Dropout Rate of Participants with Cancer in Randomized Clinical Trials That Use Virtual Reality to Manage Pain—A Systematic Review with Meta-Analysis and Meta-Regression
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Search Strategy
2.2. Research Question and Study Selection
- -
- P: patients diagnosed with cancer or survivors of cancer (adults and pediatric patients).
- -
- I: virtual reality-based interventions related to pain management.
- -
- C: all types of comparators, except those based on virtual reality
- -
- O: participant dropout.
- -
- S: randomized clinical trials that report the dropout rate or allow its indirect calculation.
- -
- The exclusion criteria were as follows:
- -
- Studies in which the comparator is also a virtual reality group because comparisons could not be conducted.
- -
- Studies with the same sample size as other publications.
2.3. Data Extraction
2.4. Data Analysis
3. Results
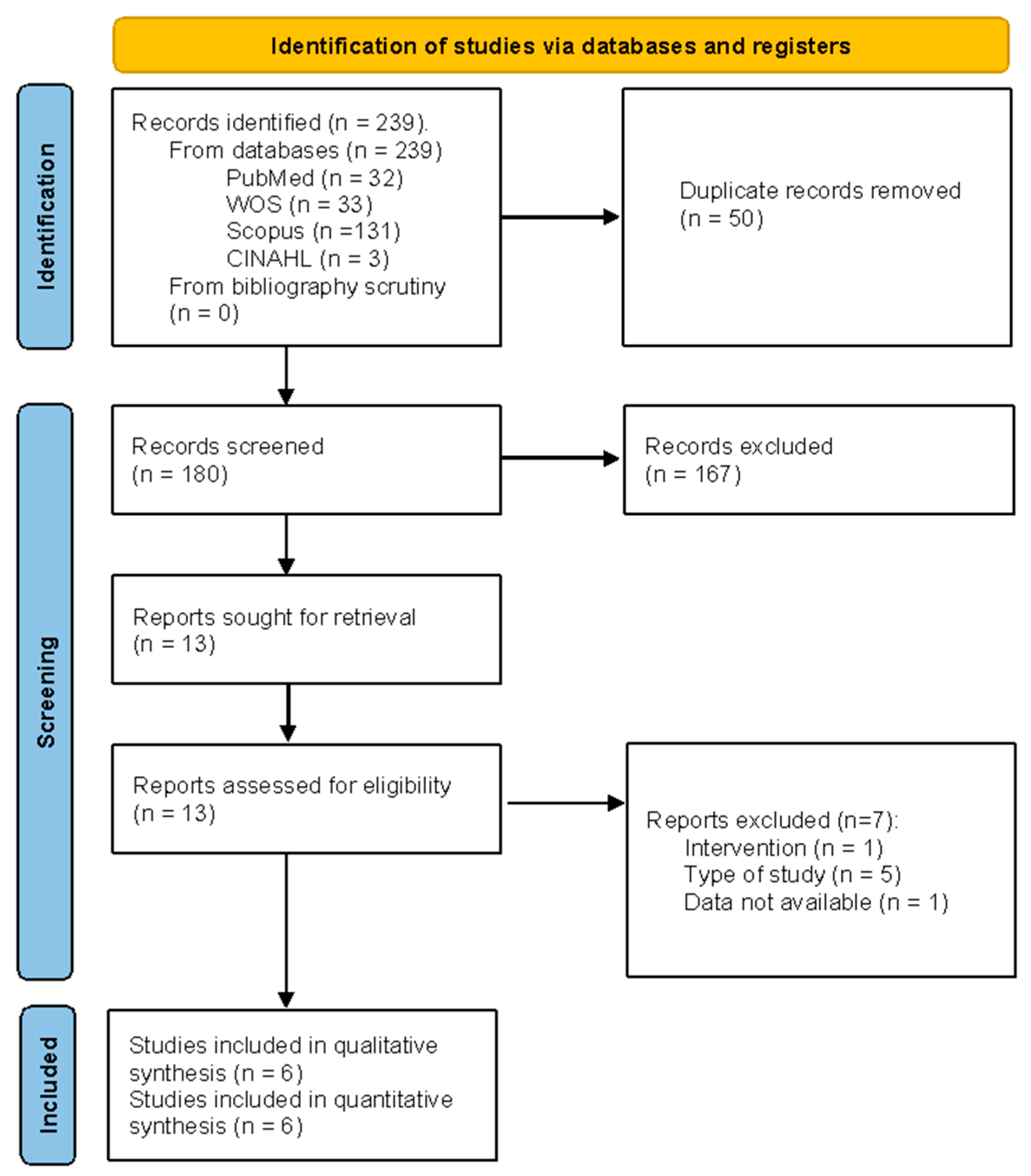
3.1. Study Selection
3.2. Methodological Quality Assessment: JBI Critical Appraisal Tool
3.3. Description of the Selected Studies
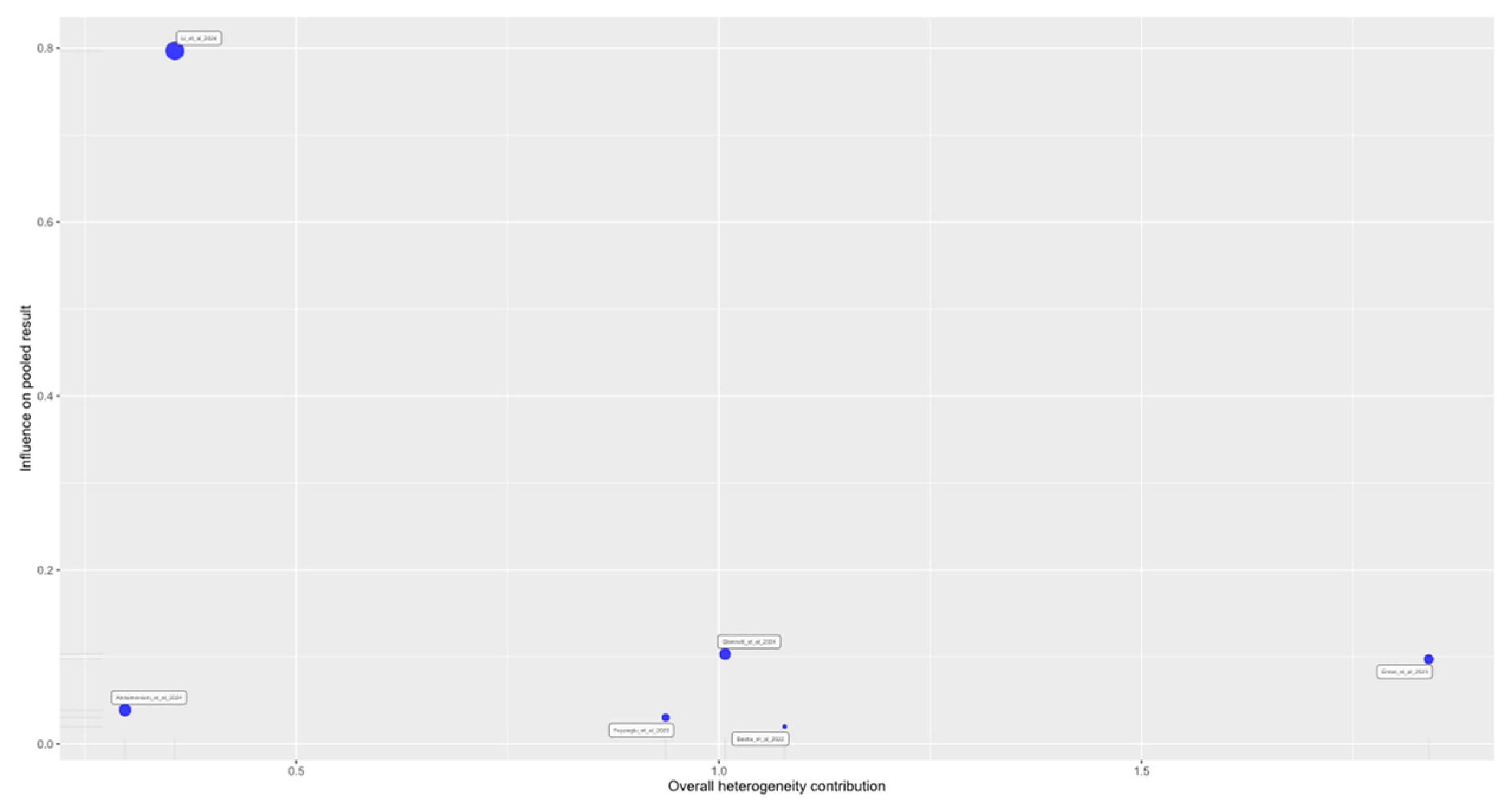
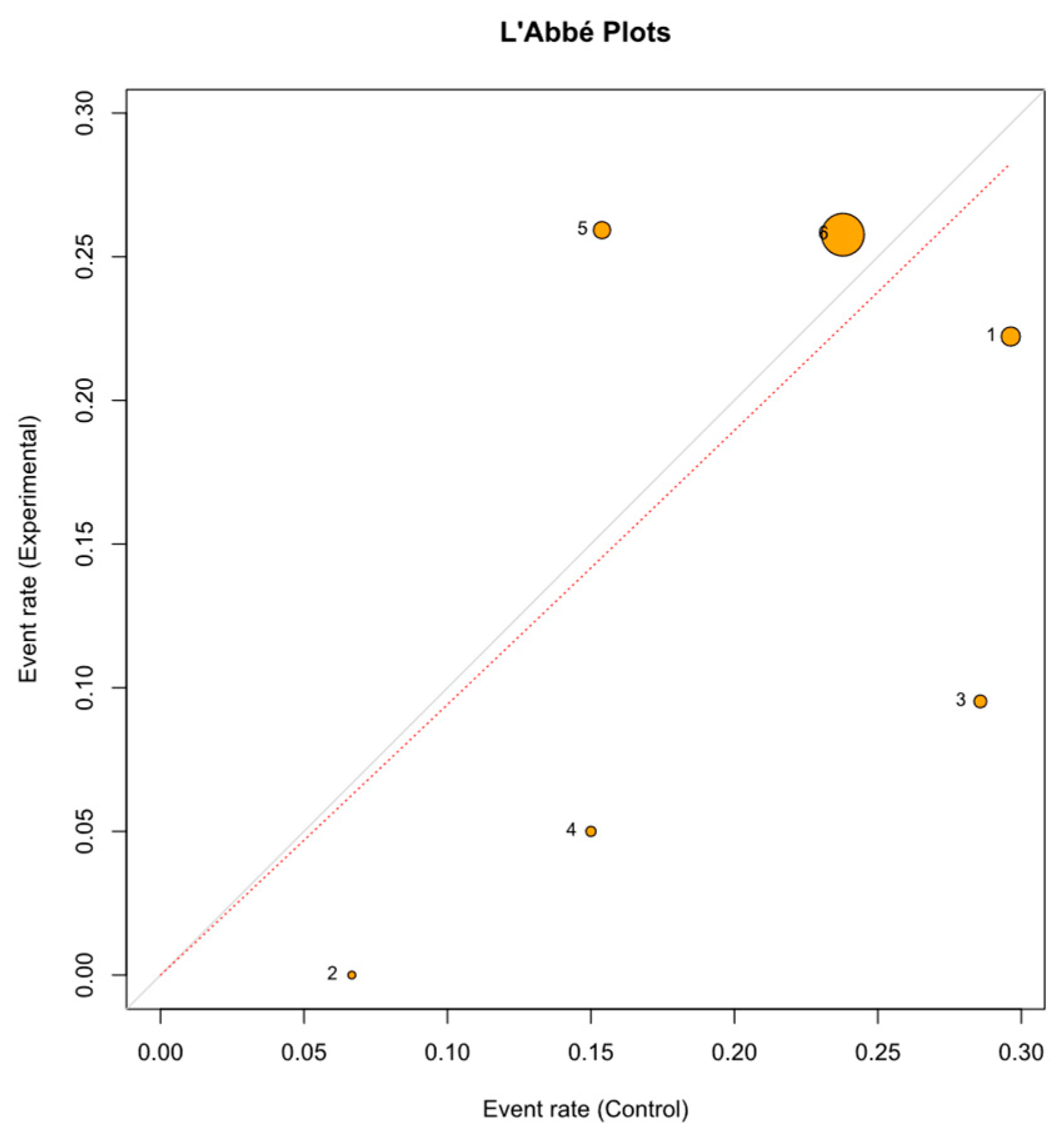
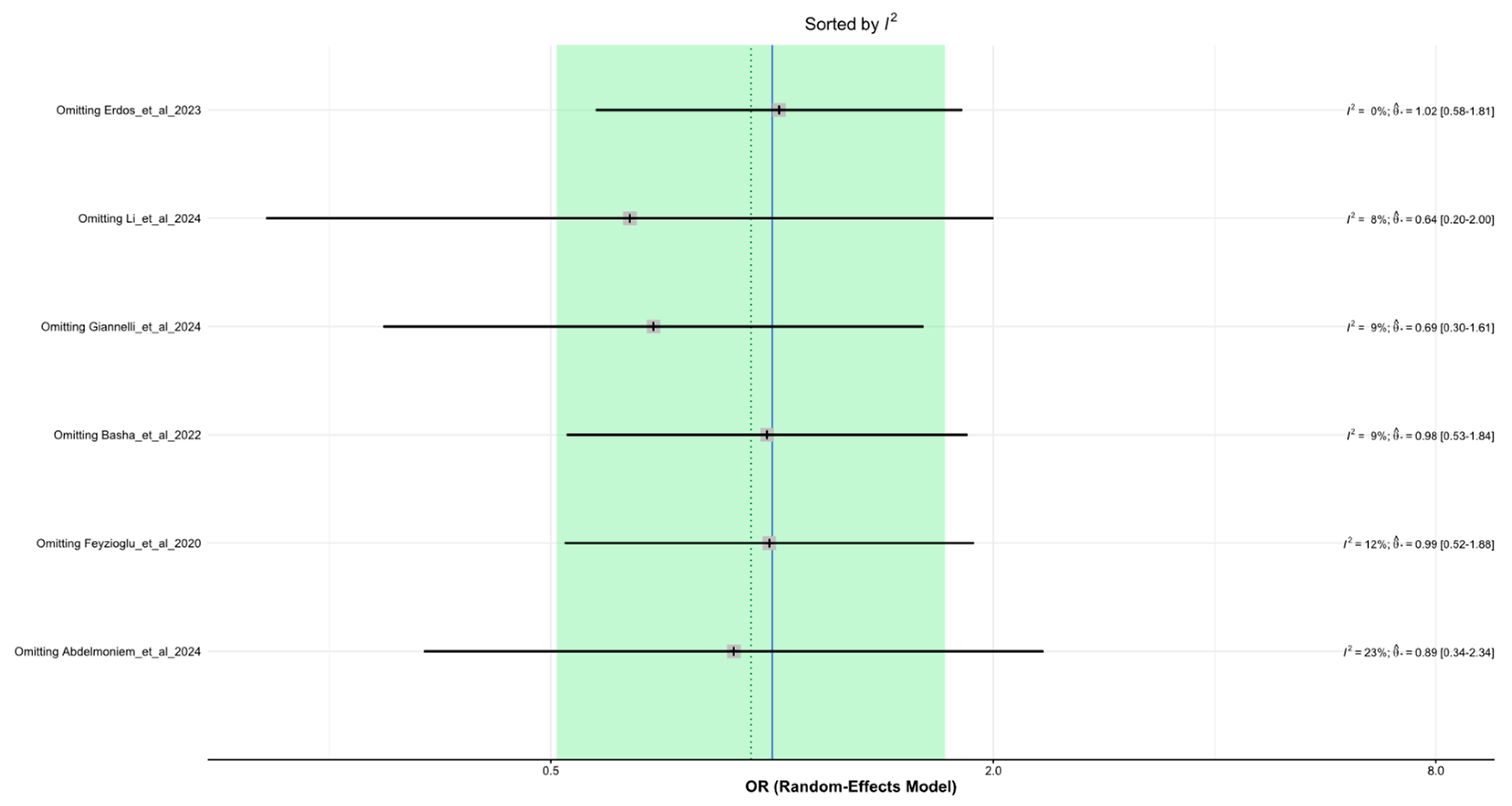
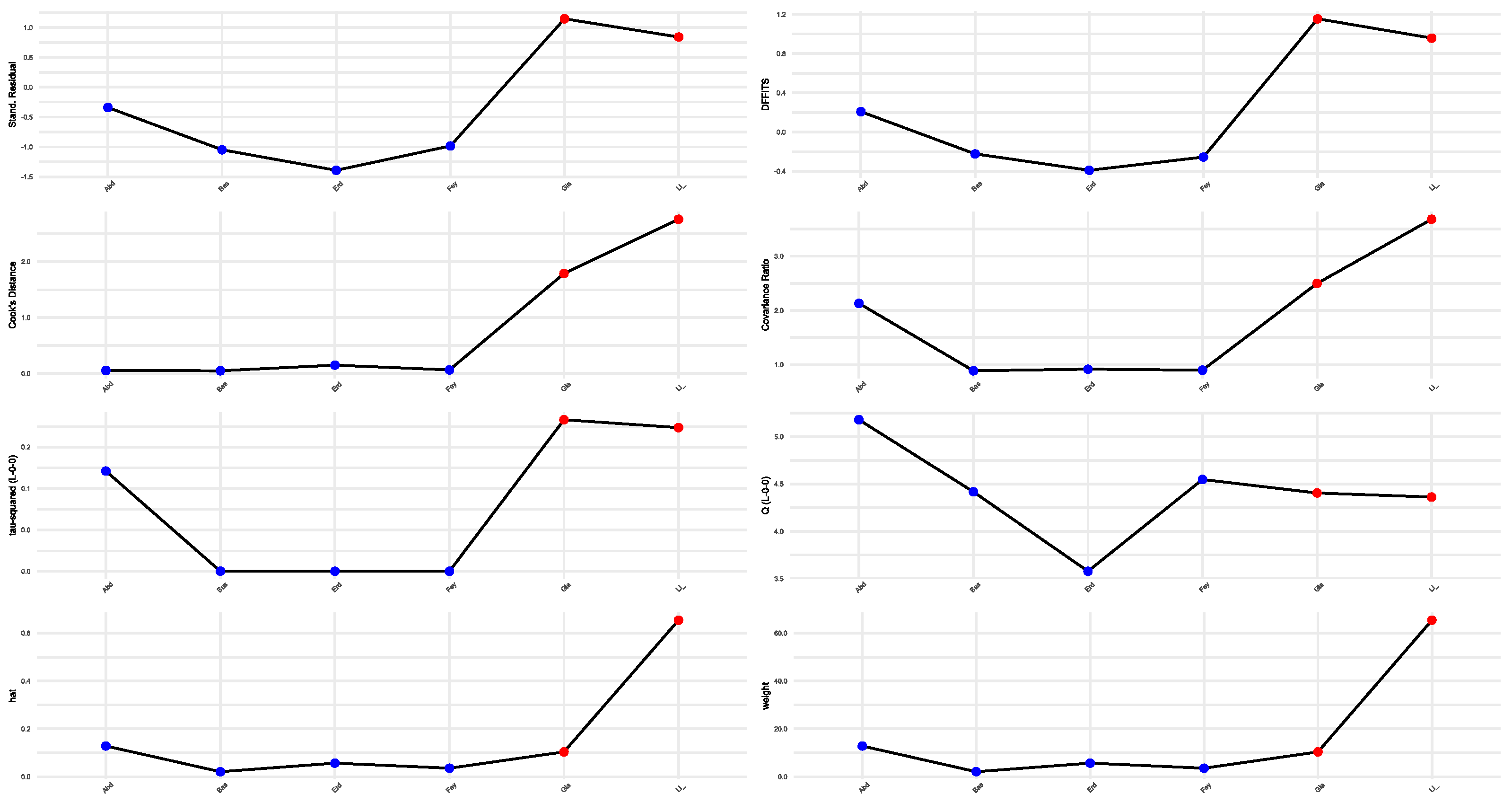
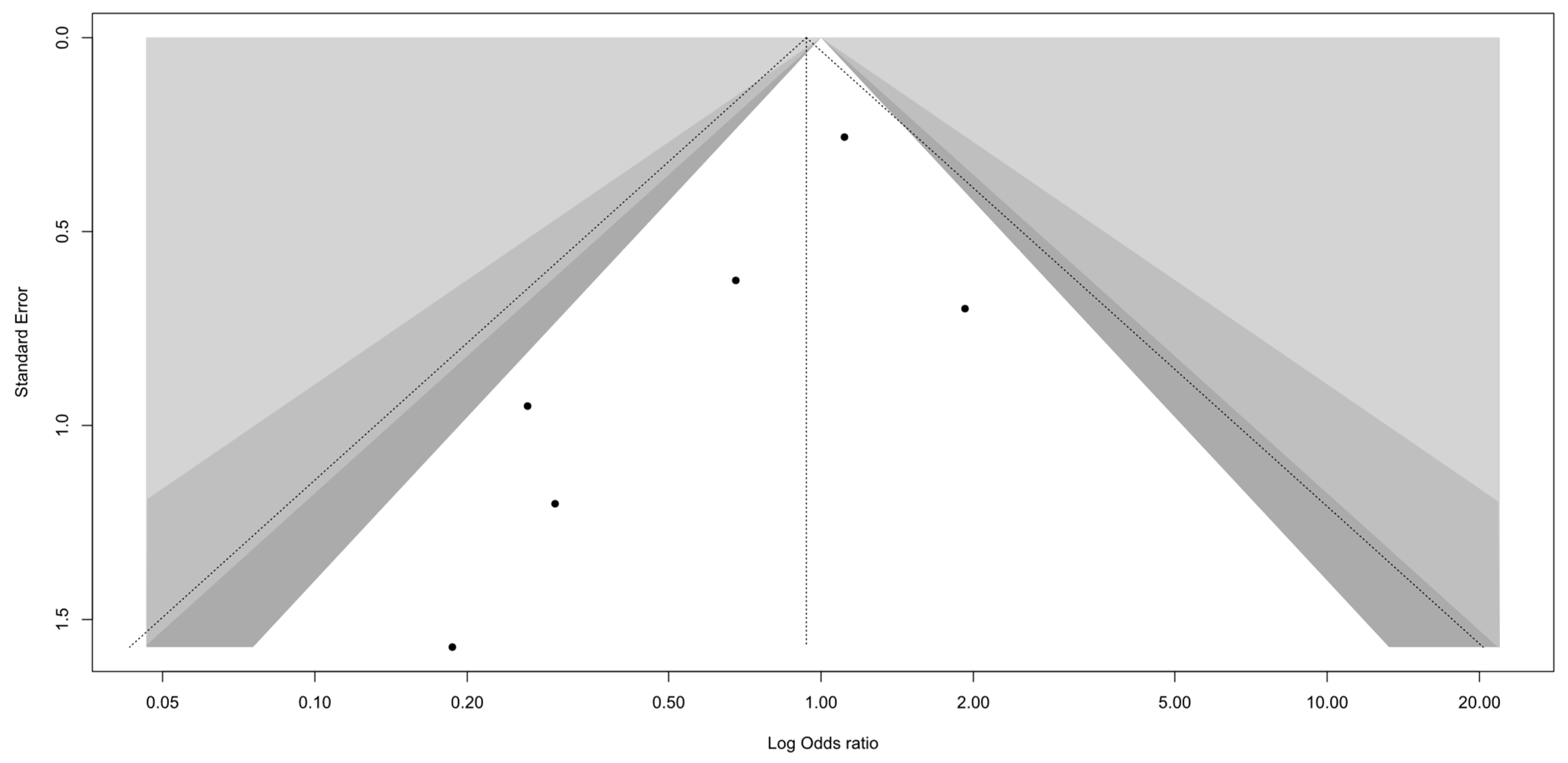
3.4. Sensitivity Analysis and Publication Bias
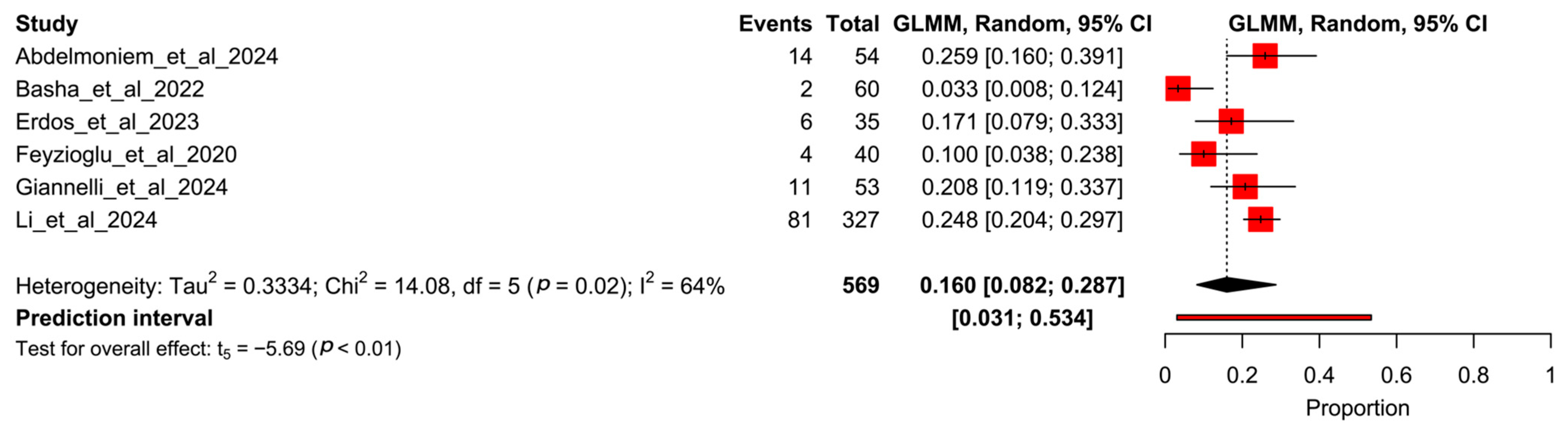
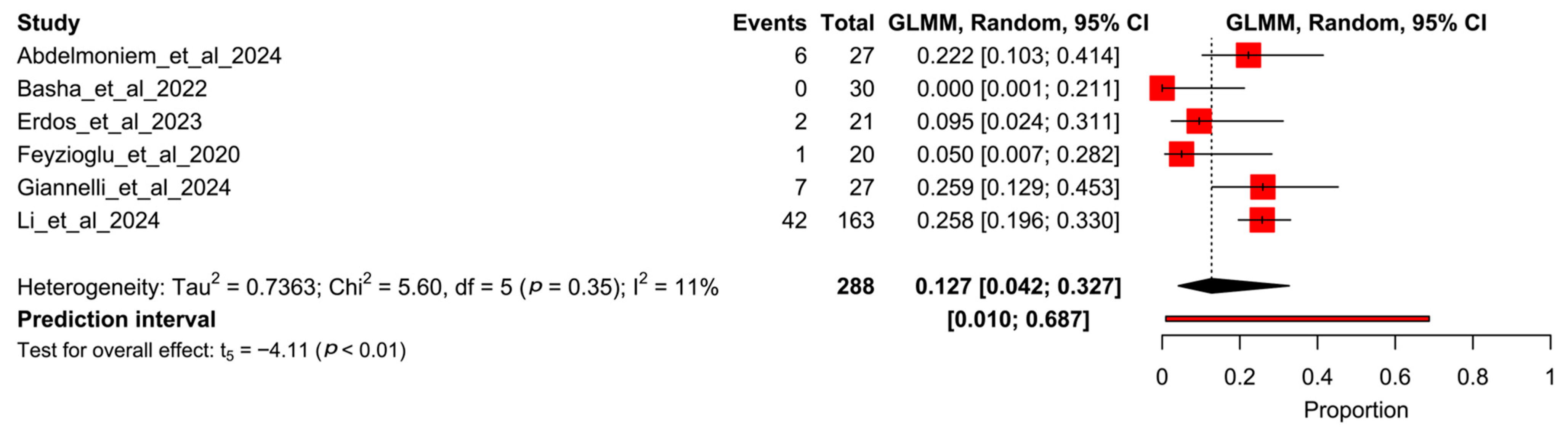
3.5. Proportion Meta-Analysis
3.6. Odds Ratio Meta-Analysis
3.7. Subgroup Meta-Analysis and Meta-Regressions
3.8. Evidence Synthesis
4. Discussion
4.1. Research and Clinical Implications
4.2. Future Research
4.3. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VR | Virtual Reality |
Appendix A
Appendix A.1
References
- World Health Organization. International Agency for Research on Cancer (GLOBOCAN). Available online: https://gco.iarc.fr/today/en (accessed on 1 April 2025).
- van den Beuken-van Everdingen, M.H.J.; Hochstenbach, L.M.J.; Joosten, E.A.J.; Tjan-Heijnen, V.C.G.; Janssen, D.J.A. Update on Prevalence of Pain in Patients With Cancer: Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2016, 51, 1070–1090.e9. [Google Scholar] [CrossRef] [PubMed]
- Evenepoel, M.; Haenen, V.; de Baerdemaecker, T.; Meeus, M.; Devoogdt, N.; Dams, L.; van Dijck, S.; van der Gucht, E.; de Groef, A. Pain Prevalence During Cancer Treatment: A Systematic Review and Meta-Analysis. J. Pain Symptom Manag. 2022, 63, e317–e335. [Google Scholar] [CrossRef] [PubMed]
- Snijders, R.A.H.; Brom, L.; Theunissen, M.; van den Beuken-van Everdingen, M.H.J. Update on Prevalence of Pain in Patients with Cancer 2022: A Systematic Literature Review and Meta-Analysis. Cancers 2023, 15, 591. [Google Scholar] [CrossRef] [PubMed]
- Emery, J.; Butow, P.; Lai-Kwon, J.; Nekhlyudov, L.; Rynderman, M.; Jefford, M. Management of common clinical problems experienced by survivors of cancer. Lancet 2022, 399, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Stansel, C.C.; McLeod, A.R.; Gulati, S.; Ivory, C.H.; Dietrich, M.S.; Murray, H.N.; Zhang, N.; Shah, K.; Patel, H.U.; Pegram, K.B.; et al. Effects of Virtual Reality on Pain, Stress, and Affect in an Outpatient Chemotherapy Infusion Clinic: A Randomized Controlled Trial. Clin. J. Oncol. Nurs. 2025, 29, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zhang, J.-E.; Cheng, A.S.K.; Cheng, H.; Wefel, J.S. Meta-Analysis of the Efficacy of Virtual Reality–Based Interventions in Cancer-Related Symptom Management. Integr. Cancer Ther. 2023, 22, 153473542311574. [Google Scholar] [CrossRef] [PubMed]
- Garrett, B.M.; Tao, G.; Taverner, T.; Cordingley, E.; Sun, C. Patients perceptions of virtual reality therapy in the management of chronic cancer pain. Heliyon 2020, 6, e03916. [Google Scholar] [CrossRef] [PubMed]
- Groninger, H.; Violanti, D.; Mete, M. Virtual reality for pain management in hospitalized patients with cancer: A randomized controlled trial. Cancer 2024, 130, 2552–2560. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.D.; Siddall, P.J.; Lovell, M.R. Feasibility and acceptability of virtual reality for cancer pain in people receiving palliative care: A randomised cross-over study. Support. Care Cancer 2022, 30, 3995–4005. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.A.; Aly, S.M.; Youssef, A.S.A.; Ragab, M.M.M.; Hussein, H.M. Using Virtual Reality Pablo Gaming in the Post-Operative Rehabilitation of Breast Cancer Patients: Randomized Controlled Trial. J. Clin. Med. 2024, 13, 7609. [Google Scholar] [CrossRef] [PubMed]
- Basha, M.A.; Aboelnour, N.H.; Alsharidah, A.S.; Kamel, F.H. Effect of exercise mode on physical function and quality of life in breast cancer–related lymphedema: A randomized trial. Support. Care Cancer 2021, 30, 2101–2110. [Google Scholar] [CrossRef] [PubMed]
- Erdős, S.; Horváth, K. The Impact of Virtual Reality (VR) on Psychological and Physiological Variables in Children Receiving Chemotherapy: A Pilot Cross-Over Study. Integr. Cancer Ther. 2023, 22, 15347354231168984. [Google Scholar] [CrossRef] [PubMed]
- Feyzioğlu, Ö.; Dinçer, S.; Akan, A.; Algun, Z.C. Is Xbox 360 Kinect-based virtual reality training as effective as standard physiotherapy in patients undergoing breast cancer surgery? Support. Care Cancer 2020, 28, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
- Giannelli, A.; Moscato, S.; Ostan, R.; Pannuti, R.; Chiari, L.; Biasco, G.; Varani, S. Virtual Reality for advanced cancer patients assisted at home: A randomized controlled interventional study. Psycho Oncol. 2024, 33, e6368. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yu, Z.; Li, H.; Cao, L.; Yu, H.; Deng, N.; Liu, Y. Effects of Virtual Reality Therapy for Patients With Breast Cancer During Chemotherapy: Randomized Controlled Trial. JMIR Serious Games 2024, 12, e53825. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.; Glitza, I.; Chisholm, G.; Yennu, S.; Bruera, E. Attrition rates, reasons, and predictive factors in supportive care and palliative oncology clinical trials. Cancer 2012, 119, 1098–1105. [Google Scholar] [CrossRef] [PubMed]
- Roick, J.; Danker, H.; Kersting, A.; Briest, S.; Dietrich, A.; Dietz, A.; Einenkel, J.; Papsdorf, K.; Lordick, F.; Meixensberger, J.; et al. Factors associated with non-participation and dropout among cancer patients in a cluster-randomised controlled trial. Eur. J. Cancer Care 2017, 27, e12645. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, M.G.; Lim, K.M. Participation in and withdrawal from cancer clinical trials: A survey of clinical research coordinators. Asia Pac. J. Oncol. Nurs. 2022, 9, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Erlik, M.; Timm, H.; Larsen, A.T.S.; Quist, M. Reasons for non-participation in cancer rehabilitation: A scoping literature review. Support. Care Cancer 2024, 32, 346. [Google Scholar] [CrossRef] [PubMed]
- Lundin, R.M.; Yeap, Y.; Menkes, D.B. Adverse Effects of Virtual and Augmented Reality Interventions in Psychiatry: Systematic Review. JMIR Ment. Heal. 2023, 10, e43240. [Google Scholar] [CrossRef] [PubMed]
- Simón-Vicente, L.; Rodríguez-Cano, S.; Delgado-Benito, V.; Ausín-Villaverde, V.; Delgado, E.C. Cybersickness. A systematic literature review of adverse effects related to virtual reality. Neurología 2024, 39, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Montaño, Z.; Chen, V.J.; Gold, J.I. Virtual reality and pain management: Current trends and future directions. Pain Manag. 2011, 1, 147–157. [Google Scholar] [CrossRef] [PubMed]
- Garrett, B.; Taverner, T.; Masinde, W.; Gromala, D.; Shaw, C.; Negraeff, M. A rapid evidence assessment of immersive virtual reality as an adjunct therapy in acute pain management in clinical practice. Clin. J. Pain 2014, 30, 1089–1098. [Google Scholar] [CrossRef] [PubMed]
- Mallari, B.; Spaeth, E.K.; Goh, H.; Boyd, B.S. Virtual reality as an analgesic for acute and chronic pain in adults: A systematic review and meta-analysis. J. Pain Res. 2019, 12, 2053–2085. [Google Scholar] [CrossRef] [PubMed]
- Eijlers, R.; Utens, E.M.W.J.; Staals, L.M.; de Nijs, P.F.A.; Berghmans, J.M.; Wijnen, R.M.H.; Hillegers, M.H.J.; Dierckx, B.; Legerstee, J.S. Systematic review and meta-analysis of virtual reality in pediatrics: Effects on pain and anxiety. Anesth. Analg. 2019, 129, 1344–1353. [Google Scholar] [CrossRef] [PubMed]
- Triberti, S.; Repetto, C.; Riva, G. Psychological factors influencing the effectiveness of virtual reality–based analgesia: A systematic review. Cyberpsychol. Behav. Soc. Netw. 2014, 17, 335–345. [Google Scholar] [CrossRef] [PubMed]
- Chirico, A.; Lucidi, F.; De Laurentiis, M.; Milanese, C.; Napoli, A.; Giordano, A. Virtual Reality in Health System: Beyond Entertainment. A mini-review on the efficacy of VR during cancer treatment. J. Cell. Physiol. 2016, 231, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Lier, E.J.; de Vries, M.; Steggink, E.M.; ten Broek, R.P.G.; van Goor, H. Effect modifiers of virtual reality in pain management: A systematic review and meta-regression analysis. Pain 2023, 164, 1658–1665. [Google Scholar] [CrossRef] [PubMed]
- Alvarado-Omenat, J.J.; Llamas-Ramos, R.; García-García, D.; Correyero-León, M.; Fonseca-Sánchez, E.; Llamas-Ramos, I. Effectiveness of virtual reality in cancer patients undergoing chemotherapy. Systematic review. Int. J. Cancer 2025, 156, 1419–1428. [Google Scholar] [CrossRef] [PubMed]
- Burrai, F.; Sguanci, M.; Petrucci, G.; De Marinis, M.G.; Piredda, M. Effectiveness of immersive virtual reality on anxiety, fatigue and pain in patients with cancer undergoing chemotherapy: A systematic review and meta-analysis. Eur. J. Oncol. Nurs. 2023, 64, 102340. [Google Scholar] [CrossRef] [PubMed]
- Blom, J.W.; Muth, C.; Glasziou, P.; McCormack, J.P.; Perera, R.; Poortvliet, R.K.E.; Numans, M.E.; Thürmann, P.; Thiem, U.; Thio, S.L.; et al. Describing deprescribing trials better: An elaboration of the CONSORT statement. J. Clin. Epidemiol. 2020, 127, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Chuan, A.; Hatty, M.; Shelley, M.; Lan, A.; Chow, H.; Dai, E.; Haider, S.; Bogdanovych, A.; Chua, W. Feasibility of virtual reality-delivered pain psychology therapy for cancer-related neuropathic pain: A pilot randomised controlled trial. Anaesthesia 2023, 78, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Cîmpean, A.I. A pilot study to compare cognitive behavioral therapy with virtual reality vs. Standard cognitive behavioral therapy for patients who suffer from cervical cancer. J. Evid.-Based Psychother. 2019, 19, 115–127. [Google Scholar] [CrossRef]
- Ioannou, A.; Paikousis, L.; Papastavrou, E.; Avraamides, M.N.; Astras, G.; Charalambous, A. Effectiveness of Virtual Reality Vs Guided Imagery on mood changes in cancer patients receiving chemotherapy treatment: A crossover trial. Eur. J. Oncol. Nurs. 2022, 61, 102188. [Google Scholar] [CrossRef] [PubMed]
- Saeidi, M.; Bostanabad, M.A.; Jabraeili, M. The Effect of Virtual Reality and Hugo Point Massage on the Pain and Anxiety of School-aged Children with Cancer: Crossover Clinical Trial. Open Nurs. J. 2023, 17. [Google Scholar] [CrossRef]
- Tennant, M.; Youssef, G.J.; McGillivray, J.; Clark, T.-J.; McMillan, L.; McCarthy, M.C. Exploring the use of Immersive Virtual Reality to enhance Psychological Well-Being in Pediatric Oncology: A pilot randomized controlled trial. Eur. J. Oncol. Nurs. 2020, 48, 101804. [Google Scholar] [CrossRef] [PubMed]
- Wong, C.L.; Li, C.K.; Chan, C.W.H.; Choi, K.C.; Chen, J.; Yeung, M.T.; Chan, O.N. Virtual Reality Intervention Targeting Pain and Anxiety Among Pediatric Cancer Patients Undergoing Peripheral Intravenous Cannulation. Cancer Nurs. 2021, 44, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Mogahed, H.G.; Hamoda, R.E.; Elkalla, R.A. Virtual reality on pain and anxiety after modified radical mastectomy in menopause. Res. J. Pharm. Technol. 2024, 17, 1657–1661. [Google Scholar] [CrossRef]
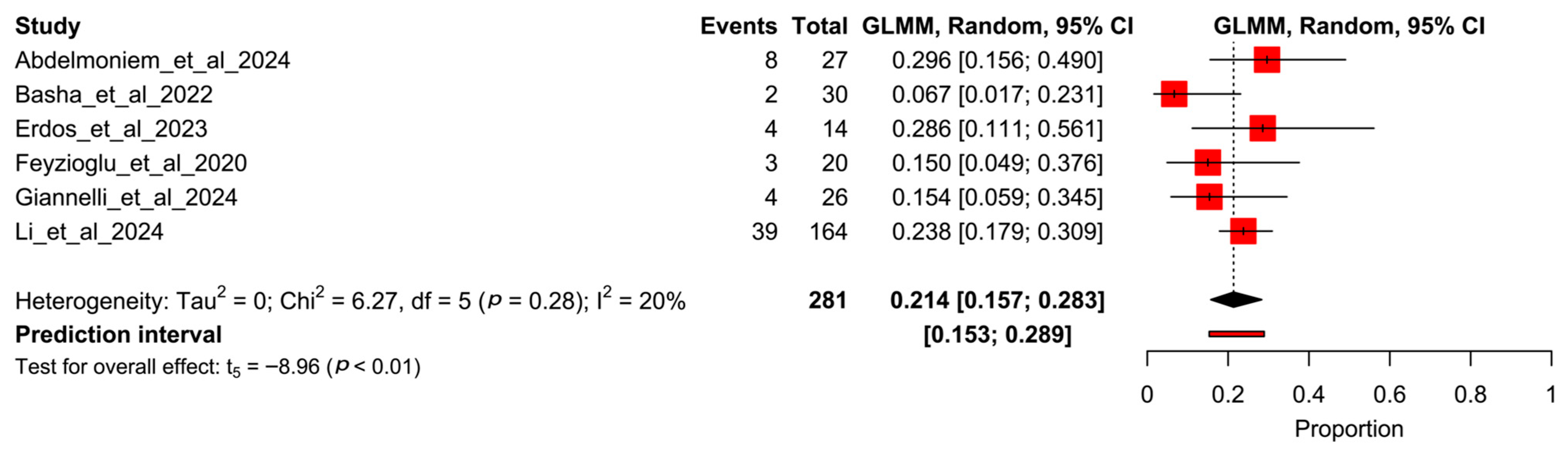
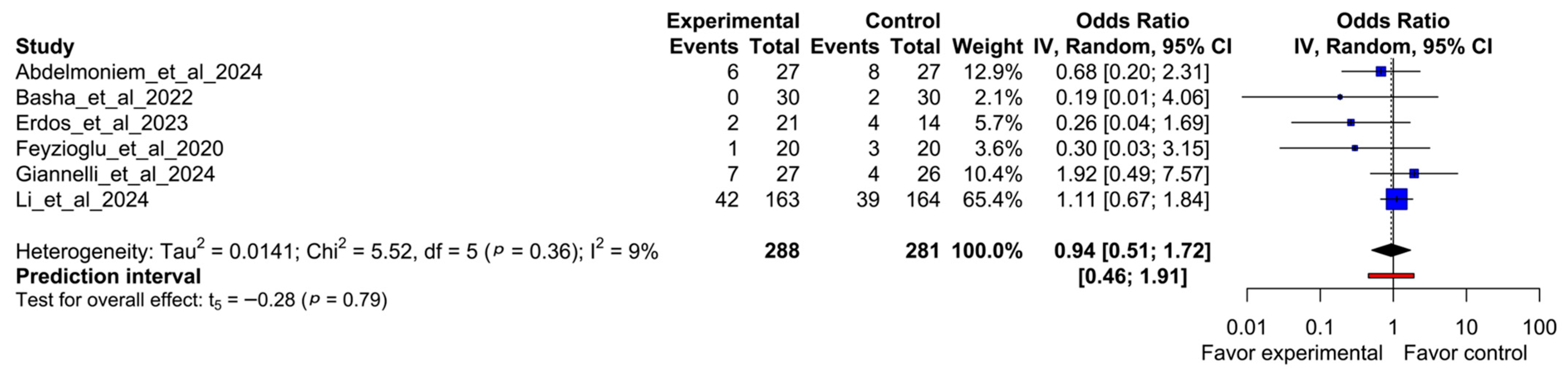
| Author (Year) | Item 1 | Item 2 | Item 3 | Item 4 | Item 5 | Item 6 | Item 7 | Item 8 | Item 9 | Item 10 | Item 11 | Item 12 | Item 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdelmoniem et al., 2024 [12] | Y | Y | Y | Y | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Basha et al., 2022 [13] | Y | Y | U | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Erdős et al., 2023 [14] | Y | N | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Feyzioğlu et al., 2020 [15] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Giannelli et al., 2024 [16] | Y | Y | U | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Li et al., 2024 [17] | Y | Y | Y | N | N | Y | Y | Y | Y | Y | Y | Y | Y |
| Study | Population | Intervention | Comparation | Outcomes | ||||
|---|---|---|---|---|---|---|---|---|
| N CG EG | Type of Cancer Gender Mean Age (Years) | Type (Duration) | Type (Duration) | % Overall Retention | % Dropout | Reason for Dropouts | Adverse Events | |
| Abdelmoniem et al., 2024 [12] | N: 54 CG: 27 EG: 27 | Post-operative breast cancer F 100% M 0% CG: 48 ± 4.60 GE: 47 ± 3.94 | Exercises using VR technology and standard treatment (3/week; 8 weeks). Exercises using VR technology (Pablo© Handle Training): 5 VR games (3 min/game; 15 min). Non-immersive virtual reality. Standard treatment: upper limb exercises (2 sets, 15 repetitions 15 min) and electronic intermittent compression therapy. | Standard treatment: upper limb exercises (2 sets, 15 repetitions 15 min) and electronic intermittent compression therapy (3/week; 8 weeks). | N: 74.07% (40/54) CG: 77.78% (21/27) CG: 70.37% (19/27) | N: 25.93% (14/54) EG: 22.22% (6/27) CG: 29.63% (8/27) | Not indicated | None |
| Basha et al., 2022 [13] | N: 60 CG: 30 EG: 30 | Unilateral breast cancer-related lymphedema F 100% M 0% CG: 52.07 ± 7.48 GE: 48.83 ± 7.0 | VR Kinect-based games and complex decongestive physiotherapy in small groups (1–4 women) supervised by a physiotherapist (1 session/day, 5 days/week, 8 weeks) VR Kinect-based games (Xbox Kinect): active movements of all joints of the upper limbs. Non-immersive virtual reality Complex decongestive physiotherapy: manual lymphatic drainage, compression bandages, skin care, and exercises. | Upper limb exercises and complex decongestive physiotherapy in small groups (1–4 women) supervised by a physiotherapist (1 session/day, 5 days/week, 8 weeks) Stretching and strength exercises in upper limb (2–3 sets; 10–12; 2 min rest allowed between sets) Complex decongestive physiotherapy: manual lymphatic drainage, compression bandages, skin care, and exercises. | N: 96.67% (58/60) CG: 93.33% (28/30) EG: 100% (30/30) | N: 3.33% (2/60) CG: 6.67% (2/30) EG: 0% (0/30) | Not indicated | Not indicated |
| Erdős et al., 2023 [14] | N: 35 CG: 14 EG: 21 | Pediatric cancer patients underwent chemotherapy F 27.59% M 72.41% N: 15 ± 2.44 | VR game (during the chemotherapy process). VR game (A Night Sky): Immersive virtual reality. | Playing a mobile game according to participants’ own preferences (during chemotherapy process) | N: 82.86% (29/35) CG: 71.43% (10/14) EG: 90.48% (19/21) | N: 17.14% (6/35) CG: 28.57% (4/14) EG: 9.52% (2/21) | CG: declined to participate (n = 4) EG: declined to participate (n = 2) | Not indicated |
| Feyzioğlu et al., 2020 [15] | N: 40 CG: 20 EG: 20 | Breast cancer patients who underwent unilateral mastectomy with axillary lymph node dissection and were receiving adjuvant therapy F 100% M 0% CG: 51 ± 7.06 GE: 50.84 ± 8.53 | VR-exercise using Xbox Kinect-based games (45 min/session; 2 sessions/week, 6 weeks) VR-exercise: functional and strength exercises for upper limbs using a VR system (Xbox Kinect-based games). Supervised by an experienced physiotherapist. Non-immersive virtual reality. | Standard physiotherapy (45 min/session; 2 sessions/week, 6 weeks) Standard physiotherapy: exercises not using any VR system. Supervised by an experienced physiotherapist. | N: 90% (36/40) CG: 85% (17/20) EG: 95% (19/20) | N: 10% (4/40) CG: 15% (3/20) EG: 1% (1/20) | EG: declined to participate (n = 1) CG: new metastasis focus (n =1), declined to participate (n = 1), chemotherapy side effect (n = 1) | None found |
| Giannelli et al., 2024 [16] | N: 56 CG: 26 EG: 27 | Advanced cancer patients F 58% M 42% N: 55.7 ± (10.7) CG: 58.23 ± 8 GE: 53.2 ± 12.4 | VR headset with interactive and non-interactive content (4 days; to avoid forced use of the device, the investigator did not specify a minimum or maximum usage time or number of sessions; supervised by psychologists) VR headset: interactive content based on a basic three-level skill game called ‘Yuma’s World’. Non-interactive content based on 10 immersive 360° videos with natural and relaxing scenarios. Immersive virtual reality. | Tablet (TAB) which played 10 non-interactive 2D videos depicting natural and relaxing scenarios. (4 days; to avoid a forced use of the device, the investigator did not specify a minimum or maximum usage time or number of sessions) | N: 80.36% (45/56) CG: 84.62% (22/26) EG: 74.07% (20/27) | N: 19.64% (11/56) CG: 15.38% (4/26) EG: 25.92% (7/27) | All dropouts were because of no autonomous device use | None found |
| Li et al., 2024 [17] | N: 327 CG: 125 EG: 163 | Breast cancer undergoing chemotherapy F 100% M 0% MA: 54.3 ± 9.6 CG: 53.6 ± 9.4 GE: 55.2 ± 9.7 | Relax VR intervention (during chemotherapy intervals/breaks and 15–20 min 1 to 2 sessions/week; 12 weeks) Relax VR intervention: two parts. Firstly, using a headset (VIVES110) and secondly using a hand controller. In the hospital supervised by nurses. Immersive virtual reality. | Traditional care during chemotherapy. They refrained from the beginning any VR treatment. | N: 75.23% (246/327) CG: 76.23% (125/164) EG: 74.23% (121/163) | N: 24.77% (81/327) CG: 23.78% (39/164) EG: 25.77% (42/163) | EG: intervention times < 12 (n = 10), physical problems (n = 15), loss of interest (n = 8), other reasons (n = 9) CG: Physical problems (n =17), loss of interest (n = 12), other reasons (n = 10) | Not indicated |
| Covariate (k) | Coefficient β (95% CI) 1 | p-Value |
|---|---|---|
| Age (5) | 0.001 (−0.06 to 0.09) | 0.64 |
| Cancer type: | ||
| Breast (4) | −0.26 (−1.38 to 0.85) | 0.55 |
| Experimental interventions: | ||
| Non-immersive virtual reality (3) | −0.77 (−2.30 to 0.77) | 0.23 |
| Immersive virtual reality (3) | 0.08 (−0.54 to 0.71) | 0.73 |
| Frequency of interventions (4) | 0.27 (−2.71 to 3.25) | 0.74 |
| Number of female participants (6) | 0.001 (−0.004 to 0.01) | 0.41 |
| Number of male participants (6) | 0.004 (−0.08 to 0.09) | 0.91 |
| Number of sessions (4) | 0.11 (−0.06 to 0.27) | 0.11 |
| Number of weeks of interventions (4) | 0.19 (−0.13 to 0.50) | 0.12 |
| Sample size (6) | 0.002(−0.004 to 0.009) | 0.39 |
| Summary of Findings | Certainty of Evidence Based on the GRADE Approach | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome | Studies (n/k) | Participants (N) | Risk of Bias | Inconsistency | Indirectness | Imprecision | Level of Evidence | Importance |
| Odds ratio meta-analysis | 6 | 569 | Very Serious 1 (−2) | No | Very Serious 2 (−2) | No | Very Low | Critical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-Muñoz, C.; Cortés-Vega, M.-D.; Martínez-Miranda, P. Dropout Rate of Participants with Cancer in Randomized Clinical Trials That Use Virtual Reality to Manage Pain—A Systematic Review with Meta-Analysis and Meta-Regression. Healthcare 2025, 13, 1708. https://doi.org/10.3390/healthcare13141708
García-Muñoz C, Cortés-Vega M-D, Martínez-Miranda P. Dropout Rate of Participants with Cancer in Randomized Clinical Trials That Use Virtual Reality to Manage Pain—A Systematic Review with Meta-Analysis and Meta-Regression. Healthcare. 2025; 13(14):1708. https://doi.org/10.3390/healthcare13141708
Chicago/Turabian StyleGarcía-Muñoz, Cristina, María-Dolores Cortés-Vega, and Patricia Martínez-Miranda. 2025. "Dropout Rate of Participants with Cancer in Randomized Clinical Trials That Use Virtual Reality to Manage Pain—A Systematic Review with Meta-Analysis and Meta-Regression" Healthcare 13, no. 14: 1708. https://doi.org/10.3390/healthcare13141708
APA StyleGarcía-Muñoz, C., Cortés-Vega, M.-D., & Martínez-Miranda, P. (2025). Dropout Rate of Participants with Cancer in Randomized Clinical Trials That Use Virtual Reality to Manage Pain—A Systematic Review with Meta-Analysis and Meta-Regression. Healthcare, 13(14), 1708. https://doi.org/10.3390/healthcare13141708







