Healthcare Complexities in Neurodegenerative Proteinopathies: A Narrative Review
Abstract
1. Introduction
2. Methods
3. Results
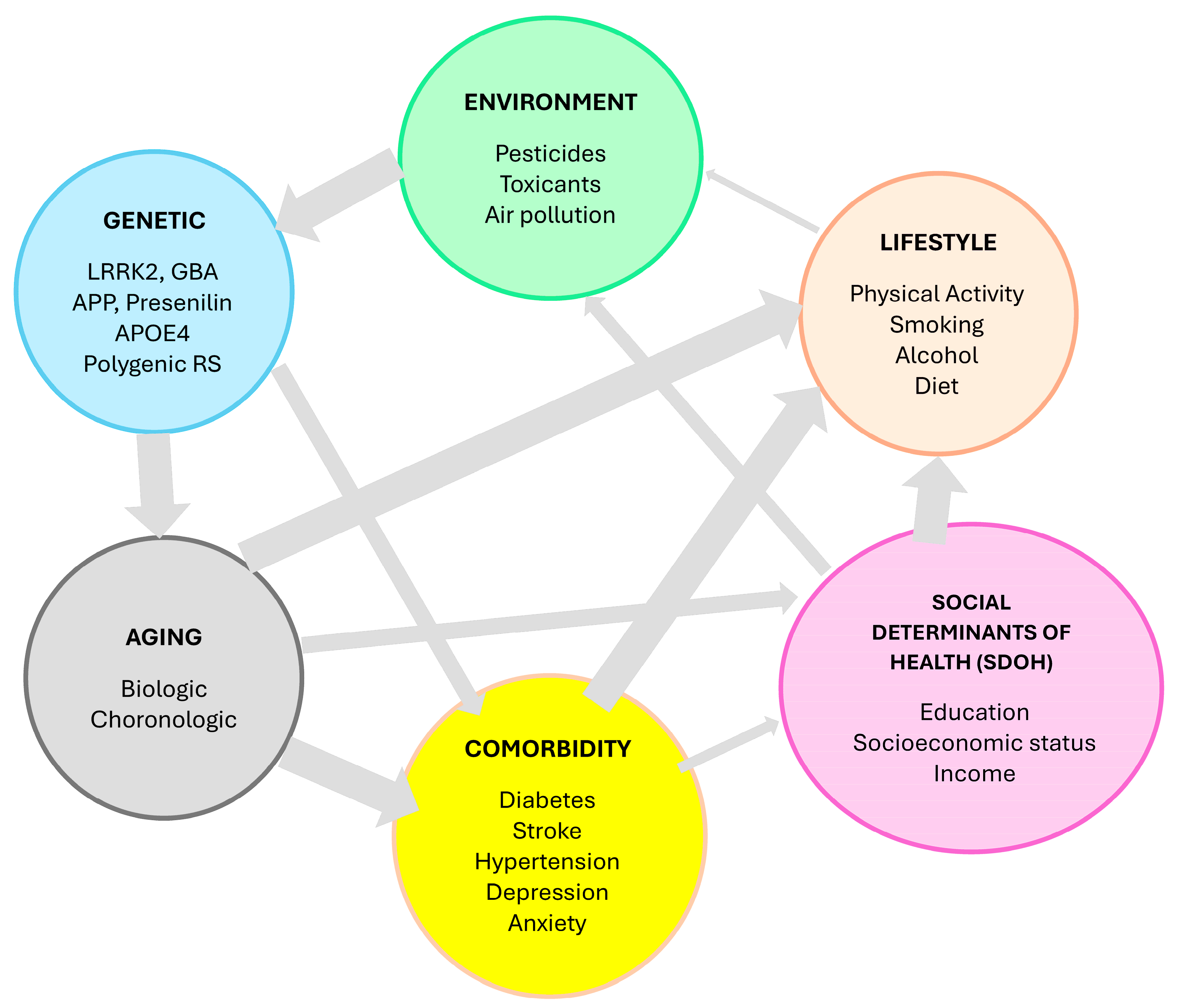
3.1. Multifactorial Etiology
3.1.1. Alzheimer’s Disease
3.1.2. Parkinson’s Disease
3.2. Long Prodromal Stage
3.2.1. Alzheimer’s Disease
3.2.2. Parkinson’s Disease
3.2.3. Frontotemporal Dementia
3.3. Co-Pathologies
3.4. Progressive Nature
3.5. Sex Differences
3.6. Lack of Efficient Disease-Modifying Therapies
3.6.1. Alzheimer’s Disease
3.6.2. Parkinson’s Disease
3.7. Multi-Domain Multidisciplinary Care
3.8. Caregiver Burden
3.9. Costly Management
3.9.1. Alzheimer’s Disease
3.9.2. Parkinson’s Disease
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- GBD 2021 Nervous System Disorders Collaborators. Global, regional, and national burden of disorders affecting the nervous system, 1990-2021: A systematic analysis for the Global Burden of Disease Study 2021. Lancet Neurol. 2024, 23, 344–381. [Google Scholar] [CrossRef]
- GBD 2016 Dementia Collaborators. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990-2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 88–106. [Google Scholar] [CrossRef]
- Su, D.; Cui, Y.; He, C.; Yin, P.; Bai, R.; Zhu, J.; Lam, J.S.T.; Zhang, J.; Yan, R.; Zheng, X.; et al. Projections for prevalence of Parkinson’s disease and its driving factors in 195 countries and territories to 2050: Modelling study of Global Burden of Disease Study 2021. BMJ 2025, 388, e080952. [Google Scholar] [CrossRef] [PubMed]
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ye, X.; Huang, Z.; Ye, L.; Chen, C. Global burden of Parkinson’s disease from 1990 to 2021: A population-based study. BMJ Open 2025, 15, e095610. [Google Scholar] [CrossRef] [PubMed]
- Hendriks, S.; Peetoom, K.; Bakker, C.; van der Flier, W.M.; Papma, J.M.; Koopmans, R.; Verhey, F.R.J.; de Vugt, M.; Kohler, S.; the Young-Onset Dementia Epidemiology Study Group; et al. Global Prevalence of Young-Onset Dementia: A Systematic Review and Meta-analysis. JAMA Neurol. 2021, 78, 1080–1090. [Google Scholar] [CrossRef]
- Golde, T.E. Disease-Modifying Therapies for Alzheimer’s Disease: More Questions than Answers. Neurotherapeutics 2022, 19, 209–227. [Google Scholar] [CrossRef]
- Dyer, O. Aduhelm: Biogen abandons Alzheimer’s drug after controversial approval left it unfunded by Medicare. BMJ 2024, 384, q281. [Google Scholar] [CrossRef]
- Gharat, R.; Dixit, G.; Khambete, M.; Prabhu, A. Targets, trials and tribulations in Alzheimer therapeutics. Eur. J. Pharmacol. 2024, 962, 176230. [Google Scholar] [CrossRef]
- Hung, A.Y.; Schwarzschild, M.A. Approaches to Disease Modification for Parkinson’s Disease: Clinical Trials and Lessons Learned. Neurotherapeutics 2020, 17, 1393–1405. [Google Scholar] [CrossRef]
- Grand, J.H.; Caspar, S.; Macdonald, S.W. Clinical features and multidisciplinary approaches to dementia care. J. Multidiscip. Healthc. 2011, 4, 125–147. [Google Scholar] [CrossRef]
- Dujardin, S.; Commins, C.; Lathuiliere, A.; Beerepoot, P.; Fernandes, A.R.; Kamath, T.V.; De Los Santos, M.B.; Klickstein, N.; Corjuc, D.L.; Corjuc, B.T.; et al. Tau molecular diversity contributes to clinical heterogeneity in Alzheimer’s disease. Nat. Med. 2020, 26, 1256–1263. [Google Scholar] [CrossRef]
- Giannakopoulos, P.; Herrmann, F.R.; Bussiere, T.; Bouras, C.; Kovari, E.; Perl, D.P.; Morrison, J.H.; Gold, G.; Hof, P.R. Tangle and neuron numbers, but not amyloid load, predict cognitive status in Alzheimer’s disease. Neurology 2003, 60, 1495–1500. [Google Scholar] [CrossRef]
- Kurkinen, M.; Fulek, M.; Fulek, K.; Beszlej, J.A.; Kurpas, D.; Leszek, J. The Amyloid Cascade Hypothesis in Alzheimer’s Disease: Should We Change Our Thinking? Biomolecules 2023, 13, 453. [Google Scholar] [CrossRef]
- Kepp, K.P.; Robakis, N.K.; Hoilund-Carlsen, P.F.; Sensi, S.L.; Vissel, B. The amyloid cascade hypothesis: An updated critical review. Brain 2023, 146, 3969–3990. [Google Scholar] [CrossRef] [PubMed]
- Mendes, A.J.; Ribaldi, F.; Pievani, M.; Boccalini, C.; Garibotto, V.; Frisoni, G.B.; Alzheimer’s Disease Neuroimaging, I. Validating the Amyloid Cascade Through the Revised Criteria of Alzheimer’s Association Workgroup 2024 for Alzheimer Disease. Neurology 2025, 104, e213675. [Google Scholar] [CrossRef] [PubMed]
- Raj, A.; Torok, J.; Ranasinghe, K. Understanding the complex interplay between tau, amyloid and the network in the spatiotemporal progression of Alzheimer’s disease. Prog. Neurobiol. 2025, 249, 102750. [Google Scholar] [CrossRef] [PubMed]
- Thal, D.R.; Tome, S.O. The central role of tau in Alzheimer’s disease: From neurofibrillary tangle maturation to the induction of cell death. Brain Res. Bull. 2022, 190, 204–217. [Google Scholar] [CrossRef]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated Tau in Alzheimer’s Disease and Other Tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Congdon, E.E.; Ji, C.; Tetlow, A.M.; Jiang, Y.; Sigurdsson, E.M. Tau-targeting therapies for Alzheimer disease: Current status and future directions. Nat. Rev. Neurol. 2023, 19, 715–736. [Google Scholar] [CrossRef]
- Levy-Lahad, E.; Wasco, W.; Poorkaj, P.; Romano, D.M.; Oshima, J.; Pettingell, W.H.; Yu, C.E.; Jondro, P.D.; Schmidt, S.D.; Wang, K.; et al. Candidate gene for the chromosome 1 familial Alzheimer’s disease locus. Science 1995, 269, 973–977. [Google Scholar] [CrossRef]
- Sherrington, R.; Rogaev, E.I.; Liang, Y.; Rogaeva, E.A.; Levesque, G.; Ikeda, M.; Chi, H.; Lin, C.; Li, G.; Holman, K.; et al. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer’s disease. Nature 1995, 375, 754–760. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Stolk, R.P.; van Harskamp, F.; Pols, H.A.; Hofman, A.; Breteler, M.M. Diabetes mellitus and the risk of dementia: The Rotterdam Study. Neurology 1999, 53, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Di Domenico, F.; Barone, E. Elevated risk of type 2 diabetes for development of Alzheimer disease: A key role for oxidative stress in brain. Biochim. Biophys. Acta 2014, 1842, 1693–1706. [Google Scholar] [CrossRef]
- Stampfer, M.J. Cardiovascular disease and Alzheimer’s disease: Common links. J. Intern. Med. 2006, 260, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Akbaraly, T.N.; Singh-Manoux, A.; Dugravot, A.; Brunner, E.J.; Kivimaki, M.; Sabia, S. Association of Midlife Diet With Subsequent Risk for Dementia. JAMA 2019, 321, 957–968. [Google Scholar] [CrossRef]
- Mielke, M.M.; Ransom, J.E.; Mandrekar, J.; Turcano, P.; Savica, R.; Brown, A.W. Traumatic Brain Injury and Risk of Alzheimer’s Disease and Related Dementias in the Population. J. Alzheimers Dis. 2022, 88, 1049–1059. [Google Scholar] [CrossRef]
- Wallensten, J.; Ljunggren, G.; Nager, A.; Wachtler, C.; Bogdanovic, N.; Petrovic, P.; Carlsson, A.C. Stress, depression, and risk of dementia—A cohort study in the total population between 18 and 65 years old in Region Stockholm. Alzheimers Res. Ther. 2023, 15, 161. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- Sironi, L.; Restelli, L.M.; Tolnay, M.; Neutzner, A.; Frank, S. Dysregulated Interorganellar Crosstalk of Mitochondria in the Pathogenesis of Parkinson’s Disease. Cells 2020, 9, 233. [Google Scholar] [CrossRef]
- Dorsey, E.R.; Bloem, B.R. Parkinson’s Disease Is Predominantly an Environmental Disease. J. Parkinsons Dis. 2024, 14, 451–465. [Google Scholar] [CrossRef]
- Dorsey, E.R.; De Miranda, B.R.; Horsager, J.; Borghammer, P. The Body, the Brain, the Environment, and Parkinson’s Disease. J. Parkinsons Dis. 2024, 14, 363–381. [Google Scholar] [CrossRef]
- Wareham, L.K.; Liddelow, S.A.; Temple, S.; Benowitz, L.I.; Di Polo, A.; Wellington, C.; Goldberg, J.L.; He, Z.; Duan, X.; Bu, G.; et al. Solving neurodegeneration: Common mechanisms and strategies for new treatments. Mol. Neurodegener. 2022, 17, 23. [Google Scholar] [CrossRef]
- Shvetcov, A.; Johnson, E.C.B.; Winchester, L.M.; Walker, K.A.; Wilkins, H.M.; Thompson, T.G.; Rothstein, J.D.; Krish, V.; Imam, F.B.; The Global Neurodegeneration Proteomics Consortium (GNPC); et al. APOE epsilon4 carriers share immune-related proteomic changes across neurodegenerative diseases. Nat. Med. 2025. [Google Scholar] [CrossRef]
- Palmer, J.M.; Huentelman, M.; Ryan, L. More than just risk for Alzheimer’s disease: APOE epsilon4’s impact on the aging brain. Trends Neurosci. 2023, 46, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Verghese, P.B.; Castellano, J.M.; Holtzman, D.M. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neurol. 2011, 10, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Song, S.Y. Genomic and Transcriptomic Approaches Advance the Diagnosis and Prognosis of Neurodegenerative Diseases. Genes 2025, 16, 135. [Google Scholar] [CrossRef] [PubMed]
- Kodam, P.; Sai Swaroop, R.; Pradhan, S.S.; Sivaramakrishnan, V.; Vadrevu, R. Integrated multi-omics analysis of Alzheimer’s disease shows molecular signatures associated with disease progression and potential therapeutic targets. Sci. Rep. 2023, 13, 3695. [Google Scholar] [CrossRef]
- Arnold, M.; Nho, K.; Kueider-Paisley, A.; Massaro, T.; Huynh, K.; Brauner, B.; MahmoudianDehkordi, S.; Louie, G.; Moseley, M.A.; Thompson, J.W.; et al. Sex and APOE epsilon4 genotype modify the Alzheimer’s disease serum metabolome. Nat. Commun. 2020, 11, 1148. [Google Scholar] [CrossRef]
- Wang, E.; Lemos Duarte, M.; Rothman, L.E.; Cai, D.; Zhang, B. Non-coding RNAs in Alzheimer’s disease: Perspectives from omics studies. Hum. Mol. Genet. 2022, 31, R54–R61. [Google Scholar] [CrossRef]
- Madrid, L.; Moreno-Grau, S.; Ahmad, S.; Gonzalez-Perez, A.; de Rojas, I.; Xia, R.; Martino Adami, P.V.; Garcia-Gonzalez, P.; Kleineidam, L.; Yang, Q.; et al. Multiomics integrative analysis identifies APOE allele-specific blood biomarkers associated to Alzheimer’s disease etiopathogenesis. Aging 2021, 13, 9277–9329. [Google Scholar] [CrossRef]
- Sancesario, G.M.; Bernardini, S. Alzheimer’s disease in the omics era. Clin. Biochem. 2018, 59, 9–16. [Google Scholar] [CrossRef]
- Powell, W.R.; Buckingham, W.R.; Larson, J.L.; Vilen, L.; Yu, M.; Salamat, M.S.; Bendlin, B.B.; Rissman, R.A.; Kind, A.J.H. Association of Neighborhood-Level Disadvantage With Alzheimer Disease Neuropathology. JAMA Netw. Open 2020, 3, e207559. [Google Scholar] [CrossRef] [PubMed]
- Vermunt, L.; Sikkes, S.A.M.; van den Hout, A.; Handels, R.; Bos, I.; van der Flier, W.M.; Kern, S.; Ousset, P.J.; Maruff, P.; Skoog, I.; et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimers Dement. 2019, 15, 888–898. [Google Scholar] [CrossRef] [PubMed]
- Sperling, R.; Mormino, E.; Johnson, K. The evolution of preclinical Alzheimer’s disease: Implications for prevention trials. Neuron 2014, 84, 608–622. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Hardy, J.; Blennow, K.; Chen, C.; Perry, G.; Kim, S.H.; Villemagne, V.L.; Aisen, P.; Vendruscolo, M.; Iwatsubo, T.; et al. The Amyloid-beta Pathway in Alzheimer’s Disease. Mol. Psychiatry 2021, 26, 5481–5503. [Google Scholar] [CrossRef]
- Palmqvist, S.; Zetterberg, H.; Mattsson, N.; Johansson, P.; Alzheimer’s Disease Neuroimaging Initiative; Minthon, L.; Blennow, K.; Olsson, M.; Hansson, O.; Swedish Bio, F.S.G. Detailed comparison of amyloid PET and CSF biomarkers for identifying early Alzheimer disease. Neurology 2015, 85, 1240–1249. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Yao, C.; Pelletier, A.; Montplaisir, J.Y.; Gagnon, J.F.; Postuma, R.B. Evolution of prodromal Parkinson’s disease and dementia with Lewy bodies: A prospective study. Brain 2019, 142, 2051–2067. [Google Scholar] [CrossRef]
- Postuma, R.B. Prodromal Parkinson’s disease--using REM sleep behavior disorder as a window. Park. Relat. Disord. 2014, 20 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Montplaisir, J.Y.; Pelletier, A.; Gagnon, J.F.; Berg, D.; Postuma, R.B. Validation of the MDS research criteria for prodromal Parkinson’s disease: Longitudinal assessment in a REM sleep behavior disorder (RBD) cohort. Mov. Disord. 2017, 32, 865–873. [Google Scholar] [CrossRef]
- Postuma, R.B.; Iranzo, A.; Hu, M.; Hogl, B.; Boeve, B.F.; Manni, R.; Oertel, W.H.; Arnulf, I.; Ferini-Strambi, L.; Puligheddu, M.; et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: A multicentre study. Brain 2019, 142, 744–759. [Google Scholar] [CrossRef]
- Iranzo, A.; Tolosa, E.; Gelpi, E.; Molinuevo, J.L.; Valldeoriola, F.; Serradell, M.; Sanchez-Valle, R.; Vilaseca, I.; Lomena, F.; Vilas, D.; et al. Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: An observational cohort study. Lancet Neurol. 2013, 12, 443–453. [Google Scholar] [CrossRef]
- Stefani, A.; Antelmi, E.; Arnaldi, D.; Arnulf, I.; During, E.; Hogl, B.; Hu, M.M.T.; Iranzo, A.; Luke, R.; Peever, J.; et al. From mechanisms to future therapy: A synopsis of isolated REM sleep behavior disorder as early synuclein-related disease. Mol. Neurodegener. 2025, 20, 19. [Google Scholar] [CrossRef] [PubMed]
- Wittenberg, D.; Possin, K.L.; Rascovsky, K.; Rankin, K.P.; Miller, B.L.; Kramer, J.H. The early neuropsychological and behavioral characteristics of frontotemporal dementia. Neuropsychol. Rev. 2008, 18, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Chatzidimitriou, E.; Ioannidis, P.; Moraitou, D.; Konstantinopoulou, E.; Aretouli, E. The cognitive and behavioral correlates of functional status in patients with frontotemporal dementia: A pilot study. Front. Hum. Neurosci. 2023, 17, 1087765. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.; Borghammer, P.; Fereshtehnejad, S.M.; Heinzel, S.; Horsager, J.; Schaeffer, E.; Postuma, R.B. Prodromal Parkinson disease subtypes—Key to understanding heterogeneity. Nat. Rev. Neurol. 2021, 17, 349–361. [Google Scholar] [CrossRef]
- Koszla, O.; Solek, P. Misfolding and aggregation in neurodegenerative diseases: Protein quality control machinery as potential therapeutic clearance pathways. Cell Commun. Signal 2024, 22, 421. [Google Scholar] [CrossRef]
- Allegri, R.F. Moving from neurodegenerative dementias, to cognitive proteinopathies, replacing “where” by “what”. Dement. Neuropsychol. 2020, 14, 237–242. [Google Scholar] [CrossRef]
- Robinson, J.L.; Xie, S.X.; Baer, D.R.; Suh, E.; Van Deerlin, V.M.; Loh, N.J.; Irwin, D.J.; McMillan, C.T.; Wolk, D.A.; Chen-Plotkin, A.; et al. Pathological combinations in neurodegenerative disease are heterogeneous and disease-associated. Brain 2023, 146, 2557–2569. [Google Scholar] [CrossRef]
- Wisse, L.E.M.; Wuestefeld, A.; Murray, M.E.; Jagust, W.; La Joie, R. Role of tau versus TDP-43 pathology on medial temporal lobe atrophy in aging and Alzheimer’s disease. Alzheimers Dement. 2025, 21, e14582. [Google Scholar] [CrossRef]
- Robinson, J.L.; Richardson, H.; Xie, S.X.; Suh, E.; Van Deerlin, V.M.; Alfaro, B.; Loh, N.; Porras-Paniagua, M.; Nirschl, J.J.; Wolk, D.; et al. The development and convergence of co-pathologies in Alzheimer’s disease. Brain 2021, 144, 953–962. [Google Scholar] [CrossRef]
- Hampel, H.; Cummings, J.; Blennow, K.; Gao, P.; Jack, C.R., Jr.; Vergallo, A. Developing the ATX(N) classification for use across the Alzheimer disease continuum. Nat. Rev. Neurol. 2021, 17, 580–589. [Google Scholar] [CrossRef]
- Dubois, B.; Villain, N.; Schneider, L.; Fox, N.; Campbell, N.; Galasko, D.; Kivipelto, M.; Jessen, F.; Hanseeuw, B.; Boada, M.; et al. Alzheimer Disease as a Clinical-Biological Construct-An International Working Group Recommendation. JAMA Neurol. 2024, 81, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Goedert, M.; Masuda-Suzukake, M.; Falcon, B. Like prions: The propagation of aggregated tau and alpha-synuclein in neurodegeneration. Brain 2017, 140, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Vijayakumari, A.A.; Saadatpour, L.; Floden, D.; Fernandez, H.; Walter, B.L. Neuroanatomical heterogeneity drives divergent cognitive and motor trajectories in Parkinson’s disease subtypes. J. Neurol. Sci. 2024, 468, 123335. [Google Scholar] [CrossRef] [PubMed]
- Zeighami, Y.; Fereshtehnejad, S.M.; Dadar, M.; Collins, D.L.; Postuma, R.B.; Misic, B.; Dagher, A. A clinical-anatomical signature of Parkinson’s disease identified with partial least squares and magnetic resonance imaging. Neuroimage 2019, 190, 69–78. [Google Scholar] [CrossRef]
- Hassan, A.; Whitwell, J.L.; Josephs, K.A. The corticobasal syndrome-Alzheimer’s disease conundrum. Expert. Rev. Neurother. 2011, 11, 1569–1578. [Google Scholar] [CrossRef]
- Burrell, J.R.; Hodges, J.R.; Rowe, J.B. Cognition in corticobasal syndrome and progressive supranuclear palsy: A review. Mov. Disord. 2014, 29, 684–693. [Google Scholar] [CrossRef]
- Chow, T.W.; Hynan, L.S.; Lipton, A.M. MMSE scores decline at a greater rate in frontotemporal degeneration than in AD. Dement. Geriatr. Cogn. Disord. 2006, 22, 194–199. [Google Scholar] [CrossRef]
- Mestre, T.A.; Fereshtehnejad, S.M.; Berg, D.; Bohnen, N.I.; Dujardin, K.; Erro, R.; Espay, A.J.; Halliday, G.; van Hilten, J.J.; Hu, M.T.; et al. Parkinson’s Disease Subtypes: Critical Appraisal and Recommendations. J. Parkinsons Dis. 2021, 11, 395–404. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Zeighami, Y.; Dagher, A.; Postuma, R.B. Clinical criteria for subtyping Parkinson’s disease: Biomarkers and longitudinal progression. Brain 2017, 140, 1959–1976. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Romenets, S.R.; Anang, J.B.; Latreille, V.; Gagnon, J.F.; Postuma, R.B. New Clinical Subtypes of Parkinson Disease and Their Longitudinal Progression: A Prospective Cohort Comparison With Other Phenotypes. JAMA Neurol. 2015, 72, 863–873. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, A.; Baschi, R.; Cicero, C.E.; Iacono, S.; Re, V.L.; Luca, A.; Schiro, G.; Monastero, R.; Gender Neurology Study Group of the Italian Society of Neurology. Sex and gender differences in Alzheimer’s disease, Parkinson’s disease, and Amyotrophic Lateral Sclerosis: A narrative review. Mech. Ageing Dev. 2023, 212, 111821. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.S.; Jiang, Q.W.; Chen, S.D. Sex difference in biological change and mechanism of Alzheimer’s disease: From macro- to micro-landscape. Ageing Res. Rev. 2023, 87, 101918. [Google Scholar] [CrossRef] [PubMed]
- Farhadi, F.; Vosoughi, K.; Shahidi, G.A.; Delbari, A.; Lokk, J.; Fereshtehnejad, S.M. Sexual dimorphism in Parkinson’s disease: Differences in clinical manifestations, quality of life and psychosocial functioning between males and females. Neuropsychiatr. Dis. Treat. 2017, 13, 329–338. [Google Scholar] [CrossRef]
- DuMont, M.; Agostinis, A.; Singh, K.; Swan, E.; Buttle, Y.; Tropea, D. Sex representation in neurodegenerative and psychiatric disorders’ preclinical and clinical studies. Neurobiol. Dis. 2023, 184, 106214. [Google Scholar] [CrossRef]
- Hosseinzadeh, S.; Afshari, S.; Molaei, S.; Rezaei, N.; Dadkhah, M. The role of genetics and gender specific differences in neurodegenerative disorders: Insights from molecular and immune landscape. J. Neuroimmunol. 2023, 384, 578206. [Google Scholar] [CrossRef]
- Casaletto, K.B.; Nichols, E.; Aslanyan, V.; Simone, S.M.; Rabin, J.S.; La Joie, R.; Brickman, A.M.; Dams-O’Connor, K.; Palta, P.; Kumar, R.G.; et al. Sex-specific effects of microglial activation on Alzheimer’s disease proteinopathy in older adults. Brain 2022, 145, 3536–3545. [Google Scholar] [CrossRef]
- Korf, J.M.; Ganesh, B.P.; McCullough, L.D. Gut dysbiosis and age-related neurological diseases in females. Neurobiol. Dis. 2022, 168, 105695. [Google Scholar] [CrossRef]
- Saha, P.; Weigle, I.Q.; Slimmon, N.; Poli, P.B.; Patel, P.; Zhang, X.; Cao, Y.; Michalkiewicz, J.; Gomm, A.; Zhang, C.; et al. Early modulation of the gut microbiome by female sex hormones alters amyloid pathology and microglial function. Sci. Rep. 2024, 14, 1827. [Google Scholar] [CrossRef]
- Zhang, M.; Zhai, Z.; Yang, B.; He, L.; Wang, J.; Dai, W.; Xue, L.; Yang, X.; Feng, Y.; Wang, H. Exploring the alteration of gut microbiota and brain function in gender-specific Parkinson’s disease based on metagenomic sequencing. Front. Aging Neurosci. 2023, 15, 1148546. [Google Scholar] [CrossRef]
- Castro-Aldrete, L.; Moser, M.V.; Putignano, G.; Ferretti, M.T.; Schumacher Dimech, A.; Santuccione Chadha, A. Sex and gender considerations in Alzheimer’s disease: The Women’s Brain Project contribution. Front. Aging Neurosci. 2023, 15, 1105620. [Google Scholar] [CrossRef]
- Castro-Aldrete, L.; Einsiedler, M.; Novakova Martinkova, J.; Depypere, H.; Alvin Ang, T.F.; Mielke, M.M.; Sindi, S.; Eyre, H.A.; Au, R.; Schumacher Dimech, A.M.; et al. Alzheimer disease seen through the lens of sex and gender. Nat. Rev. Neurol. 2025, 21, 235–249. [Google Scholar] [CrossRef]
- Bianco, A.; Antonacci, Y.; Liguori, M. Sex and Gender Differences in Neurodegenerative Diseases: Challenges for Therapeutic Opportunities. Int. J. Mol. Sci. 2023, 24, 6354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Liu, J.; Huang, S.; Wang, X.Y.; Chen, X.; Liu, G.H.; Ye, K.; Song, W.; Masters, C.L.; Wang, J.; et al. Antiageing strategy for neurodegenerative diseases: From mechanisms to clinical advances. Signal Transduct. Target. Ther. 2025, 10, 76. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, U.; Kayed, R. Amyloid beta, Tau, and alpha-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Moda, F.; Ciullini, A.; Dellarole, I.L.; Lombardo, A.; Campanella, N.; Bufano, G.; Cazzaniga, F.A.; Giaccone, G. Secondary Protein Aggregates in Neurodegenerative Diseases: Almost the Rule Rather than the Exception. Front. Biosci. (Landmark Ed.) 2023, 28, 255. [Google Scholar] [CrossRef]
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Cummings, J.; Osse, A.M.L.; Cammann, D.; Powell, J.; Chen, J. Anti-Amyloid Monoclonal Antibodies for the Treatment of Alzheimer’s Disease. BioDrugs 2024, 38, 5–22. [Google Scholar] [CrossRef]
- Hampel, H.; Elhage, A.; Cho, M.; Apostolova, L.G.; Nicoll, J.A.R.; Atri, A. Amyloid-related imaging abnormalities (ARIA): Radiological, biological and clinical characteristics. Brain 2023, 146, 4414–4424. [Google Scholar] [CrossRef]
- Pagano, G.; Taylor, K.I.; Anzures-Cabrera, J.; Marchesi, M.; Simuni, T.; Marek, K.; Postuma, R.B.; Pavese, N.; Stocchi, F.; Azulay, J.P.; et al. Trial of Prasinezumab in Early-Stage Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 421–432. [Google Scholar] [CrossRef]
- Lang, A.E.; Siderowf, A.D.; Macklin, E.A.; Poewe, W.; Brooks, D.J.; Fernandez, H.H.; Rascol, O.; Giladi, N.; Stocchi, F.; Tanner, C.M.; et al. Trial of Cinpanemab in Early Parkinson’s Disease. N. Engl. J. Med. 2022, 387, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Cummings, J. Disease modification and Neuroprotection in neurodegenerative disorders. Transl. Neurodegener. 2017, 6, 25. [Google Scholar] [CrossRef] [PubMed]
- Fereshtehnejad, S.M. Strategies to maintain quality of life among people with Parkinson’s disease: What works? Neurodegener. Dis. Manag. 2016, 6, 399–415. [Google Scholar] [CrossRef] [PubMed]
- Callahan, C.M.; Boustani, M.A.; Unverzagt, F.W.; Austrom, M.G.; Damush, T.M.; Perkins, A.J.; Fultz, B.A.; Hui, S.L.; Counsell, S.R.; Hendrie, H.C. Effectiveness of collaborative care for older adults with Alzheimer disease in primary care: A randomized controlled trial. JAMA 2006, 295, 2148–2157. [Google Scholar] [CrossRef]
- Reuben, D.B.; Tan, Z.S.; Romero, T.; Wenger, N.S.; Keeler, E.; Jennings, L.A. Patient and Caregiver Benefit From a Comprehensive Dementia Care Program: 1-Year Results From the UCLA Alzheimer’s and Dementia Care Program. J. Am. Geriatr. Soc. 2019, 67, 2267–2273. [Google Scholar] [CrossRef]
- Lidstone, S.C.; Bayley, M.; Lang, A.E. The evidence for multidisciplinary care in Parkinson’s disease. Expert. Rev. Neurother. 2020, 20, 539–549. [Google Scholar] [CrossRef]
- Radder, D.L.M.; Nonnekes, J.; van Nimwegen, M.; Eggers, C.; Abbruzzese, G.; Alves, G.; Browner, N.; Chaudhuri, K.R.; Ebersbach, G.; Ferreira, J.J.; et al. Recommendations for the Organization of Multidisciplinary Clinical Care Teams in Parkinson’s Disease. J. Parkinsons Dis. 2020, 10, 1087–1098. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Rodriguez-Violante, M.; Martinez-Ramirez, D.; Ramirez-Zamora, A. Editorial: Managing Parkinson’s Disease With a Multidisciplinary Perspective. Front. Neurol. 2021, 12, 799017. [Google Scholar] [CrossRef]
- Brauer, S.G.; Lamont, R.M.; O’Sullivan, J.D. A physiotherapy group exercise and self-management approach to improve physical activity in people with mild-moderate Parkinson’s disease: A randomized controlled trial. Trials 2024, 25, 76. [Google Scholar] [CrossRef]
- Stark, S.; Landsbaum, A.; Palmer, J.L.; Somerville, E.K.; Morris, J.C. Client-centred home modifications improve daily activity performance of older adults. Can. J. Occup. Ther. 2009, 76, 235–245. [Google Scholar] [CrossRef]
- Suttrup, I.; Warnecke, T. Dysphagia in Parkinson’s Disease. Dysphagia 2016, 31, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.; Lee, J.H. Oro-Pharyngeal Dysphagia in Parkinson’s Disease and Related Movement Disorders. J. Mov. Disord. 2019, 12, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Parmera, J.B.; Rodriguez, R.D.; Studart Neto, A.; Nitrini, R.; Brucki, S.M.D. Corticobasal syndrome: A diagnostic conundrum. Dement. Neuropsychol. 2016, 10, 267–275. [Google Scholar] [CrossRef] [PubMed]
- Marshall, C.R.; Hardy, C.J.D.; Volkmer, A.; Russell, L.L.; Bond, R.L.; Fletcher, P.D.; Clark, C.N.; Mummery, C.J.; Schott, J.M.; Rossor, M.N.; et al. Primary progressive aphasia: A clinical approach. J. Neurol. 2018, 265, 1474–1490. [Google Scholar] [CrossRef]
- Burrell, J.R.; Ballard, K.J.; Halliday, G.M.; Hodges, J.R. Aphasia in Progressive Supranuclear Palsy: As Severe as Progressive Non-Fluent Aphasia. J. Alzheimers Dis. 2018, 61, 705–715. [Google Scholar] [CrossRef]
- Pu, T.; Huang, M.; Kong, X.; Wang, M.; Chen, X.; Feng, X.; Wei, C.; Weng, X.; Xu, F. Lee Silverman Voice Treatment to Improve Speech in Parkinson’s Disease: A Systemic Review and Meta-Analysis. Parkinson’s Dis. 2021, 2021, 3366870. [Google Scholar] [CrossRef]
- Gonzalez-Ramos, G.; Cohen, E.V.; Luce, V.; Gonzalez, M.J. Clinical social work in the care of Parkinson’s disease: Role, functions, and opportunities in integrated health care. Soc. Work. Health Care 2019, 58, 108–125. [Google Scholar] [CrossRef]
- Frank, C.; Chiu, R.; Lee, J. Parkinson disease primer, part 2: Management of motor and nonmotor symptoms. Can. Fam. Physician 2023, 69, 91–96. [Google Scholar] [CrossRef]
- Plouvier, A.O.A.; Olde Hartman, T.C.; Verhulst, C.E.M.; Bloem, B.R.; van Weel, C.; Lagro-Janssen, A.L.M. Parkinson’s disease: Patient and general practitioner perspectives on the role of primary care. Fam. Pract. 2017, 34, 227–233. [Google Scholar] [CrossRef][Green Version]
- Dijk, J.M.; Espay, A.J.; Katzenschlager, R.; de Bie, R.M.A. The Choice Between Advanced Therapies for Parkinson’s Disease Patients: Why, What, and When? J. Parkinson’s Dis. 2020, 10, S65–S73. [Google Scholar] [CrossRef] [PubMed]
- Hunka, K.; Suchowersky, O.; Wood, S.; Derwent, L.; Kiss, Z.H. Nursing time to program and assess deep brain stimulators in movement disorder patients. J. Neurosci. Nurs. 2005, 37, 204–210. [Google Scholar] [CrossRef] [PubMed]
- Thyrian, J.R.; Hertel, J.; Wucherer, D.; Eichler, T.; Michalowsky, B.; Dreier-Wolfgramm, A.; Zwingmann, I.; Kilimann, I.; Teipel, S.; Hoffmann, W. Effectiveness and Safety of Dementia Care Management in Primary Care: A Randomized Clinical Trial. JAMA Psychiatry 2017, 74, 996–1004. [Google Scholar] [CrossRef] [PubMed]
- Reuben, D.B.; Gill, T.M.; Stevens, A.; Williamson, J.; Volpi, E.; Lichtenstein, M.; Jennings, L.A.; Galloway, R.; Summapund, J.; Araujo, K.; et al. Health System, Community-Based, or Usual Dementia Care for Persons With Dementia and Caregivers: The D-CARE Randomized Clinical Trial. JAMA 2025, 333, 950–961. [Google Scholar] [CrossRef]
- Fereshtehnejad, S.M.; Lokk, J. Challenges of Teleneurology in the Care of Complex Neurodegenerative Disorders: The Case of Parkinson’s Disease with Possible Solutions. Healthcare 2023, 11, 3187. [Google Scholar] [CrossRef]
- Hu, Y.; Li, Y.; Li, J.; Liu, J.Y.W.; Gustin, S.M.; Li, M.; Leung, A.Y.M. Effectiveness of Telehealth Interventions on Cognitive Function and Quality of Life in Adults With Neurological Disorders: A Systematic Review and Meta-Analysis. J. Am. Med. Dir. Assoc. 2025, 26, 105491. [Google Scholar] [CrossRef]
- Dhamija, R.K.; Saluja, A.; Garg, D.; Chauhan, S.; Majumdar, R.; Bhardwaj, S.B.; Preenja, R.; Kashyap, D.; Owolabi, M.O. Teleneurorehabilitation and Motor and Nonmotor Symptoms and Quality of Life in Parkinson Disease: The TELEPARK Randomized Clinical Trial. JAMA Neurol. 2025, 82, 376–383. [Google Scholar] [CrossRef]
- Schneider, R.B.; Biglan, K.M. The promise of telemedicine for chronic neurological disorders: The example of Parkinson’s disease. Lancet Neurol. 2017, 16, 541–551. [Google Scholar] [CrossRef]
- Lima, D.P.; Queiroz, I.B.; Carneiro, A.H.S.; Pereira, D.A.A.; Castro, C.S.; Viana-Junior, A.B.; Nogueira, C.B.; Coelho Filho, J.M.; Lobo, R.R.; Roriz-Filho, J.S.; et al. Feasibility indicators of telemedicine for patients with dementia in a public hospital in Northeast Brazil during the COVID-19 pandemic. PLoS ONE 2022, 17, e0268647. [Google Scholar] [CrossRef]
- Guterman, E.L.; Kiekhofer, R.E.; Wood, A.J.; Allen, I.E.; Kahn, J.G.; Dulaney, S.; Merrilees, J.J.; Lee, K.; Chiong, W.; Bonasera, S.J.; et al. Care Ecosystem Collaborative Model and Health Care Costs in Medicare Beneficiaries With Dementia: A Secondary Analysis of a Randomized Clinical Trial. JAMA Intern. Med. 2023, 183, 1222–1228. [Google Scholar] [CrossRef]
- Tso, J.V.; Farinpour, R.; Chui, H.C.; Liu, C.Y. A Multidisciplinary Model of Dementia Care in an Underserved Retirement Community, Made Possible by Telemedicine. Front. Neurol. 2016, 7, 225. [Google Scholar] [CrossRef][Green Version]
- Pirtosek, Z. Breaking barriers in Parkinson’s care: The multidisciplinary team approach. J. Neural. Transm. 2024, 131, 1349–1361. [Google Scholar] [CrossRef]
- Sorensen, S.; Conwell, Y. Issues in dementia caregiving: Effects on mental and physical health, intervention strategies, and research needs. Am. J. Geriatr. Psychiatry 2011, 19, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Henry, R.S.; Lageman, S.K.; Perrin, P.B. The relationship between Parkinson’s disease symptoms and caregiver quality of life. Rehabil. Psychol. 2020, 65, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Aamodt, W.W.; Kluger, B.M.; Mirham, M.; Job, A.; Lettenberger, S.E.; Mosley, P.E.; Seshadri, S. Caregiver Burden in Parkinson Disease: A Scoping Review of the Literature from 2017–2022. J. Geriatr. Psychiatry Neurol. 2024, 37, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Ornstein, K.; Gaugler, J.E. The problem with “problem behaviors”: A systematic review of the association between individual patient behavioral and psychological symptoms and caregiver depression and burden within the dementia patient-caregiver dyad. Int. Psychogeriatr. 2012, 24, 1536–1552. [Google Scholar] [CrossRef]
- Abdelhalim, D.S.; Ahmed, M.M.; Hussein, H.A.; Khalaf, O.O.; Sarhan, M.D. Burden of Care, Depression, and Anxiety Among Family Caregivers of People With Dementia. J. Prim. Care Community Health 2024, 15, 21501319241288029. [Google Scholar] [CrossRef]
- Alves, L.C.S.; Monteiro, D.Q.; Bento, S.R.; Hayashi, V.D.; Pelegrini, L.N.C.; Vale, F.A.C. Burnout syndrome in informal caregivers of older adults with dementia: A systematic review. Dement. Neuropsychol. 2019, 13, 415–421. [Google Scholar] [CrossRef]
- Duru, E.E.; Ben-Umeh, K.C.; Mattingly, T.J. Cost of long-term care and balancing caregiver wellbeing: A narrative review. Expert Rev. Pharmacoecon. Outcomes Res. 2024, 24, 883–897. [Google Scholar] [CrossRef]
- Elliott, A.F.; Burgio, L.D.; Decoster, J. Enhancing caregiver health: Findings from the resources for enhancing Alzheimer’s caregiver health II intervention. J. Am. Geriatr. Soc. 2010, 58, 30–37. [Google Scholar] [CrossRef]
- Roberts, E.; Struckmeyer, K.M. The Impact of Respite Programming on Caregiver Resilience in Dementia Care: A Qualitative Examination of Family Caregiver Perspectives. Inquiry 2018, 55, 46958017751507. [Google Scholar] [CrossRef] [PubMed]
- Schindler, N.; Bay, A.A.; Perkins, M.M.; Jackson, J.; Ni, L.; Pothineni, S.; Wincek, R.; Hackney, M.E. Remote and in-person research education for people with Parkinson’s disease and their care partners. Fam. Syst. Health 2023, 41, 26–43. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Thornton, J.D.; Sambamoorthi, U.; Innes, K. Direct and indirect cost of managing alzheimer’s disease and related dementias in the United States. Expert Rev. Pharmacoecon. Outcomes Res. 2017, 17, 189–202. [Google Scholar] [CrossRef] [PubMed]
- Deb, A.; Sambamoorthi, U.; Thornton, J.D.; Schreurs, B.; Innes, K. Direct medical expenditures associated with Alzheimer’s and related dementias (ADRD) in a nationally representative sample of older adults—An excess cost approach. Aging Ment. Health 2018, 22, 619–624. [Google Scholar] [CrossRef]
- Turro-Garriga, O.; Vinas-Diez, V.; Conde-Sala, J.L.; Calvo-Perxas, L.; Cullell-Junca, M.; Mas-Vall-Llosera, G.; Flaque, M.; Turon-Estrada, A.; Juvinya-Canal, D.; Mioshi, E.; et al. Caregivers’ Sense of Coherence: Implications on Direct and Indirect Costs of Dementia Care. J. Alzheimer’s Dis. 2020, 78, 117–126. [Google Scholar] [CrossRef]
- Aamodt, W.W.; Sun, C.; Dahodwala, N.; Elser, H.; Schneider, A.L.C.; Farrar, J.T.; Coe, N.B.; Willis, A.W. End-of-Life Health Care Service Use and Cost Among Medicare Decedents With Neurodegenerative Diseases. Neurology 2024, 103, e209925. [Google Scholar] [CrossRef]
- Lastuka, A.; Bliss, E.; Breshock, M.R.; Iannucci, V.C.; Sogge, W.; Taylor, K.V.; Pedroza, P.; Dieleman, J.L. Societal Costs of Dementia: 204 Countries, 2000-2019. J. Alzheimers Dis. 2024, 101, 277–292. [Google Scholar] [CrossRef]
- Guzzon, A.; Rebba, V.; Paccagnella, O.; Rigon, M.; Boniolo, G. The value of supportive care: A systematic review of cost-effectiveness of non-pharmacological interventions for dementia. PLoS ONE 2023, 18, e0285305. [Google Scholar] [CrossRef]
- Findley, L.J. The economic impact of Parkinson’s disease. Park. Relat. Disord. 2007, 13, S8–S12. [Google Scholar] [CrossRef]
- Koerts, J.; Konig, M.; Tucha, L.; Tucha, O. Working capacity of patients with Parkinson’s disease—A systematic review. Park. Relat. Disord. 2016, 27, 9–24. [Google Scholar] [CrossRef]
- Bramble, M.; Wong, A.; Carroll, V.; Schwebel, D.; Rossiter, R. Using an economic evaluation approach to support specialist nursing services for people with Parkinson’s in a regional community. J. Adv. Nurs. 2021, 77, 4722–4732. [Google Scholar] [CrossRef]
| Team Member | Primary Roles and Contributions |
|---|---|
| Neurologist | Diagnosis and management of neurodegenerative disorders; coordination of specialist care (e.g., movement disorders, cognitive neurology). |
| Geriatrician | Comprehensive care for older adults with multimorbidity; functional and cognitive assessments. |
| Psychiatrist | Management of mood, behavioral, and psychotic symptoms; support for psychiatric comorbidities. |
| Primary Care Physician | First point of contact; chronic disease management, comorbidities (e.g., hypertension, diabetes); care coordination and continuity; patient and caregiver education. |
| Physiotherapist | Assessment and treatment of mobility, gait, balance, and strength; fall prevention; exercise programs tailored to motor symptoms (e.g., tremor, rigidity, freezing of gait). |
| Occupational Therapist | Support for daily living activities; home safety assessments; adaptive equipment recommendations; environmental modifications. |
| Speech–Language Therapist | Assessment and management of communication and swallowing impairments; swallowing exercises; speech therapy (e.g., LSVT); use of augmentative communication devices. |
| Social Worker | Emotional support; resource navigation; financial and legal advocacy; connection to community services and caregiver support. |
| Specialist Nurse Practitioner | Expertise in advanced therapies (e.g., DuoDopa, DBS); monitoring and adjusting therapy; patient education; support across treatment continuum. |
| Dietitian | Nutritional assessments; diet modifications for swallowing difficulties or metabolic needs; support for healthy aging. |
| Psychologist | Cognitive and emotional assessments; psychotherapy for depression, anxiety, and caregiver stress; behavioral interventions. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fereshtehnejad, S.-M.; Lökk, J. Healthcare Complexities in Neurodegenerative Proteinopathies: A Narrative Review. Healthcare 2025, 13, 1873. https://doi.org/10.3390/healthcare13151873
Fereshtehnejad S-M, Lökk J. Healthcare Complexities in Neurodegenerative Proteinopathies: A Narrative Review. Healthcare. 2025; 13(15):1873. https://doi.org/10.3390/healthcare13151873
Chicago/Turabian StyleFereshtehnejad, Seyed-Mohammad, and Johan Lökk. 2025. "Healthcare Complexities in Neurodegenerative Proteinopathies: A Narrative Review" Healthcare 13, no. 15: 1873. https://doi.org/10.3390/healthcare13151873
APA StyleFereshtehnejad, S.-M., & Lökk, J. (2025). Healthcare Complexities in Neurodegenerative Proteinopathies: A Narrative Review. Healthcare, 13(15), 1873. https://doi.org/10.3390/healthcare13151873






