Assessing Grief in Cancer Care: A Systematic Review of Observational Studies Using Psychometric Instruments
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Protocol
2.2. Eligibility Criteria
2.3. Search Strategy and Information Sources
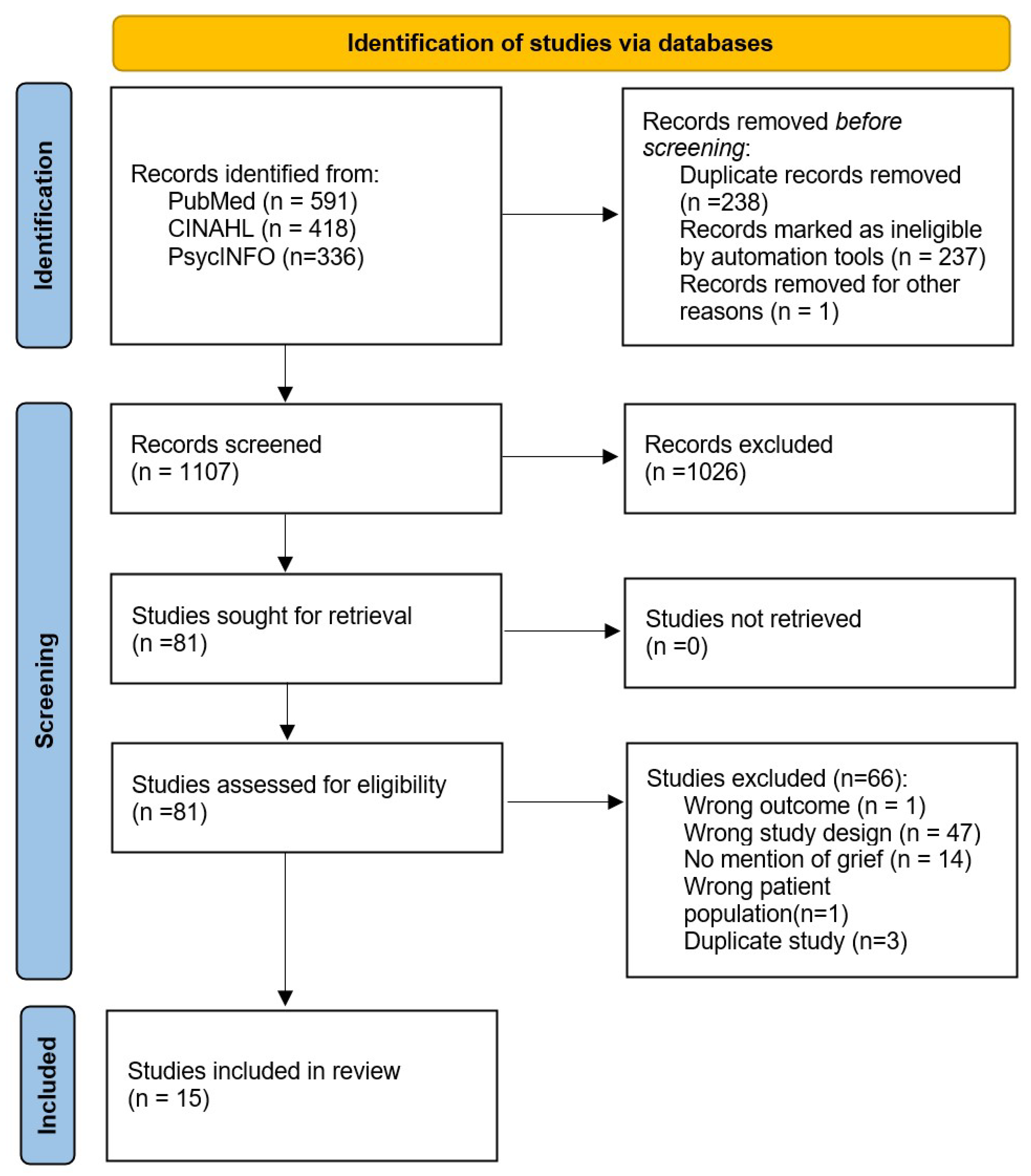
2.4. Study Selection
2.5. Data Extraction and Synthesis
2.6. Quality Appraisal
2.7. Generative AI Statement
3. Results
3.1. Demographics
| Study | Location | Psychometric Grief Instrument | Demographics |
|---|---|---|---|
| Gökler-Danışman, 2017 [34] | Turkey | PG-12 | n = 250, 67.6% female, 32.4% male, mean age 55.8 ± 12.96 |
| Kostopoulou, 2018 [21] | Greece | PGAC | n = 120, 55% female, 45% male. mean age 66.4 ± 13.64 (range 29–95) |
| Mystakidou, 2006 [22] | Greece | PGAC | n = 200, 55.5% female, 44.5% male, mean age 61.7 (range 31–87) |
| Mystakidou, 2008 [23] | Greece | PGAC | n = 94, 56.4% female, 43.6% male, mean age 57.65 ±14.0 (range: 24–85) |
| Mystakidou, 2008 [24] | Greece | PGAC | n = 100, 53% female, 47% male, mean age 63.17 ±15.3 |
| Mystakidou, 2011 [25] | Greece | PGAC | n = 94, 51% female, 49% male, mean age 63.37 years ± 12.03, (range: 41–93) |
| Mystakidou, 2012 [26] | Greece | PGAC | n = 195, mean age of 64.41 ± 12.37, (range from 34–93) |
| Parpa, 2019 [27] | Greece | PGAC | n = 120, 55% female, 45% male, mean age 66.45 years ±13.64 (range 29–95). |
| Siedentopf, 2010 [29] | Germany | PO-Bado | n = 333 |
| Tacón, 2011 [30] | Texas, USA | GDI | n = 65 women, mean age 45.4 (range 32–63) |
| Trevino, 2011 [31] | Massachusetts, USA | PG-12 | n = 53, 66% female, 34% male, age range 20–40 |
| Trevino, 2013 [32] | Massachusetts, USA | PG-12 | n = 71, 70.42% female, 29.58% male, age range 20–40 |
| Trevino, 2013 [33] | Massachusetts, USA | PG-13 | n = 97, 68.4% female, 31.6% male, mean age 33.4 ± 5.51 (range 20–40) |
| Tsilika, 2009 [28] | Greece | PGAC | n = 94, 51% female, 49% male, mean age 63.37 ± 12.03 (range: 41–93) |
| Vergo, 2017 [19] | Multiple states, USA | PGAC | n = 53, 53% female, 47% male, mean age 63.37 years ± 12.03 (range: 41–93) |
3.2. Grief Scales
3.2.1. Grief Findings
| Study | Location | Article Title | Psychometric Grief Instrument | Finding 1 | Comparison 1 | Comparison 2 | Confounders | Cronbach’s Alpha |
|---|---|---|---|---|---|---|---|---|
| Tacón, 2011 [30] | Lubbock, Texas, USA | Mindfulness: Existential, Loss, and Grief Factors in Women with Breast Cancer | GDI | Mean (Pre-Intervention): 20.12 (SD = 4.67) Mean (Post-Intervention): 17.72 (SD = 4.14) t = 3.56 | Anxious Preoccupation (M = 17.38, SD = 3.20), t = 5.74, p < 0.001 | Essential Wellbeing M = 32.17, SD = 8. 02), t = 4.63, p < 0.001 | Gender, Cancer Type, Time | Not reported |
| Mystakidou et al., 2006 [22] | Athens, Greece | Demographic and Clinical Predictors of Preparatory Grief | PGAC | Mean = 44.5 (SD = (13.6)) | Anxiety symptoms: r = 0.818, p < 0.0005 (HADS-A). | HADS-D: r = 0.659, p < 0.0005 (HADS-D). | Age, ECOG status, gender, opioids, other surgery, surgery | PGAC α = 0.0838 |
| Mystakidou et al., 2008 [23] | Athens, Greece | Preparatory grief, psychological distress and hopelessness | PGAC | Mean = 42.49 (SD = (13.97)) | HADS-A PGAC: r = 0.629, p < 0.0005 | HADS-D: r = 0.489, p < 0.0005 | Age, metastasis, gender, education, chemo-, radiotherapy, cancer type | PGAC α = 0.70 |
| Mystakidou et al., 2008 [24] | Athens, Greece | Screening for Preparatory Grief in Advanced Cancer Patients | PGAC | Mean = 44.5 (SD = (13.6)) | Anxiety AUC 0.968 SE 0.014 p < 0.0005 | Depression AUC 0.867 SE 0.036 p < 0.0005 | ECOG status, diagnosis, treatment, opioid use | PGAC α = 0.838 (0.823–0.864) |
| Mystakidou et al., 2011 [25] | Athens, Greece | The Mediation Effect of Anxiety Between PTSD and Grief | PGAC | Mean = 35.88 (SD = (10.42)) | Anxiety (r = 0.527); PTSD symptoms (IES-R total) r = 0.433–0.579 | IES-R- hyperarousal (1.65, 0.70) | Post-traumatic stress, ECOG Status, Cancer type, Treatment | PGAC (no alpha, reported as Spearman’srhocoefficient) HAD α = 0.887 Anxiety 0.703 Depression |
| Parpa et al., 2019 [27] | Greece | Depression as Mediator Between Grief and Dignity | PGAC | Mean = 27.58 (SD = (14.34)) | Patient Dignity Inventory (PDI) score r = 0.637, p < 0.001 | HADS-D r = 0.565, p < 0.001 | Cancer stage, Gender | PGAC α = 0.0838 |
| Vergo et al., 2017 [19] | Chicago, IL, USA | Assessing Preparatory Grief in Advanced Cancer Patients as an Independent Predictor of Distress in an American Population | PGAC | Mean PGAC = 26.7 (range = 12.0–41.4) | HADS-A Score Change %, CI 1.72 (0.99, 2.46) p < 0.0001 | HADS-D Score Change %, CI 1.74 (0.98, 2.51). | Distress thermometer, HADS, satisfaction with QoL | PGAC α = 0.0838 |
| Kostopoulou et al., 2018 [21] | Athens, Greece | Advanced Cancer Patients’ Perceptions of Dignity: The Impact of Psychologically Distressing Symptoms and Preparatory Grief | PGAC | Mean = 27.58 (SD = (14.34)) | PGAC scores associated with HADS-Anxiety range r = 0.711 to 0.330 across subscales. | HADS-D r = 0.573 to 0.314 across subscales | Age, gender, education, ECOG, stage, chemo-, hormonotherapy | PGAC α = 0.838 |
| Tsilika et al., 2009 [28] | Athens, Greece | The Influence of Cancer Impact on Patients’ Preparatory Grief | PGAC | Mean PGAC = 35.88, SD = (10.42) | PTSD subscales: Avoidance r = 0.537, Intrusion r = 0.607, Hyperarousal r = 0.645 p < 0.0005 | IES-R Total r = 0.70, p < 0.0005 | Age, ECOG, intrusion, hyperarousal | PGAC α = 0.838 |
| Siedentopf et al., 2010 [29] | Germany | Experiences with a specific screening instrument to identify psychosocial support needs in breast cancer patients | PO-Bado | Grief/despondency/depression: M = 1.59, SD = (1.17) | Anxiety/worries/tension: M = 1.57, SD = (1.29); p < 0.001 | Age-grouped means (<50 y) grief scores (M = 1.90) than older (>64 y; M = 1.38), p = 0.009 | Age, psychiatric history, tumor size, type of surgery | Not reported |
| Gökler-Danışman et al., 2017 [34] | Istanbul, Turkey | Experience of grief by patients with cancer in relation to perceptions of illness | PG-12 | Mean grief score = 26.09 (SD = (9.46)) | Illness perception (r = 22, p < 0.01) | Negative experience discrimination (r = 0.22, p < 0.01),hopeful ness (r = 0.25, p < 0.01), identity Centrality (r = 0.28, p < 0.01), | Gender, Education Level, Economic Level, Employment Status, Residence, Type of Cancer | PG-12 α = 0.89 |
| Trevino et al., 2011 [31] | Dana-Farber Cancer Institute, Boston, MA | Grief and Life Disruption in Young Adults with Advanced Cancer | PG-12 | Mean PG-12 = 23.80, SD = (7.05) | Life Disruption F (2, 49) = 13.06, p < 0.001 | Education 15.49 (2.30), Spearmans rho −16; −0.40 Performance Status 77.55 (11.420, −0.39, 0.61) | Gender, marital status, dependents, income, metastatic status, trial status | PG-12 α = 0.76 |
| Trevino et al., 2013 [32] | USA | Correlates of social support in young adults with advanced cancer | PG-12 | Mean = 24.34, SD = (7.15) | QoL (r = –0.67, p < 0.1) | QoL (r = –0.53, p < 0.001) | Appraisal support, metastasis | PG-12 α = 0.76 |
| Trevino et al. 2013 [33] | USA | Patient-oncologist alliance, psychosocial well-being, and treatment adherence among young adults with advanced cancer | PG-12 | Grief and stopping treatment: r = 0.28, p = 0.009 | Appraisal support r = 0.32, p = 0.002 | Alliance r = −0.28, p = 0.01 | Metastatic disease | PG-12 α = 0.77 |
3.2.2. Quality Assessment
| Study | Validity | Reliability | Sensitivity and Specificity | Measurement Error | Other Sources of Bias |
|---|---|---|---|---|---|
| Gökler-Danışman, 2017 [34] |  |  |  |  |  |
| Kostopoulou, 2018 [21] |  |  |  |  |  |
| Mystakidou, 2006 [22] |  |  |  |  |  |
| Mystakidou, 2008 [23] |  |  |  |  |  |
| Mystakidou, 2008 [24] |  |  |  |  |  |
| Mystakidou, 2011 [25] |  |  |  |  |  |
| Mystakidou, 2012 [26] |  |  |  |  |  |
| Parpa, 2019 [27] |  |  |  |  |  |
| Siedentopf, 2010 [29] |  |  |  |  |  |
| Tacón, 2011 [30] |  |  |  |  |  |
| Trevino, 2011 [31] |  |  |  |  |  |
| Trevino, 2013 [32] |  |  |  |  |  |
| Trevino, 2013 [33] |  |  |  |  |  |
| Tsilika, 2009 [28] |  |  |  |  |  |
| Vergo, 2017 [19] |  |  |  |  |  |
 Low Risk of Bias;
Low Risk of Bias;  Unclear Risk of Bias
Unclear Risk of Bias4. Discussion
4.1. Grief Related to Emotional Distress
4.2. Limitations
4.3. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PGAC | Preparatory Grief in Advanced Cancer Patients |
| G-HADS | Greek Hospital Anxiety and Depression Scale |
| G-SAHD | Greek Schedule for Attitudes toward Hastened Death |
| GDI | Grief Diagnostic Instrument |
| HADS | Hospital Anxiety and Depression Scale |
| HADS-D | Hospital Anxiety and Depression Scale—Depression Subscale |
| IES-R-Gr | Impact Events Scale Revised (Greek) |
| PDI | Patient Dignity Inventory |
| PG | Prolonged Grief Disorder Scale |
| DOAJ | Directory of open access journals |
| PO-Bado | Psychological-Oncological Base Documentation |
| QoL | Quality of Life |
Appendix A. Search Strategies Used in PubMed, CINAHL, and PsycInfo
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Jaglarz, K.; Kuca, M.; Cholewa, M.; Wójcik, J.; Pamuła, K.W.; Szymańska, W.M.; Plewniok, J.; Partyka, M.; Janeczek, M.; Kantor, K.K. Role of Psychooncology in Cancer Treatment—A Literature Review. J. Educ. Health Sport 2024, 70, 55663. [Google Scholar] [CrossRef]
- Demkhosei Vaiphei, S. Understanding End-of-Life Care: Psychological Approaches to Cancer Care, 1st ed.; Taylor & Francis Group: Oxford, UK, 2025; ISBN 978-1-040-33076-0. [Google Scholar]
- Karacan, Y.; Yılmaz, H.; Akkus, Y. Grief and Spiritual Well-Being after Cancer Loss: Insights from Family Caregivers. Eur. J. Oncol. Nurs. Off. J. Eur. Oncol. Nurs. Soc. 2025, 76, 102887. [Google Scholar] [CrossRef] [PubMed]
- Tyrrell, P.; Harberger, S.; Schoo, C.; Siddiqui, W. Kubler-Ross Stages of Dying and Subsequent Models of Grief. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Sun, D.; Zhang, X.; Li, J.; Liu, M.; Zhang, L.; Zhang, J.; Cui, M. Mediating Effect of Cognitive Appraisal and Coping on Anticipatory Grief in Family Caregivers of Patients with Cancer: A Bayesian Structural Equation Model Study. BMC Nurs. 2024, 23, 636. [Google Scholar] [CrossRef] [PubMed]
- Christodoulou, L.; Parpottas, P.; Vogazianos, P.; Prigerson, H.G.; Lichtenthal, W.; Petkari, E. The Effectiveness of a Psychological Intervention Targeting Bereaved Caregivers of Cancer Patients: Study Protocol for the EMPOWER-Cancer-Grief RCT. Contemp. Clin. Trials 2025, 154, 107949. [Google Scholar] [CrossRef] [PubMed]
- Meer, S.; Buckle, P.; Miller, R.; Murray, L.; Ziegler, L.; Piil, K.; Boele, F. End-of-Life Care Experiences and Long-Term Outcomes of Bereaved Neuro-Oncology Caregivers: A Cross-Sectional Survey. Palliat. Med. 2025, 39, 814–826. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, A.; McVittie, C.; Goodall, K.; Ellison, M. “It Is Kind of Invisible Work”: Lived Experiences of Informal Caregivers of People with a Brain Tumor. Semin. Oncol. Nurs. 2025, 151938. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Albuquerque, S.; Neto, D.D. Bereavement Support Guidelines for Caregivers in Palliative Care: A Scoping Review. Front. Psychol. 2025, 16, 1541783. [Google Scholar] [CrossRef] [PubMed]
- Treml, J.; Schmidt, V.; Nagl, M.; Kersting, A. Pre-Loss Grief and Preparedness for Death among Caregivers of Terminally Ill Cancer Patients: A Systematic Review. Soc. Sci. Med. 2021, 284, 114240. [Google Scholar] [CrossRef] [PubMed]
- Svop, K.; Dieperink, K.B.; Livingston, T.; Marcussen, J. Families’ Experience of Anticipatory Grief in Home-Based Palliative Cancer Care and Their Support Needs: A Qualitative Study. Eur. J. Oncol. Nurs. 2025, 76, 102880. [Google Scholar] [CrossRef] [PubMed]
- Aydın, A.; Bingöl, H.; Kebudi, R.; Savaş, E.H.; Koç, B.; Büyükkapu Bay, S.; Yıldırım, Ü.M.; Zülfikar, B. Religious Coping Strategies of Mothers Who Lost Their Children to Cancer in Türkiye: A Qualitative Study. J. Relig. Health 2025, 64, 2152–2164. [Google Scholar] [CrossRef] [PubMed]
- Laor-Maayany, R.; Goldzweig, G.; Hasson-Ohayon, I.; Bar-Sela, G.; Engler-Gross, A.; Braun, M. Compassion Fatigue among Oncologists: The Role of Grief, Sense of Failure, and Exposure to Suffering and Death. Support. Care Cancer 2020, 28, 2025–2031. [Google Scholar] [CrossRef] [PubMed]
- Brenner, K.O.; Nathanson, A.; Sławkowski-Rode, M. Oncology and Suffering: Strategies on Coping With Grief for Health Care areareProfessionals. Am. Soc. Clin. Oncol. Educ. Book 2025, 45, e482244. [Google Scholar] [CrossRef] [PubMed]
- Peña-Vargas, C.; Del Río-Rodriguez, P.; Rosario, L.P.; Laporte-Estela, G.; Torres-Blasco, N.; Rodriguez-Castro, Z.; Tollinchi-Natali, N.; Guerrero, W.I.; Torres, P.; Armaiz-Pena, G.N.; et al. Losses Related to Breast Cancer Diagnosis: The Impact on Grief and Depression Symptomatology Within the Context of Hispanic/Latina Patients with Breast Cancer. Healthcare 2025, 13, 624. [Google Scholar] [CrossRef] [PubMed]
- Gabay, G. A Nonheroic Cancer Narrative: Body Deterioration, Grief, Disenfranchised Grief, and Growth. OMEGA—J. Death Dying 2021, 83, 287–309. [Google Scholar] [CrossRef] [PubMed]
- Schoo, C.; Azhar, Y.; Mughal, S.; Rout, P. Grief and Prolonged Grief Disorder. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Vergo, M.T.; Whyman, J.; Li, Z.; Kestel, J.; James, S.L.; Rector, C.; Salsman, J.M. Assessing Preparatory Grief in Advanced Cancer Patients as an Independent Predictor of Distress in an American Population. J. Palliat. Med. 2017, 20, 48–52. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Tsilika, E.; Parpa, E.; Katsouda, E.; Sakkas, P.; Soldatos, C. Life before Death: Identifying Preparatory Grief through the Development of a New Measurement in Advanced Cancer Patients (PGAC). Support. Care Cancer 2005, 13, 834–841. [Google Scholar] [CrossRef] [PubMed]
- Kostopoulou, S.; Parpa, E.; Tsilika, E.; Katsaragakis, S.; Papazoglou, I.; Zygogianni, A.; Galanos, A.; Mystakidou, K. Advanced Cancer Patients’ Perceptions of Dignity: The Impact of Psychologically Distressing Symptoms and Preparatory Grief. J. Palliat. Care 2018, 33, 88–94. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Tsilika, E.; Parpa, E.; Katsouda, E.; Sakkas, P.; Galanos, A.; Vlahos, L. Demographic and Clinical Predictors of Preparatory Grief in a Sample of Advanced Cancer Patients. Psychooncology 2006, 15, 828–833. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Athanasouli, P.; Pathiaki, M.; Galanos, A.; Pagoropoulou, A.; Vlahos, L. Preparatory Grief, Psychological Distress and Hopelessness in Advanced Cancer Patients. Eur. J. Cancer Care 2008, 17, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Tsilika, E.; Parpa, E.; Galanos, A.; Vlahos, L. Screening for Preparatory Grief in Advanced Cancer Patients. Cancer Nurs. 2008, 31, 326–332. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Tsilika, E.; Parpa, E.; Panagiotou, I.; Galanos, A.; Gouliamos, A. The Mediation Effect of Anxiety Between Post-Traumatic Stress Symptoms and Preparatory Grief in Advanced Cancer Patients. J. Pain Symptom Manag. 2011, 41, 511–521. [Google Scholar] [CrossRef] [PubMed]
- Mystakidou, K.; Parpa, E.; Tsilika, E.; Panagiotou, I.; Galanos, A.; Sakkas, P.; Gouliamos, A. Posttraumatic Stress Disorder and Preparatory Grief in Advanced Cancer. J. BUON Off. J. Balk. Union Oncol. 2012, 17, 155–159. [Google Scholar]
- Parpa, E.; Kostopoulou, S.; Tsilika, E.; Galanos, A.; Mystakidou, K. Depression as a Mediator or Moderator Between Preparatory Grief and Sense of Dignity in Patients With Advanced Cancer. Am. J. Hosp. Palliat. Med. 2019, 36, 1063–1067. [Google Scholar] [CrossRef] [PubMed]
- Tsilika, E.; Mystakidou, K.; Parpa, E.; Galanos, A.; Sakkas, P.; Vlahos, L. The Influence of Cancer Impact on Patients’ Preparatory Grief. Psychol. Health 2009, 24, 135–148. [Google Scholar] [CrossRef] [PubMed]
- Siedentopf, F.; Marten-Mittag, B.; Utz-Billing, I.; Schoenegg, W.; Kentenich, H.; Dinkel, A. Experiences with a Specific Screening Instrument to Identify Psychosocial Support Needs in Breast Cancer Patients. Eur. J. Obstet. Gynecol. Reprod. Biol. 2010, 148, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Tacón, A.M. Mindfulness: Existential, Loss, and Grief Factors in Women with Breast Cancer. J. Psychosoc. Oncol. 2011, 29, 643–656. [Google Scholar] [CrossRef] [PubMed]
- Trevino, K.M.; Maciejewski, P.K.; Fasciano, K.; Prigerson, H.G. Grief and Life Disruption in Young Adults with Advanced Cancer. J. Adolesc. Young Adult Oncol. 2011, 1, 168–172. [Google Scholar] [CrossRef]
- Trevino, K.M.; Fasciano, K.; Block, S.; Prigerson, H.G. Correlates of Social Support in Young Adults with Advanced Cancer. Support. Care Cancer 2013, 21, 421–429. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Trevino, K.M.; Fasciano, K.; Prigerson, H.G. Patient-Oncologist Alliance, Psychosocial Well-Being, and Treatment Adherence Among Young Adults With Advanced Cancer. J. Clin. Oncol. 2013, 31, 1683–1689. [Google Scholar] [CrossRef] [PubMed]
- Gökler-Danışman, I.; Yalçınay-İnan, M.; Yiğit, İ. Experience of Grief by Patients with Cancer in Relation to Perceptions of Illness: The Mediating Roles of Identity Centrality, Stigma-Induced Discrimination, and Hopefulness. J. Psychosoc. Oncol. 2017, 35, 776–796. [Google Scholar] [CrossRef] [PubMed]
- Perna, G.; Pinto, E.; Spiti, A.; Torti, T.; Cucchi, M.; Caldirola, D. Foundations for a Personalized Psycho-Oncology: The State of the Art. J. Pers. Med. 2024, 14, 892. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattson, R.; Henderson, M.; Carlson, S.S. Assessing Grief in Cancer Care: A Systematic Review of Observational Studies Using Psychometric Instruments. Healthcare 2025, 13, 1722. https://doi.org/10.3390/healthcare13141722
Mattson R, Henderson M, Carlson SS. Assessing Grief in Cancer Care: A Systematic Review of Observational Studies Using Psychometric Instruments. Healthcare. 2025; 13(14):1722. https://doi.org/10.3390/healthcare13141722
Chicago/Turabian StyleMattson, Rebecca, Margaret Henderson, and Savitri Singh Carlson. 2025. "Assessing Grief in Cancer Care: A Systematic Review of Observational Studies Using Psychometric Instruments" Healthcare 13, no. 14: 1722. https://doi.org/10.3390/healthcare13141722
APA StyleMattson, R., Henderson, M., & Carlson, S. S. (2025). Assessing Grief in Cancer Care: A Systematic Review of Observational Studies Using Psychometric Instruments. Healthcare, 13(14), 1722. https://doi.org/10.3390/healthcare13141722





