Safety and Anatomical Accuracy of Dry Needling of the Quadratus Femoris Muscle: A Cadaveric Study
Abstract
1. Introduction
2. Methods
2.1. Study Design
2.2. Cadaveric Specimens and Sample Characteristics
2.3. DDN Procedure and Standardization Protocol
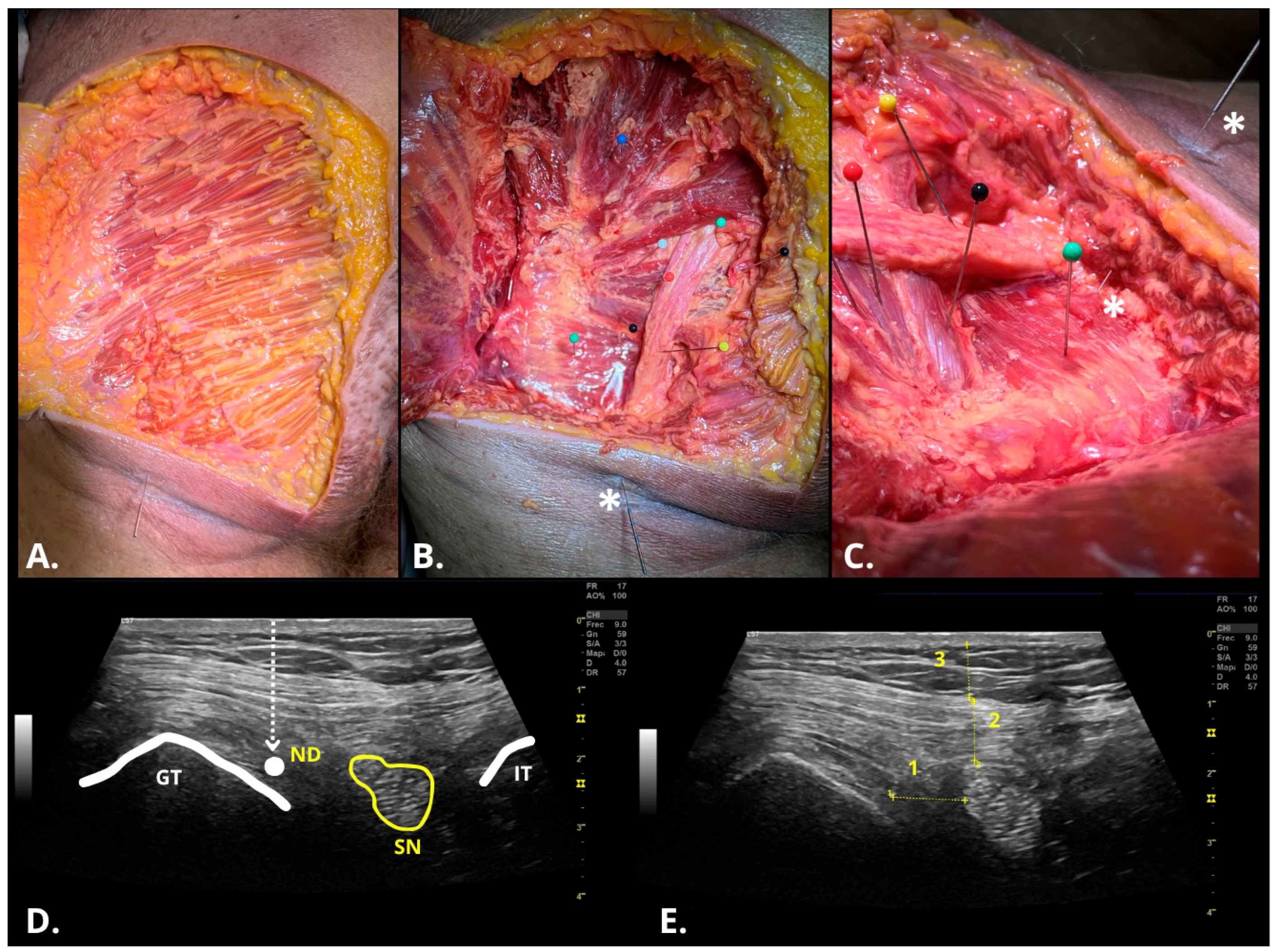
2.4. Ultrasonographic Assessment of DDN Procedure
2.5. Disecction Assessment of DDN Procedure
2.6. Statistics
3. Results
3.1. Decriptive Cadaver Characteristics
3.2. Descriptive Analysis of Procedural and Anatomical Variables
3.3. Intra and Inter-Rater Reliability
3.4. Agreement Between Ultrasound Imaging and Cadaveric Dissection
4. Discussion
4.1. Overview of Findings
4.2. Safety, Accuracy and Reliability of the Procedure
4.3. Agreement Between Ultrasound Imaging and Anatomical Dissection
4.4. Clinical Relevance and Rationale for Protocol Development
4.5. Limitations and Future Directions
4.6. Practical Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernando, M.F.; Cerezal, L.; Pérez-Carro, L.; Abascal, F.; Canga, A. Deep gluteal syndrome: Anatomy, imaging, and management of sciatic nerve entrapments in the subgluteal space. Skelet. Radiol. 2015, 44, 919–934. [Google Scholar] [CrossRef]
- Torriani, M.; Souto, S.C.L.; Thomas, B.J.; Ouellette, H.; Bredella, M.A. Ischiofemoral impingement syndrome: An entity with hip pain and abnormalities of the quadratus femoris muscle. Am. J. Roentgenol. 2009, 193, 186–190. [Google Scholar] [CrossRef]
- Laumonerie, P.; Dalmas, Y.; Tibbo, M.E.; Robert, S.; Durant, T.; Caste, T.; Vialla, T.; Tiercelin, J.; Gracia, G.; Chaynes, P. Sensory Innervation of the Hip Joint and Referred Pain: A Systematic Review of the Literature. Pain Med. 2021, 22, 1149–1157. [Google Scholar] [CrossRef]
- Olewnik, Ł.; Zielinska, N.; Karauda, P.; Piagkou, M.; Koptas, K.; Maślanka, K.; Ruzik, K.; Triantafyllou, G.; Balcerzak, A.; Klejman, E.; et al. The quadratus femoris muscle anatomy: Do we know everything? Ann. Anat.-Anat. Anz. 2024, 255, 152284. [Google Scholar] [CrossRef]
- Smoll, N.R. Variations of the piriformis and sciatic nerve with clinical consequence: A review. Clin. Anat. 2010, 23, 8–17. [Google Scholar] [CrossRef]
- Kearns, G.; Gilbert, K.K.; Allen, B.; Sizer, P.S.; Brismée, J.M.; Pendergrass, T.; Lierly, M.; York, D. Accuracy and safety of dry needle placement in the piriformis muscle in cadavers. J. Man. Manip. Ther. 2018, 26, 89–96. [Google Scholar] [CrossRef]
- Kim, D.-H.; Yoon, D.M.; Yoon, K.B. Ultrasound-Guided Quadratus Femoris Muscle Injection in Patients with Lower Buttock Pain: Novel Ultrasound-Guided Approach and Clinical Effectiveness. Pain Physician 2016, 19, E863–E870. [Google Scholar]
- Shin, H.; Woo, H.; Han, Y.; Choi, S.; Jo, J.; Jeon, S.; Ha, W.; Lee, J. Analysis of Research Trends in Ultrasound-Guided Acupuncture and Dry-Needling: A Scoping Review. J. Clin. Med. 2024, 13, 4962. [Google Scholar] [CrossRef]
- Mesa-Jiménez, J.A.; Sánchez-Gutiérrez, J.; De-La-Hoz-Aizpurua, J.L.; Fernández-De-Las-Peñas, C. Cadaveric validation of dry needle placement in the lateral pterygoid muscle. J. Manip. Physiol. Ther. 2015, 38, 145–150. [Google Scholar] [CrossRef]
- Pérez-Bellmunt, A.; López-de-Celis, C.; Rodríguez-Sanz, J.; Hidalgo-García, C.; Donnelly, J.M.; Cedeño-Bermúdez, S.A.; Fernández-De-Las-Peñas, C. Dorsal dry needling to the pronator quadratus muscle is a safe and valid technique: A cadaveric study. Physiother. Theory Pract. 2023, 39, 1033–1037. [Google Scholar] [CrossRef]
- Calderón-Díez, L.; Sánchez-Sánchez, J.L.; Belón-Pérez, P.; Robles-García, M.; Pérez-Robledo, F.; Fernández-de-las-Peñas, C. Cadaveric and Ultrasound Validation of Percutaneous Electrolysis Approach at the Distal Biceps Tendon: A Potential Treatment for Biceps Tendinopathy. Diagnostics 2022, 12, 3051. [Google Scholar] [CrossRef]
- Mesa-Jiménez, J.A.; Fernández-de-las-Peñas, C.; Koppenhaver, S.L.; Sánchez-Gutiérrez, J.; Arias-Buría, J.L. Cadaveric and in vivo validation of needle placement in the medial pterygoid muscle. Musculoskelet. Sci. Pract. 2020, 49, 102197. [Google Scholar] [CrossRef]
- Pérez-Bellmunt, A.; López-de-Celis, C.; Rodríguez-Sanz, J.; Koppenhaver, S.L.; Zegarra-Chávez, D.; Ortiz-Miguel, S.; Fernández-de-Las-Peñas, C. The posterior/medial dry needling approach of the tibialis posterior muscle is an accurate and safe procedure: A cadaveric study. BMC Musculoskelet. Disord. 2022, 23, 570. [Google Scholar] [CrossRef]
- Rodríguez-Sanz, J.; Pérez-Bellmunt, A.; López-de-Celis, C.; Hidalgo-García, C.; Koppenhaver, S.L.; Canet-Vintró, M.; Fernández-de-las-Peñas, C. Accuracy and safety of dry needling placement in the popliteus muscle: A cadaveric study. Int. J. Clin. Pract. 2021, 75, e14669. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; López-De-Celis, C.; Rodríguez-Sanz, J.; Hidalgo-García, C.; Donelly, J.M.; Cedeño-Bermúdez, S.A.; Pérez-Bellmunt, A. Safety of Dry Needling of the Pronator Teres Muscle in Cadavers: A Potential Treatment for Pronator Syndrome. Pain Med. 2022, 23, 1158–1161. [Google Scholar] [CrossRef]
- Wilke, J.; Krause, F.; Niederer, D.; Engeroff, T.; Nürnberger, F.; Vogt, L.; Banzer, W. Appraising the methodological quality of cadaveric studies: Validation of the QUACS scale. J. Anat. 2015, 226, 440–446. [Google Scholar] [CrossRef]
- Vas, L.C. Ultrasound guided dry needling: Relevance in chronic pain. J. Postgrad. Med. 2022, 68, 1–9. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Kwong, J.S.W.; Zhang, C.; Li, S.; Sun, F.; Niu, Y.; Du, L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: A systematic review. J. Evid.-Based Med. 2015, 8, 2–10. [Google Scholar] [CrossRef]
- Thiel, W. Photographic Atlas of Practical Anatomy I, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1997. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The measurement of observer agreement for categorical data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef]
- Belón-Pérez, P.; Calderón-Díez, L.; Sánchez-Sánchez, J.L.; Robles-García, M.; Plaza-Manzano, G.; Fernández-De-las-peñas, C. Cadaveric and Ultrasound Validation of Percutaneous Electrolysis Approaches at the Arcade of Frohse: A Potential Treatment for Radial Tunnel Syndrome. Int. J. Environ. Res. Public Health 2022, 19, 2476. [Google Scholar] [CrossRef]
- Sánchez-Montoya, M.; Almazán-Polo, J.; González-de-la-Flor, Á. Safety and Anatomical Accuracy of Dry Needling in Musculoskeletal System: A Systematic Review of Cadaveric Studies. J. Man. Manip. Ther. 2025, 1–18. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Varol, U.; Plaza-Manzano, G.; Fernández-de-las-Peñas, C.; Belón-Pérez, P.; López-Redondo, M.; Navarro-Santana, M.J. Testing the Safety of Piriformis Dry Needling Interventions: An Observational Study Evaluating the Predictive Value of Anthropometric and Demographic Factors. J. Clin. Med. 2024, 13, 6674. [Google Scholar] [CrossRef]
- Duijn, E.A.H.D.; Roy van, S.; Karel, Y.H.J.M.; Provyn, S.; Pouliart, N. An interexaminer agreement and reliability study on cadavers with musculoskeletal ultrasound of the shoulder performed by physiotherapists and radiologists compared with dissection. Musculoskelet. Sci. Pract. 2022, 60, 102569. [Google Scholar] [CrossRef]
- Vitt, M.; Macaraeg, S.; Stapleton, Z.; Mata, A.; Ross, B.S. Ultrasound verification of palpation-based dry needling techniques of rotator cuff muscles: A prospective feasibility trial. J. Man. Manip. Ther. 2024, 32, 166–172. [Google Scholar] [CrossRef]
- Dinges, H.C.; Hoeft, J.; Cornelius, V.M.; Steinfeldt, T.; Wiesmann, T.; Wulf, H.; Schubert, A.K. Nominal logistic regression analysis of variables determining needle visibility in ultrasound images–a full factorial cadaver study. BMC Anesthesiol. 2023, 23, 369. [Google Scholar] [CrossRef]
- Kearns, G.A.; Brismée, J.M.; Riley, S.P.; Wang-Price, S.; Denninger, T.; Vugrin, M. Lack of standardization in dry needling dosage and adverse event documentation limits outcome and safety reports: A scoping review of randomized clinical trials. J. Man. Manip. Ther. 2023, 31, 72–83. [Google Scholar] [CrossRef]
- Valera-Calero, J.A.; Plaza-Manzano, G.; Rabanal-Rodríguez, G.; Díaz-Arribas, M.J.; Kobylarz, M.D.; Buffet-García, J.; Fernández-De-Las-Peñas, C.; Navarro-Santana, M.J. Current State of Dry Needling Practices: A Comprehensive Analysis on Use, Training, and Safety. Medicina 2024, 60, 1869. [Google Scholar] [CrossRef]
- Ellis, R.; Helsby, J.; Naus, J.; Bassett, S.; Fernández-de-Las-Peñas, C.; Carnero, S.F.; Hides, J.; O’SUllivan, C.; Teyhen, D.; Stokes, M.; et al. Exploring the use of ultrasound imaging by physiotherapists: An international survey. Musculoskelet. Sci. Pract. 2020, 49, 102213. [Google Scholar] [CrossRef]

| Variables (n = 5) | Mean ± SD (Min.–Max.) | Median ± IQR (Min.–Max.) | 95% CI | Shapiro–Wilk | |||||
|---|---|---|---|---|---|---|---|---|---|
| Lower Bound | Upper Bound | Skewness | Kurtosis | Statistic | df | p-Value | |||
| Age, years | 75.60 ± 14.10 (53.00–92.00) | 78.00 ± 1.00 (53.00–92.00) | 58.1 | 93.1 | −1.06 | 2.59 | 0.86 | 4 | 0.239 |
| Height, m | 1.63 ± 5.22 (1.55–1.68) | 1.65 ± 5.00 (1.55–1.68) | 1.57 | 1.70 | −1.31 | 1.40 | 0.89 | 4 | 0.347 |
| Weight | 71.00 ± 9.85 (55.00–80.00) | 72.00 ± 8.00 (55.00–80.00) | 58.8 | 83.2 | −1.32 | 1.90 | 0.89 | 4 | 0.34 |
| Estimated BMI | 54.48 ± 4.30 (21.00–32.50) | 26.40 ± 3.89 (21.00–32.50) | 21.3 | 32 | 0.05 | 0.149 | 0.99 | 4 | 0.99 |
| Hip Perimeter | 97.80 ± 6.42 (90.00–107.00) | 98.00 ± 6.00 (90.00–107) | 89.8 | 106 | 0.410 | 0.172 | 0.99 | 4 | 0.964 |
| Events (n = 24) | Nº (n = 5) | Side (L/R) | Physio. (n = 2) | Age (Years) | Height (m) | Weight (kg) | Sex (M/F) | Needle Size (mm) | Sub.Fat.Th (cm) | G.Max.Th (cm) | IFS (Y/N) | Success (Y/N) | SN. Punct. (Y/N) | SN.Dis. (cm) | Needle Depth (cm) (n = 14) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1a | R | 1 | 92 | 1.55 | 78 | F | 0.30 × 50 | 0.85 | 1.24 | No | No | No | 1.29 | – |
| 2 | 1b | L | 1 | 92 | 1.55 | 78 | F | 0.30 × 50 | 0.85 | 1.24 | No | No | No | 1.30 | – |
| 3 | 2a | R | 1 | 77 | 1.65 | 72 | M | 0.30 × 40 | 1.00 | 1.27 | Yes | No | No | 1.88 | – |
| 4 | 2b | L | 1 | 77 | 1.65 | 72 | M | 0.30 × 40 | 1.11 | 1.21 | Yes | No | No | 1.23 | – |
| 5 | 3a | R | 1 | 78 | 1.68 | 70 | M | 0.30 × 40 | 0.94 | 1.36 | Yes | Yes | No | 1.86 | – |
| 6 | 3b | L | 1 | 78 | 1.68 | 70 | M | 0.30 × 40 | 1.05 | 1.32 | Yes | No | No | 0.51 | – |
| 7 | 4a | R | 1 | 53 | 1.62 | 55 | M | 0.30 × 40 | 0.50 | 1.11 | Yes | No | No | 1.51 | – |
| 8 | 4b | L | 1 | 53 | 1.62 | 55 | M | 0.30 × 40 | 0.43 | 1.36 | Yes | Yes | No | 1.89 | – |
| 9 | 5a | R | 1 | 78 | 1.67 | 80 | M | 0.30 × 40 | 1.16 | 0.86 | Yes | Yes | No | 3.23 | – |
| 10 | 5b | L | 1 | 78 | 1.67 | 80 | M | 0.30 × 40 | 1.14 | 1.11 | Yes | No | No | 3.00 | – |
| 11 | 1a | R | 2 | 92 | 1.55 | 78 | F | 0.30 × 60 | 1.22 | 1.75 | Yes | Yes | No | 1.78 | 1.85 |
| 12 | 1b | L | 2 | 92 | 1.55 | 78 | F | 0.30 × 60 | 0.78 | 1.80 | Yes | Yes | No | 0.52 | 1.85 |
| 13 | 2a | R | 2 | 77 | 1.65 | 72 | M | 0.30 × 60 | 0.76 | 0.92 | Yes | No | Yes | 0.00 | 5.10 |
| 14 | 2b | L | 2 | 77 | 1.65 | 72 | M | 0.30 × 60 | 0.84 | 0.96 | Yes | Yes | No | 1.65 | 2.90 |
| 15 | 3a | R | 2 | 78 | 1.68 | 70 | M | 0.30 × 60 | 0.89 | 1.67 | Yes | Yes | No | 0.80 | 4.20 |
| 16 | 3b | R | 2 | 78 | 1.68 | 70 | M | 0.30 × 60 | 0.72 | 1.50 | Yes | Yes | No | 0.19 | 4.00 |
| 17 | 4a | R | 2 | 53 | 1.62 | 55 | M | 0.30 × 30 | 0.46 | 0.95 | Yes | No | Yes | 0.00 | 2.10 |
| 18 | 4b | L | 2 | 53 | 1.62 | 55 | M | 0.30 × 30 | 0.53 | 1.00 | Yes | Yes | No | 0.29 | 2.20 |
| 19 | 5a | R | 2 | 78 | 1.67 | 80 | M | 0.30 × 60 | 0.95 | 0.94 | Yes | Yes | No | 0.21 | 3.50 |
| 20 | 5b | L | 2 | 78 | 1.67 | 80 | M | 0.30 × 60 | 1.11 | 1.16 | Yes | Yes | No | 0.83 | 3.50 |
| 21 | 1a | R | 2 | 92 | 1.55 | 78 | F | 0.30 × 60 | 0.74 | 1.44 | Yes | Yes | No | 1.13 | 5.00 |
| 22 | 1b | L | 2 | 92 | 1.55 | 78 | F | 0.30 × 60 | 0.80 | 2.06 | Yes | No | No | 1.17 | 3.70 |
| 23 | 3a | R | 2 | 78 | 1.68 | 70 | M | 0.30 × 60 | 0.70 | 0.79 | Yes | Yes | No | 1.31 | 2.00 |
| 24 | 3b | L | 2 | 78 | 1.68 | 70 | M | 0.30 × 60 | 0.89 | 0.79 | Yes | No | No | 3.45 | 2.40 |
| Variables | Mean ± SD [Min–Max) * or Frequency (%) † | ||
|---|---|---|---|
| Physiotherapist | Ph.1: 10 (41.7%) † | ||
| Ph.2: 14 (58.3%) † | |||
| Sex | Male | 18 (75%) † | |
| Female | 6 (18%) † | ||
| Needle Size | 0.30 × 30: | 2 (8.3%) † | Ph.1: 0 (0%) ‡ |
| Ph.2: 2 (100%) ‡ | |||
| 0.30 × 40: | 8 (33.3%) † | Ph.1: 8 (100%) ‡ | |
| Ph.2: 0 (0%) ‡ | |||
| 0.30 × 50: | 2 (8.3%) † | Ph.1: 2 (100%) ‡ | |
| Ph.2: 0 (0%) ‡ | |||
| 0.30 × 60: | 12 (50%) † | Ph.1: 0 (0%) ‡ | |
| Ph.2: 12 (100%) ‡ | |||
| IFS | Yes | 22 (91.7%) † | Ph.1: 8 (36.4%) ‡ |
| Ph.2: 14 (63.6%) ‡ | |||
| No | (8.3%) † | Ph.1: 2 (100%) ‡ | |
| Ph.2: 0 (0%) ‡ | |||
| Success | Yes | 13 (54.2%) † | Ph.1: 3 (23.1%) ‡ |
| Ph.2: 10 (76.9%) ‡ | |||
| No | 13 (54.2%) † | Ph.1: 7 (63.7%) ‡ | |
| Ph.2: 4 (36.3%) ‡ | |||
| SN.Punct. | Yes | 2 (8.3%) † | Ph.1: 0 (0%) ‡ |
| Ph.2: 2 (100%) ‡ | |||
| No | 22 (91.7%) † | Ph.1: 10 (44.5%) ‡ | |
| Ph.2: 12 (54.5%) ‡ | |||
| Sub.Fat.Th (cm) | 0.86 ± 0.22 (0.43–1.22) * | Ph.1: 0.90 ± 0.26 (0.43–1.16) * | |
| Ph.2: 0.81 ± 0.20 (0.46–1.22) * | |||
| G.Max.Th (cm) | 1.24 ± 0.33 (0.79–2.06) * | Ph.1: 1.21 ± 0.15 (0.86–1.36) * | |
| Ph.2: 1.27 ± 0.43 (0.79–2.06) * | |||
| SN.Dis. (cm) | 1.29 ± 0.96 (0.00–3.45) * | Ph.1: 1.77 ± 0.82 (0.51–3.23) * | |
| Ph.2: 0.95 ± 0.93 (0.00–3.45) * | |||
| Needle Depth (cm) | Ph.1: 3.16 ± 1.14 (1.85–5.10) * | ||
| Nominal Dichotomous Variables (Success/Failure) | |||||||
|---|---|---|---|---|---|---|---|
| Variables | % Ph.1/R.1 Success/Failure (Yes/No) * | % Ph.2/R.2 Success/Failure (Yes/No) * | % Agreement | Cohen’s Kappa (κ) (95% CI) | N; p-Value | ||
| IFS Location (Y/N) | Intra-rater (Ph.2 vs. Ph.2) | 100% | 100% | 100% | N/I † | 4; — ‡ | |
| Inter-rater (Ph.1 vs. Ph.2) | 80% | 100% | 80% | N/I † | 10; — ‡ | ||
| SN. Punct. (Y/N) * | Intra-rater (Ph.2 vs. Ph.2) | 0% | 0% | 100% | N/I † | 4; — ‡ | |
| Inter-rater (Ph.1 vs. Ph.2) | 20% | 0% | 80% | N/I † | 10; — ‡ | ||
| Success (Y/N) | Intra-rater (Ph.2 vs. Ph.2) | 100% | 50% | 50% | N/I † | 4; — ‡ | |
| Inter-rater (Ph.1 vs. Ph.2) | 30% | 80% | 50% | 0.19 (−0.31–0.49) | 10; 0.301 | ||
| Nominal Multicategorical Variables | |||||||
| Nominal Variables | % Ph.1/R.1 Needle Size (Fr) | % Ph.2/R.2 Needle Size (Fr) | % Agreement | Mode Frequency (%) | Cohen’s Kappa (κ) (95% CI) | N; p-Value | |
| Needle Size | Intra-rater (Ph.2 vs. Ph.2) | 0.30 × 40 mm (2) | 0.30 × 60mm (2) | 50% | 50% | N/I † | 4; — ‡ |
| Inter-rater (Ph.1 vs. Ph.2) | 0.30 × 40 mm (8) | 0.30 × 60 mm (6) | 0% | 60% | N/I † | 10; — ‡ | |
| Variables (n = 10) | US Positive (%) | Dis. Positive (%) | % Agreement | Valence Index | Cohen’s Kappa (95% CI) | N; p-Value |
|---|---|---|---|---|---|---|
| IFS Location | 100% | 100% | 100% | 1.00 | N/I † | 10; — ‡ |
| SN.Punct. | 20% | 10% | 90% | 0.80 | 0.62 (0.04–1.28) | 10; 0.035 |
| Success | 60% | 40% | 100% | 0.20 | 1.00 (1.00–1.00) | 10; 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Montoya, M.; Almazán-Polo, J.; Hernández, N.V.; Cotteret, C.; Guerineau, F.; Monreal-Redondo, D.d.G.; González-de-la-Flor, Á. Safety and Anatomical Accuracy of Dry Needling of the Quadratus Femoris Muscle: A Cadaveric Study. Healthcare 2025, 13, 1828. https://doi.org/10.3390/healthcare13151828
Sánchez-Montoya M, Almazán-Polo J, Hernández NV, Cotteret C, Guerineau F, Monreal-Redondo DdG, González-de-la-Flor Á. Safety and Anatomical Accuracy of Dry Needling of the Quadratus Femoris Muscle: A Cadaveric Study. Healthcare. 2025; 13(15):1828. https://doi.org/10.3390/healthcare13151828
Chicago/Turabian StyleSánchez-Montoya, Marta, Jaime Almazán-Polo, Néstor Vallecillo Hernández, Charles Cotteret, Fabien Guerineau, Domingo de Guzman Monreal-Redondo, and Ángel González-de-la-Flor. 2025. "Safety and Anatomical Accuracy of Dry Needling of the Quadratus Femoris Muscle: A Cadaveric Study" Healthcare 13, no. 15: 1828. https://doi.org/10.3390/healthcare13151828
APA StyleSánchez-Montoya, M., Almazán-Polo, J., Hernández, N. V., Cotteret, C., Guerineau, F., Monreal-Redondo, D. d. G., & González-de-la-Flor, Á. (2025). Safety and Anatomical Accuracy of Dry Needling of the Quadratus Femoris Muscle: A Cadaveric Study. Healthcare, 13(15), 1828. https://doi.org/10.3390/healthcare13151828








