Group-Based Trajectory Model to Assess Adjuvant Endocrine Therapy Adherence Pattern in HR-Positive Breast Cancer: Results from Rio Grande Valley Patients †
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Data
2.2. Study Cohort
2.3. Measures
2.3.1. Main Outcome
2.3.2. Variable of Interest
- Sociodemographic Variables
- Clinical Variables
2.4. Data Management
2.5. Adherence Measurements
2.5.1. Binary Adherence
2.5.2. Group-Based Trajectory Model
2.5.3. GBTM Validation
2.6. Statistical Analysis
3. Results
3.1. Baseline Characteristics
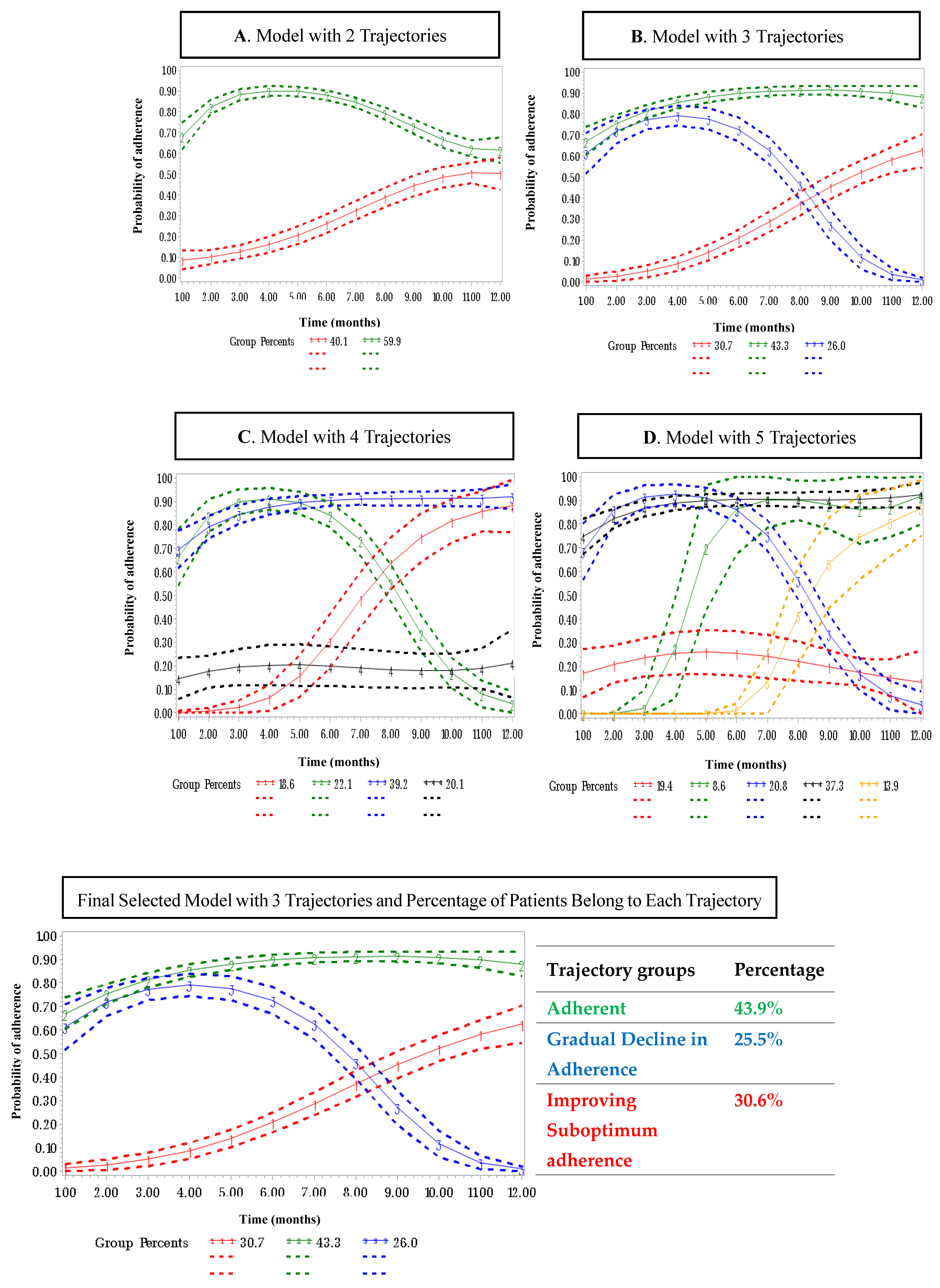
3.2. Adherence Trajectories of OET
3.3. Predictors Associated with Memberships in Adherence Trajectories
4. Discussion
4.1. Strengths
4.2. Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| ER | Estrogen receptor |
| OET | Oral endocrine therapy |
| PR | Progesterone receptor |
| HER2 | Human epidermal growth factor receptor 2 |
| PDC | Proportion of days covered |
| GBTM | Group-based trajectory model |
| RGV | Rio Grande Valley |
| OR | Odds ratio |
| CI | Confidence interval |
| SAS | Statistical Analysis System |
| AvePP | Average posterior probability of assignment |
| OCC | Odds of correct classification |
Appendix A
| Number of Groups in Trajectory Modeling | BIC (All Data Points) | BIC (Number of Subjects) | AIC | Log (2ΔBIC) | Group Membership Probabilities |
|---|---|---|---|---|---|
| 2 | −2399.19 | −2388.01 | −2370.7 | 40.10% | |
| 59.86% | |||||
| 3 | −2213.4 | −2199.73 | −2178.58 | 2.575834185 | 30.66% |
| 43.34% | |||||
| 25.99% | |||||
| 4 | −2176.84 | −2153.23 | −2116.69 | 1.968482949 | 18.60% |
| 22.05% | |||||
| 39.19% | |||||
| 20.13% | |||||
| 5 | −2153.24 | −2123.42 | −2077.27 | 1.775391972 | 19.40% |
| 8.60% | |||||
| 20.75% | |||||
| 37.34% | |||||
| 13.86% |
| Trajectory Group | Number Assigned | Proportion Assigned to Each Group | AvePP | OCC | Estimated Group Probabilities | |π–P| |
|---|---|---|---|---|---|---|
| Adherent | 152 | 0.43 | 0.96 | 31 | 0.43 | 0 |
| Improving Suboptimum Adherence | 106 | 0.30 | 0.96 | 24 | 0. 30 | 0.01 |
| Gradual Decline | 88 | 0.25 | 0.95 | 46 | 0.26 | 0.01 |
References
- Fatima, B.; Pruneda, P.S.; Mousavi, P.; Sheriff, R.; Ozuna, R.; Trivedi, M.V.; Abughosh, S. Group-Based Trajectory Modeling for Assessing Oral Endocrine Therapy Adherence Pattern in HER-positive Breast Cancer Patients at DHR Health in the Rio Grande Valley. In Proceedings of the Geriatric Medication Safety Symposium, Cooley University Life Center, Texas Medical Center, Houston, TX, USA, 25–26 April 2024. [Google Scholar]
- Rahimi, S.; Ononogbu, O.; Mohan, A.; Moussa, D.; Abughosh, S.; Trivedi, M.V. Adherence to oral endocrine therapy in racial/ethnic minority patients with low socioeconomic status before and during the COVID-19 pandemic. Int. J. Clin. Pharm. 2023, 45, 1396–1404. [Google Scholar] [CrossRef]
- Shachar, E.; Raphael, A.; Katz, U.; Kessner, R.; Shachar, S.S. Body composition measures as a determinant of Alpelisib related toxicity. Breast Cancer Res. Treat. 2024, 206, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Key, T.J.; Verkasalo, P.K.; Banks, E. Epidemiology of breast cancer. Lancet Oncol. 2001, 2, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Strauss, S.M. Oral health concerns of female breast cancer survivors on adjuvant endocrine therapy. AJN Am. J. Nurs. 2023, 123, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.; Joshi, P.; Kamra, S.; Sehgal, M. Economic Burden due to Treatment Non-Adherence in Patients with Breast Cancer: A Systematic Review. Value Health 2016, 19, A156. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: Patient-level meta-analysis of randomised trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Bradley, R.; Burrett, J.; Clarke, M.; Davies, C.; Duane, F.; Evans, V. Aromatase inhibitors versus tamoxifen in early breast cancer: Patient-level meta-analysis of the randomised trials. Lancet 2015, 386, 1341–1352. [Google Scholar]
- Bekes, I.; Huober, J. Extended Adjuvant Endocrine Therapy in Early Breast Cancer Patients—Review and Perspectives. Cancers 2023, 15, 4190. [Google Scholar] [CrossRef]
- Paranjpe, R.; Hwang, G.; Opsomer, C.; Lu, K.; Abajue, U.; Zaghloul, H.; Abughosh, S.; Trivedi, M. Evaluating medication adherence of oral endocrine therapy among breast cancer survivors in a large academic medical center. In Cancer Epidemiology Biomarkers & Prevention; American Association for Cancer Research (AACR): Philadelphia, PA, USA, 2020. [Google Scholar]
- Hershman, D.L.; Shao, T.; Kushi, L.H.; Buono, D.; Tsai, W.Y.; Fehrenbacher, L.; Kwan, M.; Gomez, S.L.; Neugut, A.I. Early discontinuation and non-adherence to adjuvant hormonal therapy are associated with increased mortality in women with breast cancer. Breast Cancer Res. Treat. 2011, 126, 529–537. [Google Scholar] [CrossRef]
- Friedman, L.; Schluchter, M.; Silverman, P.; Baar, J.; Wolff, B.; Cooper, B.; Moore, L.; Krishnamurthi, S.; Lanese, R.; Panneerselvam, A. Adherence to endocrine therapy in women with breast cancer in a clinical practice setting. Cancer Res. 2009, 69, 3108. [Google Scholar] [CrossRef]
- Ganna, S.; Rahimi, S.; Lu, A.; Laborde, K.; Trivedi, M. Interventions to improve oral endocrine therapy adherence in breast cancer patients. J. Cancer Surviv. 2024, 19, 930–939. [Google Scholar] [CrossRef]
- McQuaid, E.L.; Landier, W. Cultural issues in medication adherence: Disparities and directions. J. Gen. Intern. Med. 2018, 33, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Bialaszewski, R.P.; Gaddis, J.M.; Martin, B.; Dentino, P.; Ronnau, J. Bridging Bone Health: Osteoporosis Disparities in the Rio Grande Valley. Cureus 2023, 15, e51115. [Google Scholar] [CrossRef] [PubMed]
- Vega, W.A.; Rodriguez, M.A.; Gruskin, E. Health disparities in the Latino population. Epidemiol. Rev. 2009, 31, 99–112. [Google Scholar] [CrossRef]
- Salcedo, M.; Gowen, R.; Rodriguez, A.; Fisher-Hoch, S.; Daheri, M.; Guerra, L.; Toscano, P.; Gasca, M.; Morales, J.; Reyna-Rodriguez, F. Addressing high cervical cancer rates in the Rio Grande Valley along the Texas–Mexico border: A community-based initiative focused on education, patient navigation, and medical provider training/telementoring. Perspect. Public Health 2023, 143, 22–28. [Google Scholar] [CrossRef]
- Diop, A.; Gupta, A.; Mueller, S.; Dron, L.; Harari, O.; Berringer, H.; Kalatharan, V.; Park, J.J.; Mésidor, M.; Talbot, D. Assessing the performance of group-based trajectory modeling method to discover different patterns of medication adherence. Pharm. Stat. 2024, 23, 511–529. [Google Scholar] [CrossRef]
- Franklin, J.M.; Shrank, W.H.; Pakes, J.; Sanfélix-Gimeno, G.; Matlin, O.S.; Brennan, T.A.; Choudhry, N.K. Group-based trajectory models: A new approach to classifying and predicting long-term medication adherence. Med. Care 2013, 51, 789–796. [Google Scholar] [CrossRef]
- Memoli, V.; Ekanmian, G.; Lunghi, C.; Bouhnik, A.-D.; Lauzier, S.; Guénette, L. What methods are used to study the association between medication adherence trajectories, estimated with the group-based trajectory modeling (GBTM) method, and health-related outcomes?—a protocol for a systematic review. Syst. Rev. 2022, 11, 102. [Google Scholar] [CrossRef]
- Kini, V.; Ho, P.M. Interventions to improve medication adherence: A review. JAMA 2018, 320, 2461–2473. [Google Scholar] [CrossRef]
- Nagin, D.S. Group-based trajectory modeling: An overview. In Handbook of Quantitative Criminology; Springer: New York, NY, USA, 2010; pp. 53–67. [Google Scholar] [CrossRef]
- Nagin, D. Group-Based Modeling of Development; Harvard University Press: Cambridge, MA, USA, 2005. [Google Scholar]
- Gerber, B.S.; Cho, Y.I.; Arozullah, A.M.; Lee, S.-Y.D. Racial differences in medication adherence: A cross-sectional study of Medicare enrollees. Am. J. Geriatr. Pharmacother. 2010, 8, 136–145. [Google Scholar] [CrossRef]
- Liu, Y.; Malin, J.L.; Diamant, A.L.; Thind, A.; Maly, R.C. Adherence to adjuvant hormone therapy in low-income women with breast cancer: The role of provider–patient communication. Breast Cancer Res. Treat. 2013, 137, 829–836. [Google Scholar] [CrossRef]
- Rahimi, S.; Ononogbu, O.; Mohan, A.; Moussa, D.; Abughosh, S.; Trivedi, M. Identifying the predictors of adherence to oral endocrine therapy in racial/ethnic minority patients with low socioeconomic status. Res. Sq. 2022. [Google Scholar] [CrossRef]
- Lailler, G.; Memoli, V.; Benjamin, C.L.B.; Diane, M.-K.B.; Lauzier, S.; Mancini, J.; Bousquet, P.J.; Bouhnik, A.-D. Five-year adjuvant endocrine therapy adherence trajectories among women with breast cancer: A nationwide French study using administrative data. Clin. Breast Cancer 2021, 21, e415–e426. [Google Scholar] [CrossRef] [PubMed]
- Lambert-Côté, L.; Bouhnik, A.-D.; Bendiane, M.-K.; Bérenger, C.; Mondor, M.; Huiart, L.; Lauzier, S. Adherence trajectories of adjuvant endocrine therapy in the five years after its initiation among women with non-metastatic breast cancer: A cohort study using administrative databases. Breast Cancer Res. Treat. 2020, 180, 777–790. [Google Scholar] [CrossRef] [PubMed]
- Winn, A.N.; Dusetzina, S.B. The association between trajectories of endocrine therapy adherence and mortality among women with breast cancer. Pharmacoepidemiol. Drug Saf. 2016, 25, 953–959. [Google Scholar] [CrossRef]
- Schmidt, J.A.; Woolpert, K.M.; Hjorth, C.F.; Farkas, D.K.; Ejlertsen, B.; Cronin-Fenton, D. Social Characteristics and Adherence to Adjuvant Endocrine Therapy in Premenopausal Women with Breast Cancer. J. Clin. Oncol. 2024, 42, 3300–3307. [Google Scholar] [CrossRef]
- Tervonen, H.E.; Daniels, B.; Tang, M.; Preen, D.B.; Pearson, S.A. Patterns of endocrine therapy in a national cohort of early stage HER2-positive breast cancer patients. Pharmacoepidemiol. Drug Saf. 2019, 28, 812–820. [Google Scholar] [CrossRef]
- Vrinzen, C.E.; Delfgou, L.; Stadhouders, N.; Hermens, R.P.; Merkx, M.A.; Bloemendal, H.J.; Jeurissen, P.P. A systematic review and multilevel regression analysis reveals the comorbidity prevalence in cancer. Cancer Res. 2023, 83, 1147–1157. [Google Scholar] [CrossRef]
- Paranjpe, R.; John, G.; Trivedi, M.; Abughosh, S. Identifying adherence barriers to oral endocrine therapy among breast cancer survivors. Breast Cancer Res. Treat. 2019, 174, 297–305. [Google Scholar] [CrossRef]
- Bekele, B.B.; Lian, M.; Schmaltz, C.; Greever-Rice, T.; Shrestha, P.; Liu, Y. Preexisting Diabetes and Breast Cancer Treatment Among Low-Income Women. JAMA Netw. Open 2024, 7, e249548. [Google Scholar] [CrossRef]
- Sood, N.; Liu, Y.; Lian, M. Association of endocrine therapy initiation timeliness with adherence and continuation in low-income women with breast cancer. JAMA Netw Open 2022, 5, e2225345. [Google Scholar] [CrossRef]
- Gaddis, J.M.; Arellano, E.; Pulido, K.; Torres, T.; Chau-Zanetti, D.; Quailes, N.; Parraga, A.R.S.; Pullido, K.; Quailes, N.N. Burden of diabetes mellitus in the medically underserved Rio Grande Valley. Cureus 2024, 16, e70088. [Google Scholar] [CrossRef]
- Hwang, G.S.; Paranjpe, R.; Opsomer, C.; Lu, K.; Abajue, U.; Abughosh, S.; Zaghloul, H.; Trivedi, M.V. Oral endocrine therapy agent, race/ethnicity, and time on therapy predict adherence in breast cancer patients in a large academic institution. Clin. Breast Cancer 2020, 20, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Partridge, A.H.; Wang, P.S.; Winer, E.P.; Avorn, J. Nonadherence to adjuvant tamoxifen therapy in women with primary breast cancer. J. Clin. Oncol. 2003, 21, 602–606. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Lazaro, C.I.; Adams, D.P.; Fernandez-Lazaro, D.; Garcia-González, J.M.; Caballero-Garcia, A.; Miron-Canelo, J.A. Medication adherence and barriers among low-income, uninsured patients with multiple chronic conditions. Res. Soc. Adm. Pharm. 2019, 15, 744–753. [Google Scholar] [CrossRef] [PubMed]
- Rust, C.; Davis, C. Health literacy and medication adherence in underserved African-American breast cancer survivors: A qualitative study. Soc. Work. Health Care 2011, 50, 739–761. [Google Scholar] [CrossRef]
- Pinheiro, L.C.; Cho, J.; Kern, L.M.; Higgason, N.; O’Beirne, R.; Tamimi, R.; Safford, M. Managing diabetes during treatment for breast cancer: Oncology and primary care providers’ views on barriers and facilitators. Support. Care Cancer 2022, 30, 6901–6908. [Google Scholar] [CrossRef]
| Variable | All Patients (n = 346) | Improving Suboptimum Adherence (n = 106) | Adherent (n = 152) | Gradual Decline in Adherence (n = 88) | p Value |
|---|---|---|---|---|---|
| Age (years), n (%) | 0.029 | ||||
| <60 | 171 (49.42) | 63 (59.43) | 72 (47.37) | 36 (40.91) | |
| ≥60 | 175 (50.58) | 43 (40.57) | 80 (52.63) | 52 (59.09) | |
| Ethnicity, n (%) | 0.136 | ||||
| Hispanic | 322 (93.06) | 100 (94.34) | 137 (90.13) | 85 (96.59) | |
| Not Hispanic | 24 (6.94) | 6 (5.66) | 15 (9.87) | 3 (3.41) | |
| BMI | 0.533 | ||||
| Obese | 184 (53.18) | 61 (57.55) | 79 (51.97) | 44 (50.00) | |
| Non-obese | 162 (46.82) | 45 (42.45) | 73 (48.03) | 44 (50.00) | |
| Endocrine therapy, n (%) | 0.868 | ||||
| Aromatase inhibitors | 311 (89.88) | 94 (88.68) | 137 (90.13) | 80 (90.91) | |
| Tamoxifen | 35 (10.12) | 12 (11.32) | 15 (9.87) | 8 (9.09) | |
| Clinical Stage of Cancer, n (%) | 0.67 | ||||
| Stage 1 | 163 (47.11) | 50 (47.17) | 68 (44.74) | 45 (51.14) | |
| Stage 2 | 127 (36.71) | 37 (34.91) | 62 (40.79) | 28 (31.82) | |
| Stage 3 | 56 (16.18) | 19 (17.92) | 22 (14.47) | 15 (17.05) | |
| ER_PR_HER2, n (%) | 0.60 | ||||
| HER2+ | 56 (16.18) | 20 (18.87) | 24 (15.79) | 12 (13.64) | |
| HER2- | 290 (83.82) | 86 (81.13) | 128 (84.21) | 76 (86.36) | |
| Comorbidities | |||||
| Diabetes, n (%) | 0.0001 * | ||||
| Yes | 116 (33.53) | 30 (28.30) | 39 (25.66) | 47 (53.41) | |
| No | 230 (66.47) | 76 (74.70) | 113 (74.34) | 41 (46.59) | |
| Hypertension, n (%) | 0.07 | ||||
| Yes | 200 (57.80) | 57 (53.77) | 83 (54.61) | 60 (68.18) | |
| No | 146 (42.20) | 49 (46.23) | 69 (45.39) | 28 (31.82) | |
| Hyperlipidemia, n (%) | 0.04 * | ||||
| Yes | 168 (48.55) | 44 (41.51) | 72 (47.37) | 52 (59.09) | |
| No | 178 (51.45) | 62 (58.49) | 80 (52.63) | 36 (40.91) | |
| Depression, n (%) | 0.92 | ||||
| Yes | 53 (15.32) | 17 (16.04) | 22 (14.47) | 14 (15.91) | |
| No | 293 (84.68) | 89 (83.96) | 130 (85.53) | 74 (84.09) | |
| Number of years of therapy, n(%) | |||||
| ≤1 year | 136 (39.31) | 86 (81.13) | 31 (20.39) | 19 (21.59) | 0.0001 * |
| ≥2 years | 210 (60.69) | 20 (18.87) | 121 (79.61) | 69 (78.41) | |
| Variable | Reference | Improving Suboptimum Adherence vs. Adherent | Gradual Decline vs. Adherent | ||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | p Value | OR | 95% CI | p Value | ||
| Age | |||||||
| ≥60 | <60 | 0.67 | 0.35–1.32 | 0.25 | 1.07 | 0.58–1.96 | 0.81 |
| Ethnicity | |||||||
| Not Hispanic | Hispanic | 0.96 | 0.27–3.40 | 0.95 | 0.41 | 0.11–1.52 | 0.18 |
| BMI | |||||||
| Obese | Non-Obese | 1.32 | 0.71–2.48 | 0.37 | 1.00 | 0.56–1.78 | 0.98 |
| Endocrine therapy | |||||||
| Tamoxifen | Aromatase Inhibitors | 1.41 | 0.41–4.07 | 0.52 | 1.10 | 0.40–2.90 | 0.86 |
| Clinical Stage of Cancer | |||||||
| Stage 2 | Stage 1 | 0.96 | 0.521–1.789 | 0.91 | 0.64 | 0.343–1.191 | 0.15 |
| Stage 3 | Stage 1 | 1.24 | 0.552–2.820 | 0.59 | 1.07 | 0.472–2.438 | 0.86 |
| ER_PR_HER2 | |||||||
| HER2+ | HER2- | 1.61 | 0.355–7.304 | 0.84 | 0.773 | 0.256–2.337 | 0.61 |
| Diabetes | |||||||
| Yes | No | 1.40 | 0.66–2.97 | 0.38 | 2.96 | 1.57–5.57 | 0.0006 * |
| Hypertension | |||||||
| Yes | No | 1.82 | 0.90–3.66 | 0.09 | 1.32 | 0.69–2.53 | 0.39 |
| Hyperlipidemia | |||||||
| Yes | No | 1.11 | 0.55–2.22 | 0.76 | 1.06 | 0.56–1.99 | 0.85 |
| Depression | |||||||
| Yes | No | 1.21 | 0.50–2.88 | 0.67 | 1.19 | 0.55–2.58 | 0.65 |
| Number of years of therapy | |||||||
| ≥2 years | ≤1 year | 0.32 | 0.218–0.459 | 0.0001 * | 0.80 | 0.402–1.605 | 0.535 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fatima, B.; Pruneda, P.S.; Mousavi, P.; Sheriff, R.; Ozuna, R.; Trivedi, M.V.; Abughosh, S. Group-Based Trajectory Model to Assess Adjuvant Endocrine Therapy Adherence Pattern in HR-Positive Breast Cancer: Results from Rio Grande Valley Patients. Healthcare 2025, 13, 1777. https://doi.org/10.3390/healthcare13151777
Fatima B, Pruneda PS, Mousavi P, Sheriff R, Ozuna R, Trivedi MV, Abughosh S. Group-Based Trajectory Model to Assess Adjuvant Endocrine Therapy Adherence Pattern in HR-Positive Breast Cancer: Results from Rio Grande Valley Patients. Healthcare. 2025; 13(15):1777. https://doi.org/10.3390/healthcare13151777
Chicago/Turabian StyleFatima, Bilqees, Phillip Shayne Pruneda, Parasto Mousavi, Rheena Sheriff, Ronnie Ozuna, Meghana V. Trivedi, and Susan Abughosh. 2025. "Group-Based Trajectory Model to Assess Adjuvant Endocrine Therapy Adherence Pattern in HR-Positive Breast Cancer: Results from Rio Grande Valley Patients" Healthcare 13, no. 15: 1777. https://doi.org/10.3390/healthcare13151777
APA StyleFatima, B., Pruneda, P. S., Mousavi, P., Sheriff, R., Ozuna, R., Trivedi, M. V., & Abughosh, S. (2025). Group-Based Trajectory Model to Assess Adjuvant Endocrine Therapy Adherence Pattern in HR-Positive Breast Cancer: Results from Rio Grande Valley Patients. Healthcare, 13(15), 1777. https://doi.org/10.3390/healthcare13151777







