tools to Predict CFTR Modulator Therapy Effectiveness and to Monitor Disease Severity in Cystic Fibrosis. J. Pers. Med. 2024, 14, 93. [Google Scholar] [CrossRef] [PubMed]
- Pinto, M.C.; Silva, I.A.L.; Figueira, M.F.; Amaral, M.D.; Lopes-Pacheco, M. Pharmacological Modulation of Ion Channels for the Treatment of Cystic Fibrosis. J. Exp. Pharmacol. 2021, 13, 693–723. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Pacheco, M.; Winters, A.G.; Jackson, J.J.; Olson Rd, J.A.; Kim, M.; Ledwitch, K.V.; Tedman, A.; Jhangiani, A.R.; Schlebach, J.P.; Meiler, J.; et al. Recent developments in cystic fibrosis drug discovery: Where are we today? Expert Opin. Drug Discov. 2025, 20, 659–682. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, B.W.; Davies, J.C.; McElvaney, N.G.; Tullis, E.; Bell, S.C.; Drevinek, P.; Griese, M.; McKone, E.F.; Wainwright, C.E.; Konstan, M.W.; et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011, 365, 1663–1672. [Google Scholar] [CrossRef]
- Heijerman, H.G.M.; McKone, E.F.; Downey, D.G.; Van Braeckel, E.; Rowe, S.M.; Tullis, E.; Mall, M.A.; Welter, J.J.; Ramsey, B.W.; McKee, C.M.; et al. Efficacy and safety of the elexacaftor plus tezacaftor plus ivacaftor combination regimen in people with cystic fibrosis homozygous for the F508del mutation: A double-blind, randomised, phase 3 trial. Lancet 2019, 394, 1940–1948. [Google Scholar] [CrossRef]
- Middleton, P.G.; Mall, M.A.; Dřevínek, P.; Lands, L.C.; McKone, E.F.; Polineni, D.; Ramsey, B.W.; Taylor-Cousar, J.L.; Tullis, E.; Vermeulen, F.; et al. Elexacaftor–Tezacaftor–Ivacaftor for Cystic Fibrosis with a Single Phe508del Allele. N. Engl. J. Med. 2019, 381, 1809–1819. [Google Scholar] [CrossRef]
- Keating, C.; Yonker, L.M.; Vermeulen, F.; Prais, D.; Linnemann, R.W.; Trimble, A.; Kotsimbos, T.; Mermis, J.; Braun, A.T.; O’Carroll, M.; et al. Vanzacaftor–tezacaftor–deutivacaftor versus elexacaftor–tezacaftor–ivacaftor in individuals with cystic fibrosis aged 12 years and older (SKYLINE Trials VX20-121-102 and VX20-121-103): Results from two randomised, active-controlled, phase 3 trials. Lancet Respir. Med. 2025, 13, 256–271. [Google Scholar] [CrossRef]
- Bacalhau, M.; Camargo, M.; Magalhães-Ghiotto, G.A.V.; Drumond, S.; Castelletti, C.H.M.; Lopes-Pacheco, M. Elexacaftor-Tezacaftor-Ivacaftor: A Life-Changing Triple Combination of CFTR Modulator Drugs for Cystic Fibrosis. Pharmaceuticals 2023, 16, 410. [Google Scholar] [CrossRef]
- McKone, E.F.; Borowitz, D.; Drevinek, P.; Griese, M.; Konstan, M.W.; Wainwright, C.; Ratjen, F.; Sermet-Gaudelus, I.; Plant, B.; Munck, A.; et al. Long-term safety and efficacy of ivacaftor in patients with cystic fibrosis who have the Gly551Asp-CFTR mutation: A phase 3, open-label extension study (PERSIST). Lancet Respir. Med. 2014, 2, 902–910. [Google Scholar] [CrossRef]
- Sutharsan, S.; McKone, E.F.; Downey, D.G.; Duckers, J.; MacGregor, G.; Tullis, E.; Van Braeckel, E.; Wainwright, C.E.; Watson, D.; Ahluwalia, N.; et al. Efficacy and safety of elexacaftor plus tezacaftor plus ivacaftor versus tezacaftor plus ivacaftor in people with cystic fibrosis homozygous for F508del-CFTR: A 24-week, multicentre, randomised, double-blind, active-controlled, phase 3b trial. Lancet Respir. Med. 2022, 10, 267–277. [Google Scholar] [CrossRef]
- Volkova, N.; Moy, K.; Evans, J.; Campbell, D.; Tian, S.; Simard, C.; Higgins, M.; Konstan, M.W.; Sawicki, G.S.; Elbert, A.; et al. Disease progression in patients with cystic fibrosis treated with ivacaftor: Data from national US and UK registries. J. Cyst. Fibros. 2020, 19, 68–79. [Google Scholar] [CrossRef]
- Stanojevic, S.; Vukovojac, K.; Sykes, J.; Ratjen, F.; Tullis, E.; Stephenson, A.L. Projecting the impact of delayed access to elexacaftor/tezacaftor/ivacaftor for people with Cystic Fibrosis. J. Cyst. Fibros. 2021, 20, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; King, I.; Hill, A. International disparities in diagnosis and treatment access for cystic fibrosis. Pediatr. Pulmonol. 2024, 59, 1622–1630. [Google Scholar] [CrossRef]
- da Silva Filho, L.V.R.F.; Zampoli, M.; Cohen-Cymberknoh, M.; Kabra, S.K. Cystic fibrosis in low and middle-income countries (LMIC): A view from four different regions of the world. Paediatr. Respir. Rev. 2021, 38, 37–44. [Google Scholar] [CrossRef]
- Guo, J.; Wang, J.; Zhang, J.; Fortunak, J.; Hill, A. Current prices versus minimum costs of production for CFTR modulators. J. Cyst. Fibros. 2022, 21, 866–872. [Google Scholar] [CrossRef]
- McGarry, M.E.; Gibb, E.R.; Laguna, T.A.; O’Sullivan, B.P.; Sawicki, G.S.; Zobell, J.T. How many billions is enough? Prioritizing profits over patients with cystic fibrosis. Pediatr. Pulmonol. 2023, 58, 1595. [Google Scholar] [CrossRef] [PubMed]
- Zampoli, M.; Morrow, B.M.; Paul, G. Real-world disparities and ethical considerations with access to CFTR modulator drugs: Mind the gap! Front. Pharmacol. 2023, 14, 1163391. [Google Scholar] [CrossRef] [PubMed]
- Drugs@FDA: FDA-Approved Drugs—Kalydeco. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=203188 (accessed on 9 May 2025).
- Kalydeco (Consulta ANVISA). Available online: https://consultas.anvisa.gov.br/#/medicamentos/1274279?nomeProduto=KALYDECO (accessed on 9 May 2025).
- PORTARIA SCTIE/MS No 68, DE 30 DE DEZEMBRO DE 2020. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sctie/2020/prt0068_31_12_2020.html (accessed on 9 May 2025).
- Ivacaftor (Kalydeco) já Está Disponível no SUS: Saiba Quem é Elegível ao Medicamento. Available online: https://unidospelavida.org.br/ivacaftor-kalydeco-ja-esta-disponivel-no-sus/ (accessed on 9 May 2025).
- Drugs@FDA: FDA-Approved Drugs—Trikafta. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=212273 (accessed on 9 May 2025).
- Trikafta (Consulta ANVISA). Available online: https://consultas.anvisa.gov.br/#/medicamentos/2087366?nomeProduto=trikafta (accessed on 9 May 2025).
- Trikafta® no SUS: Saiba Mais Sobre o Trabalho do Unidos Pela Vida na Jornada pela Incorporação Deste Novo Medicamento Para Fibrose Cística. Available online: https://unidospelavida.org.br/trikafta-no-sus-saiba-mais-sobre-o-trabalho-do-unidos-pela-vida-na-jornada-pela-incorporacao-deste-novo-medicamento-para-fibrose-cistica/ (accessed on 9 May 2025).
- Lopes-Pacheco, M. CFTR Modulators: The Changing Face of Cystic Fibrosis in the Era of Precision Medicine. Front. Pharmacol. 2020, 10, 1662. [Google Scholar] [CrossRef]
- Lopez, A.; Daly, C.; Vega-Hernandez, G.; MacGregor, G.; Rubin, J.L. Elexacaftor/tezacaftor/ivacaftor projected survival and long-term health outcomes in people with cystic fibrosis homozygous for F508del. J. Cyst. Fibros. 2023, 22, 607–614. [Google Scholar] [CrossRef]
- Sosnay, P.R.; White, T.B.; Farrell, P.M.; Ren, C.L.; Derichs, N.; Howenstine, M.S.; Nick, J.A.; De Boeck, K. Diagnosis of Cystic Fibrosis in Nonscreened Populations. J. Pediatr. 2017, 181, S52–S57.e2. [Google Scholar] [CrossRef]
- Farrell, P.M.; White, T.B.; Ren, C.L.; Hempstead, S.E.; Accurso, F.; Derichs, N.; Howenstine, M.; McColley, S.A.; Rock, M.; Rosenfeld, M.; et al. Diagnosis of Cystic Fibrosis: Consensus Guidelines from the Cystic Fibrosis Foundation. J. Pediatr. 2017, 181, S4–S15.e1. [Google Scholar] [CrossRef]
- Jackson, A.D.; Goss, C.H. Epidemiology of CF: How registries can be used to advance our understanding of the CF population. J. Cyst. Fibros. 2018, 17, 297–305. [Google Scholar] [CrossRef]
- Zampoli, M.; Verstraete, J.; Baird, C.; Calligaro, G.; Morrow, B. Affordable Cystic Fibrosis (CF) Transmembrane Conductance Regulator Modulator Drugs for CF: All CF Lives Worldwide Matter! Am. J. Respir. Crit. Care Med. 2023, 208, 915–916. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Mutation Database (CFTR1 Database). Available online: http://www.genet.sickkids.on.ca/ (accessed on 9 May 2025).
- Clinical and Function Translation of CFTR (CFTR2 Database). Available online: https://cftr2.org/ (accessed on 9 May 2025).
- 2023 Patient Registry Annual Data Report—Cystic Fibrosis Foundation. Available online: https://www.cff.org/media/34491/download (accessed on 9 May 2025).
- 2022 Annual Data Report—The Canadian Cystic Fibrosis Registry. Available online: https://cystic-fibrosis.cdn.prismic.io/cystic-fibrosis/ZfbnVsmUzjad_Trg_2022-Annual-Data-Report-Web.pdf (accessed on 9 May 2025).
- 2023 Annual Report—Australian Cystic Fibrosis Data Registry. Available online: https://www.cysticfibrosis.org.au/wp-content/uploads/2024/07/ACFDR_2023_Annual-Report.pdf (accessed on 9 May 2025).
- ECFSPR Annual Highlights Report 2023. Available online: https://www.ecfs.eu/sites/default/files/general-content-files/Highlights_Report_2023_vs1.1_ECFSPR_20250508.pdf (accessed on 9 May 2025).
- Dogru, D.; Çakır, E.; Şişmanlar, T.; Çobanoğlu, N.; Pekcan, S.; Cinel, G.; Yalçın, E.; Kiper, N.; Şen, V.; S Şen, H.; et al. Cystic fibrosis in Turkey: First data from the national registry. Pediatr. Pulmonol. 2020, 55, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Goldman, A.; Graf, C.; Ramsay, M.; Leisegang, F.; Westwood, A.T.R. Molecular diagnosis of cystic fibrosis in South African populations. S. Afr. Med. J. 2003, 93, 518–519. [Google Scholar]
- Strom, C.M.; Crossley, B.; Redman, J.B.; Quan, F.; Buller, A.; McGinniss, M.J.; Sun, W. Molecular screening for diseases frequent in Ashkenazi Jews: Lessons learned from more than 100,000 tests performed in a commercial laboratory. Genet. Med. 2004, 6, 145–152. [Google Scholar] [CrossRef]
- Rendine, S.; Calafell, F.; Cappello, N.; Gagliardini, R.; Caramia, G.; Rigillo, N.; Silvetti, M.; Zanda, M.; Miano, A.; Battistini, F.; et al. Genetic history of cystic fibrosis mutations in Italy. I. Regional distribution. Ann. Hum. Genet. 1997, 61, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Anton-Păduraru, D.-T.; Azoicăi, A.N.; Trofin, F.; Mîndru, D.E.; Murgu, A.M.; Bocec, A.S.; Iliescu Halițchi, C.O.; Ciongradi, C.I.; Sȃrbu, I.; Iliescu, M.L. Diagnosing Cystic Fibrosis in the 21st Century-A Complex and Challenging Task. Diagnostics 2024, 14, 763. [Google Scholar] [CrossRef]
- Ideozu, J.E.; Liu, M.; Riley-Gillis, B.M.; Paladugu, S.R.; Rahimov, F.; Krishnan, P.; Tripathi, R.; Dorr, P.; Levy, H.; Singh, A.; et al. Diversity of CFTR variants across ancestries characterized using 454,727 UK biobank whole exome sequences. Genome Med. 2024, 16, 43. [Google Scholar] [CrossRef]
- Durmowicz, A.G.; Lim, R.; Rogers, H.; Rosebraugh, C.J.; Chowdhury, B.A. The U.S. food and drug administration’s experience with ivacaftor in cystic fibrosis: Establishing efficacy using in vitro data in lieu of a clinical trial. Ann. Am. Thorac. Soc. 2018, 15, 1–2. [Google Scholar] [CrossRef]
- Costa, E.; Girotti, S.; Pauro, F.; Leufkens, H.G.M.; Cipolli, M. The impact of FDA and EMA regulatory decision - making process on the access to CFTR modulators for the treatment of cystic fibrosis. Orphanet J. Rare Dis. 2022, 17, 188. [Google Scholar] [CrossRef]
- De Boeck, K.; Munck, A.; Walker, S.; Faro, A.; Hiatt, P.; Gilmartin, G.; Higgins, M. Efficacy and safety of ivacaftor in patients with cystic fibrosis and a non-G551D gating mutation. J. Cyst. Fibros. 2014, 13, 674–680. [Google Scholar] [CrossRef] [PubMed]
- Guimbellot, J.; Solomon, G.M.; Baines, A.; Heltshe, S.L.; VanDalfsen, J.; Joseloff, E.; Sagel, S.D.; Rowe, S.M. Effectiveness of ivacaftor in cystic fibrosis patients with non-G551D gating mutations. J. Cyst. Fibros. 2019, 18, 102–109. [Google Scholar] [CrossRef] [PubMed]
- ECFS and CF Europe Join Forces to Urge EMA to Expand Access to Kaftrio for People with CF in Europe. Available online: https://www.cf-europe.eu/ecfs-and-cf-europe-join-forces-to-urge-ema-to-expand-access-to-kaftrio-for-people-with-cf-in-europe/ (accessed on 9 May 2025).
- Buu, M.C.; Sanders, L.M.; Mayo, J.A.; Milla, C.E.; Wise, P.H. Assessing Differences in Mortality Rates and Risk Factors Between Hispanic and Non-Hispanic Patients With Cystic Fibrosis in California. Chest 2016, 149, 380–389. [Google Scholar] [CrossRef]
- Hamosh, A.; FitzSimmons, S.C.; Macek, M.; Knowles, M.R.; Rosenstein, B.J.; Cutting, G.R. Comparison of the clinical manifestations of cystic fibrosis in black and white patients. J. Pediatr. 1998, 132, 255–259. [Google Scholar] [CrossRef] [PubMed]
- McColley, S.A.; Schechter, M.S.; Morgan, W.J.; Pasta, D.J.; Craib, M.L.; Konstan, M.W. Risk factors for mortality before age 18 years in cystic fibrosis. Pediatr. Pulmonol. 2017, 52, 909–915. [Google Scholar] [CrossRef]
- Schrijver, I.; Pique, L.; Graham, S.; Pearl, M.; Cherry, A.; Kharrazi, M. The Spectrum of CFTR Variants in Nonwhite Cystic Fibrosis Patients: Implications for Molecular Diagnostic Testing. J. Mol. Diagn. 2016, 18, 39–50. [Google Scholar] [CrossRef]
- Watts, K.D.; Layne, B.; Harris, A.; McColley, S.A. Hispanic Infants with cystic fibrosis show low CFTR mutation detection rates in the Illinois newborn screening program. J. Genet. Couns. 2012, 21, 671–675. [Google Scholar] [CrossRef]
- Oliver, K.E.; Carlon, M.S.; Pedemonte, N.; Lopes-Pacheco, M. The revolution of personalized pharmacotherapies for cystic fibrosis: What does the future hold? Expert Opin. Pharmacother. 2023, 24, 1545–1565. [Google Scholar] [CrossRef]
- Terlizzi, V.; Lopes-Pacheco, M. Cystic Fibrosis: New Challenges and Perspectives Beyond Elexacaftor/Tezacaftor/Ivacaftor. Ther. Adv. Respir. Dis. 2025, 19, 17534666251323194. [Google Scholar] [CrossRef]
- Relatório do Registro Brasileiro de Fibrose Cística 2021. Available online: http://www.gbefc.org.br/ckfinder/userfiles/files/Relatorio_Rebrafc_2021(1).pdf (accessed on 9 May 2025).
- Sans, M. Admixture studies in Latin America: From the 20th to the 21st century. Hum. Biol. 2000, 72, 155–177. [Google Scholar]
- de Souza, A.M.; Resende, S.S.; de Sousa, T.N.; de Brito, C.F.A. A systematic scoping review of the genetic ancestry of the Brazilian population. Genet. Mol. Biol. 2019, 42, 495–508. [Google Scholar] [CrossRef]
- Nunes, K.; Araújo Castro E Silva, M.; Rodrigues, M.R.; Lemes, R.B.; Pezo-Valderrama, P.; Kimura, L.; de Sena, L.S.; Krieger, J.E.; Catoia Varela, M.; de Azevedo, L.O.; et al. Admixture’s impact on Brazilian population evolution and health. Science 2025, 388, eadl3564. [Google Scholar] [CrossRef]
- Faucz, F.R.; Souza, D.A.S.; Olandoski, M.; Raskin, S. CFTR allelic heterogeneity in Brazil: Historical and geographical perspectives and implications for screening and counseling for cystic fibrosis in this country. J. Hum. Genet. 2010, 55, 71–76. [Google Scholar] [CrossRef] [PubMed]
- PORTARIA SCTIE, N. 1-5 DE 14 DE JANEIRO DE 2016. Available online: http://antigo-conitec.saude.gov.br/images/Relatorios/Portaria/2016/PortariaSCTIE_1a5_2016.pdf (accessed on 9 May 2025).
- PORTARIA No 36, DE 26 DE OUTUBRO DE 2016. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/sctie/2016/prt0036_26_10_2016.html (accessed on 9 May 2025).
- Kalydeco/Ivacaftor (Consulta ANVISA). Available online: https://consultas.anvisa.gov.br/#/pareceres/q/?nomeProduto=KALYDECO (accessed on 9 May 2025).
- Symdeko/Tezacaftor-Ivacaftor (Consulta ANVISA). Available online: https://consultas.anvisa.gov.br/#/pareceres/q/?nomeProduto=Symdeko (accessed on 9 May 2025).
- Orkambi (Consulta ANVISA). Available online: https://consultas.anvisa.gov.br/#/medicamentos/1259844?substancia=26205 (accessed on 9 May 2025).
- LEI No 9.782, DE 26 DE JANEIRO DE 1999. Available online: https://www.planalto.gov.br/ccivil_03/leis/l9782.htm (accessed on 9 May 2025).
- LEI no 9.782, de 26 de Janeiro 1999. Available online: https://www.gov.br/anvisa/pt-br/acessoainformacao/institucional (accessed on 9 May 2025).
- RESOLUÇÃO—RDC No 753, De 28 De SETEMBRO De 2022. Available online: https://anvisalegis.datalegis.net/action/UrlPublicasAction.php?acao=abrirAtoPublico&num_ato=00000753&sgl_tipo=RDC&sgl_orgao=RDC/DC/ANVISA/MS&vlr_ano=2022&seq_ato=000&cod_modulo=310&cod_menu=9434 (accessed on 9 May 2025).
- Symdeko (Consulta ANVISA). Available online: https://consultas.anvisa.gov.br/#/medicamentos/1300901?numeroProcesso=25351022290201910 (accessed on 9 May 2025).
- Drugs@FDA: FDA-Approved Drugs—Orkambi. Available online: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=overview.process&ApplNo=206038 (accessed on 9 May 2025).
- Verônica del Gragnano Stasiak Bednarczuk de Olivera Critérios de Análise e Utilização das Contribuições Recebidas por Pacientes em Consultas Públicas da Conitec: O Caso dos Moduladores Para Fibrose Cística. Available online: https://acervodigital.ufpr.br/xmlui/handle/1884/83243. (accessed on 9 May 2025).
- LEI No 10.742, DE 6 DE OUTUBRO DE 2003. Available online: https://www.planalto.gov.br/ccivil_03/leis/2003/l10.742.htm (accessed on 9 May 2025).
- Câmara de Regulação do Mercado de Medicamentos—CMED. Available online: https://www.gov.br/anvisa/pt-br/assuntos/medicamentos/cmed (accessed on 9 May 2025).
- LEI No 8.080, DE 19 DE SETEMBRO DE 1990. Available online: https://www.planalto.gov.br/ccivil_03/leis/l8080.htm%0A (accessed on 9 May 2025).
- de Oliveira, V.D.G.S.B.; de Oliveira, V.B.; Amaral, M.B.; Miguel, M.D. Critérios de análise e utilização das contribuições de pacientes em consultas públicas da Conitec. Bol. Inst. Saúde—BIS 2024, 25, 89–97. [Google Scholar] [CrossRef]
- O Sistema Único de Saúde é um dos Maiores e Mais Complexos Sistemas de Saúde Pública do Mundo. Available online: https://www.gov.br/saude/pt-br/assuntos/saude-de-a-a-z/s/sus (accessed on 9 May 2025).
- PORTARIA No 4.279, DE 30 DE DEZEMBRO DE 2010. Available online: https://bvsms.saude.gov.br/bvs/saudelegis/gm/2010/prt4279_30_12_2010.html (accessed on 9 May 2025).
- Rodrigues Filho, F.J.; Pereira, M.C. O perfil das tecnologias em saúde incorporadas no SUS de 2012 a 2019: Quem são os principais demandantes? Saúde em Debate 2021, 45, 707–719. [Google Scholar] [CrossRef]
- Entenda o Fluxo de Incorporação de Tecnologias em Saúde no SUS. Available online: https://www.gov.br/saude/pt-br/assuntos/noticias/2023/marco/entenda-o-fluxo-de-incorporacao-de-tecnologias-em-saude-no-sus (accessed on 9 May 2025).
- Lopes, A.C.d.F.; Novaes, H.M.D.; Soárez, P.C.d. Patient and public involvement in health technology decision-making processes in Brazil. Rev. Saude Publica 2020, 54, 136. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.-P.; Desmartis, M.; Lepage-Savary, D.; Gagnon, J.; St-Pierre, M.; Rhainds, M.; Lemieux, R.; Gauvin, F.-P.; Pollender, H.; Légaré, F. Introducing patients’ and the public’s perspectives to health technology assessment: A systematic review of international experiences. Int. J. Technol. Assess. Health Care 2011, 27, 31–42. [Google Scholar] [CrossRef]
- Rand, L.; Dunn, M.; Slade, I.; Upadhyaya, S.; Sheehan, M. Understanding and using patient experiences as evidence in healthcare priority setting. Cost Eff. Resour. Alloc. 2019, 17, 20. [Google Scholar] [CrossRef]
- Silva, A.S.; de Sousa, M.S.A.; da Silva, E.V.; Galato, D. Social participation in the health technology incorporation process into Unified Health System. Rev. Saude Publica 2019, 53, 109. [Google Scholar] [CrossRef] [PubMed]
- DECRETO No 11.161, DE 4 DE AGOSTO DE 2022. Available online: https://www.planalto.gov.br/ccivil_03/_ato2019-2022/2022/decreto/D11161.htm (accessed on 9 May 2025).
- Lopes, A.C.d.F. Participação Social na Incorporação de Tecnologias no Sistema Único de Saúde. Ph.D. Thesis, Universidade de São Paulo, São Paulo, Brazil, 2024. [Google Scholar]
- de Paula, B.L.S.; Santana, I.J.A. Políticas do corpo: Associações de pacientes e reconfigurações da cidadania. Physis Rev. Saúde Coletiva 2021, 31, e310117. [Google Scholar] [CrossRef]
- Tobaruella, B.S.; Guerra, L.D. da S. Atuação das Associações de Pacientes na Incorporação e na Alteração de Políticas Públicas de Saúde. J. Manag. Prim. Health Care 2022, 14, e030. [Google Scholar] [CrossRef]
- Rabeharisoa, V. The multiplicity of knowledge and the trembling of institutions. Rev. D’anthropologie Des Connaiss. 2017, 11, 141–147. [Google Scholar] [CrossRef]
- Rabeharisoa, V. Evidence-Based Activism: Patients’ Organisations, Users’ and Activist’s Groups in Knowledge Society. Available online: http://tricountycc.idm.oclc.org/login?url=https://www.proquest.com/working-papers/evidence-based-activism-patients-organisations/docview/1698358922/se-2 (accessed on 9 May 2025).
- Novas, C. The Political Economy of Hope: Patients’ Organizations, Science and Biovalue. Biosocieties 2006, 1, 289–305. [Google Scholar] [CrossRef]
- Lima, A.d.N. Fibrose cística. Pulmao RJ 2006, 15, 205–206. [Google Scholar]
- United for Life Institute. Available online: https://unidospelavida.org.br/sobre/ (accessed on 9 May 2025).
- Vertex Save Us. Available online: https://www.vertexsaveus.org/ (accessed on 9 May 2025).
- Entidades Pedem licenciamento Compulsório das Patentes de Medicamentos Para a Fibrose Cística. Available online: https://fenafar.org.br/2023/02/10/entidades-pedem-licenciamento-compulsorio-das-patentes-de-medicamentos-para-a-fibrose-cistica/ (accessed on 9 May 2025).
- Conitec em Números: Acompanhe o Painel com Informações Sobre Tecnologias em Saúde Submetidas à Comissão no SUS. Available online: https://www.gov.br/conitec/pt-br/assuntos/noticias/2020/setembro/conitec-em-numeros-acompanhe-o-painel-com-informacoes-sobre-tecnologias-em-saude-submetidas-a-comissao-no-sus (accessed on 9 May 2025).
- Petersen, A. The Politics of Bioethics; Routledge: Oxfordshire, UK, 2011; ISBN 9781136821608. [Google Scholar]
- Vanstone, M.; Abelson, J.; Bidonde, J.; Bond, K.; Burgess, R.; Canfield, C.; Schwartz, L.; Tripp, L. Ethical Challenges Related to Patient Involvement in Health Technology Assessment. Int. J. Technol. Assess. Health Care 2019, 35, 253–256. [Google Scholar] [CrossRef]
- Hofmann, B.; Oortwijn, W.; Bakke Lysdahl, K.; Refolo, P.; Sacchini, D.; van der Wilt, G.J.; Gerhardus, A. Integrating ethics in health technology assessment: Many ways to Rome. Int. J. Technol. Assess. Health Care 2015, 31, 131–137. [Google Scholar] [CrossRef]
- Hofmann, B.M. Why ethics should be part of health technology assessment. Int. J. Technol. Assess. Health Care 2008, 24, 423–429. [Google Scholar] [CrossRef]
- Bellemare, C.A.; Dagenais, P.; K-Bédard, S.; Béland, J.-P.; Bernier, L.; Daniel, C.-É.; Gagnon, H.; Legault, G.-A.; Parent, M.; Patenaude, J. Ethics in Health Technology Assessment: A Systematic Review. Int. J. Technol. Assess. Health Care 2018, 34, 447–457. [Google Scholar] [CrossRef]
- Picavet, E.; Cassiman, D.; Pinxten, W.; Simoens, S. Ethical, legal and social implications of rare diseases and orphan drugs in Europe: Meeting report of a Brocher symposium. Expert Rev. Pharmacoecon. Outcomes Res. 2013, 13, 571–573. [Google Scholar] [CrossRef] [PubMed]
- Refolo, P.; Duthie, K.; Hofmann, B.; Stanak, M.; Bertelsen, N.; Bloemen, B.; Di Bidino, R.; Oortwijn, W.; Raimondi, C.; Sacchini, D.; et al. Ethical challenges for Health Technology Assessment (HTA) in the evolving evidence landscape. Int. J. Technol. Assess. Health Care 2024, 40, e39. [Google Scholar] [CrossRef] [PubMed]
- Allyse, M.A.; Agam, P.; Bombard, Y.; Feys, R.; Horstmann, M.; Kokayi, A.; Isasi, R.; Meagher, K.M.; Michie, M.; Musunuru, K.; et al. Building Better Medicine: Translational Justice and the Quest for Equity in US Healthcare. Am. J. Bioeth. 2025, 25, 11–25. [Google Scholar] [CrossRef] [PubMed]
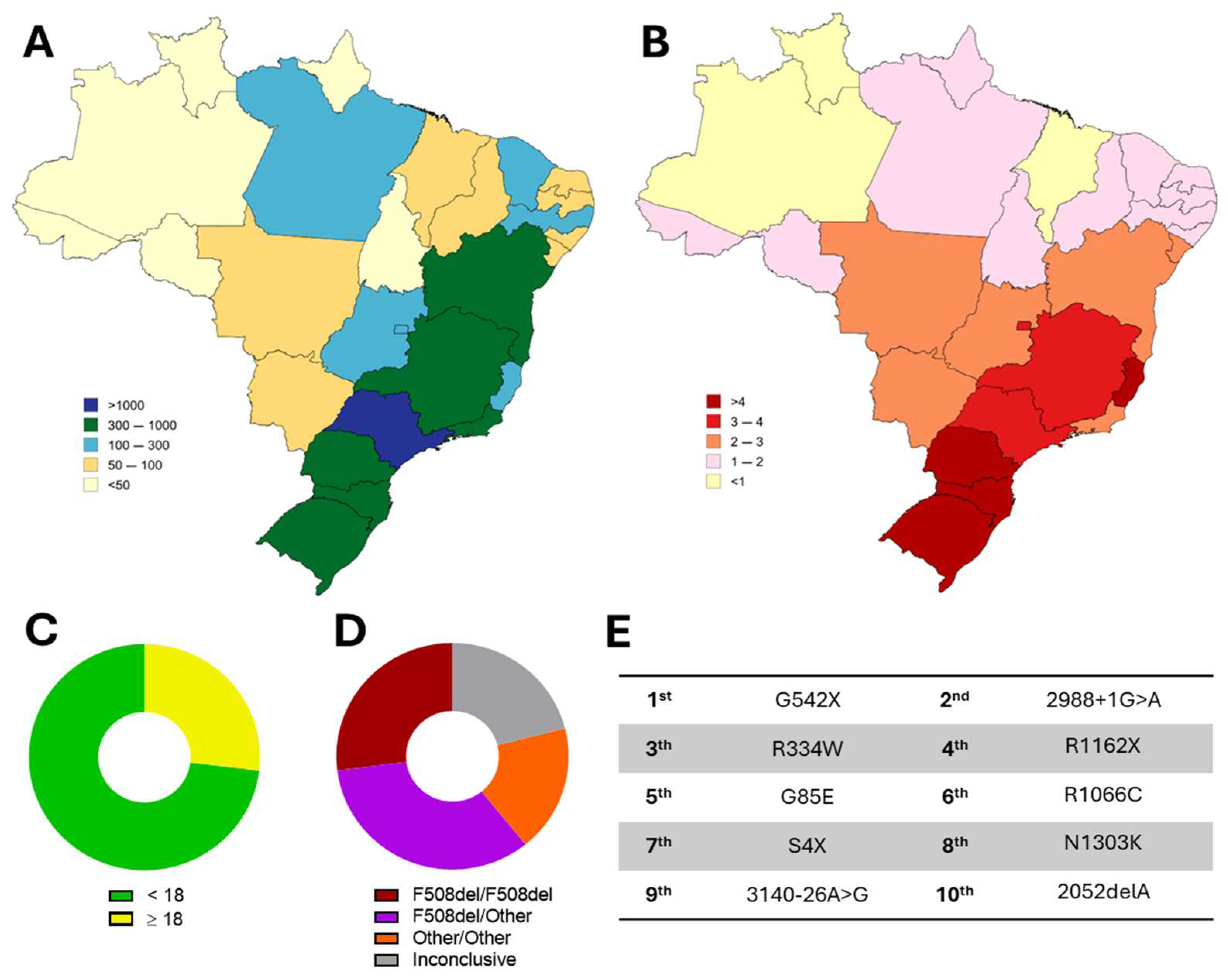
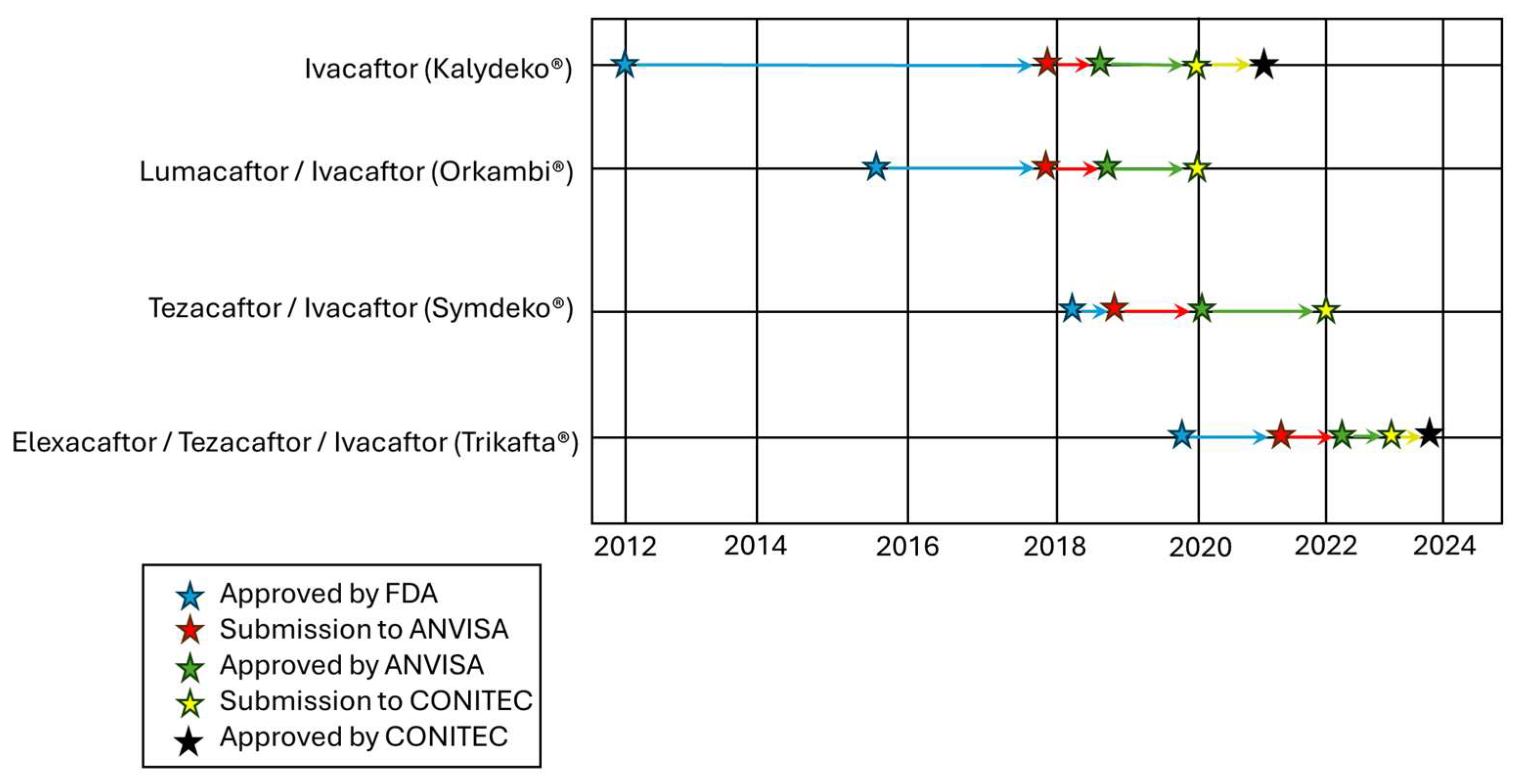
| Country Region | Regulatory Agency | IVA | LUM/IVA | TEZ/IVA | ELX/TEZ/IVA | VNZ/TEZ/dIVA |
|---|---|---|---|---|---|---|
| U.S. | Food and Drug Administration (FDA) | Jan/2012 | Jul/2015 | Feb/2018 | Oct/2019 | Dec/2024 |
| European Union | European Medicines Agency (EMA) | Jul/2012 | Nov/2015 | Oct/2018 | Aug/2020 | Jul/2025 |
| U.K. | Medicines and Healthcare Products Regulatory Agency (MHRA) | Jul/2012 | Nov/2015 | Oct/2018 | Aug/2020 | March/2025 |
| Canada | Health Canada | Nov/2012 | Jan/2016 | Jun/2018 | Jun/2021 | Jul/2025 |
| Australia | Therapeutic Goods Administration (TGA) | Jun/2019 | Aug/2018 | Mar/2018 | Mar/2021 | Submitted |
| Brazil | Brazilian Health Regulatory Agency (ANVISA) | Sept/2018 | Jul/2018 | Jan/2020 | Mar/2022 | Not Registered |
| Argentina | National Administration of Drugs, Food, and Medical Devices (ANMAT) | Not registered | Not registered | Not registered | Not registered | Not Registered |
| South Africa | South African Health Products Regulatory Authority (SAHPRA) | Not registered | Not registered | Not registered | Not registered | Not Registered |
| CFTR Modulator (Tradename) | Age (Years) | Incorporated into SUS | Variants |
|---|---|---|---|
| IVA (Kalydeco®) | ≥6 | Dec/2020 | At least one copy of the gating mutations—G551D, G1244E, G1349D, G178R, G551S, S1251N, S1255P, S549N, or S549R—and patients aged 18 years or older with one copy of the R117H mutation |
| LUM/IVA (Orkambi®) | ≥6 | Not incorporated | Two copies of F508del |
| TEZ/IVA (Symdeko®) | ≥12 | Not incorporated | Two copies of F508del or one copy of F508del and a residual functional variant on the second allele (P67L, D110H, R117C, L206W, R352Q, A455E, D579G, 711+3A>G, S945L, S977F, R1070W, D1152H, 2657+5G>A, 3140-26A>G, and 3717+12191C>T) |
| ELX/TEZ/IVA (Trikafta®) | ≥6 | Sep/2023 | At least one copy of F508del |
| Goal | Activities Led by the UPV |
|---|---|
| Community Education and Engagement |
|
| Preparing CF Patient Organizations in Brazil |
|
| Research and Data Generation |
|
| National Events about the HTA Process |
|
| Strategic Meetings to Engage the CF Community: Advocacy and Stakeholder Engagement Preparatory Actions for Public Consultation |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, V.S.B.; Amaral, M.B.; Camargo, M.; Lopes-Pacheco, M. Building Lay Society Knowledge and Education for Health Technology Assessment and Policy Engagement: Case of CFTR Modulator Access in Brazil. Healthcare 2025, 13, 1996. https://doi.org/10.3390/healthcare13161996
de Oliveira VSB, Amaral MB, Camargo M, Lopes-Pacheco M. Building Lay Society Knowledge and Education for Health Technology Assessment and Policy Engagement: Case of CFTR Modulator Access in Brazil. Healthcare. 2025; 13(16):1996. https://doi.org/10.3390/healthcare13161996
Chicago/Turabian Stylede Oliveira, Verônica Stasiak Bednarczuk, Marise Basso Amaral, Mariana Camargo, and Miquéias Lopes-Pacheco. 2025. "Building Lay Society Knowledge and Education for Health Technology Assessment and Policy Engagement: Case of CFTR Modulator Access in Brazil" Healthcare 13, no. 16: 1996. https://doi.org/10.3390/healthcare13161996
APA Stylede Oliveira, V. S. B., Amaral, M. B., Camargo, M., & Lopes-Pacheco, M. (2025). Building Lay Society Knowledge and Education for Health Technology Assessment and Policy Engagement: Case of CFTR Modulator Access in Brazil. Healthcare, 13(16), 1996. https://doi.org/10.3390/healthcare13161996







