The Adjunctive Role of Antimicrobial Photodynamic Therapy to Non-Surgical Treatment in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Focused Question and PICO
- -
- Patients = individuals with T2DM and periodontitis;
- -
- Intervention = aPDT combined with NSPT;
- -
- Comparison = NSPT (alone);
- -
- Outcome = clinical parameters.
- -
- A test group of patients with T2DM and periodontitis, receiving both aPDT and NSPT;
- -
- A control group of patients with T2DM and periodontitis, receiving only NSPT.
2.2. Eligibility Criteria
- Randomized controlled clinical trials (RCTs);
- Patients diagnosed with periodontitis;
- Patients diagnosed with T2DM, confirmed through glycosylated hemoglobin (HbA1c) measurement;
- Number of patients per group > 15;
- Primary clinical parameters: probing pocket depth (PPD), bleeding on probing (BoP).
- Studies conducted on animals;
- Studies related to dental implants;
- Studies regarding the systemic and/or topical administration of antibiotics.
2.3. Information Sources and Search Strategy
2.4. Study Selection, Screening, and Data Collection
2.5. Risk of Bias Assessment and Effect Measure
2.6. Data Synthesis and Certainty Assessment
3. Results
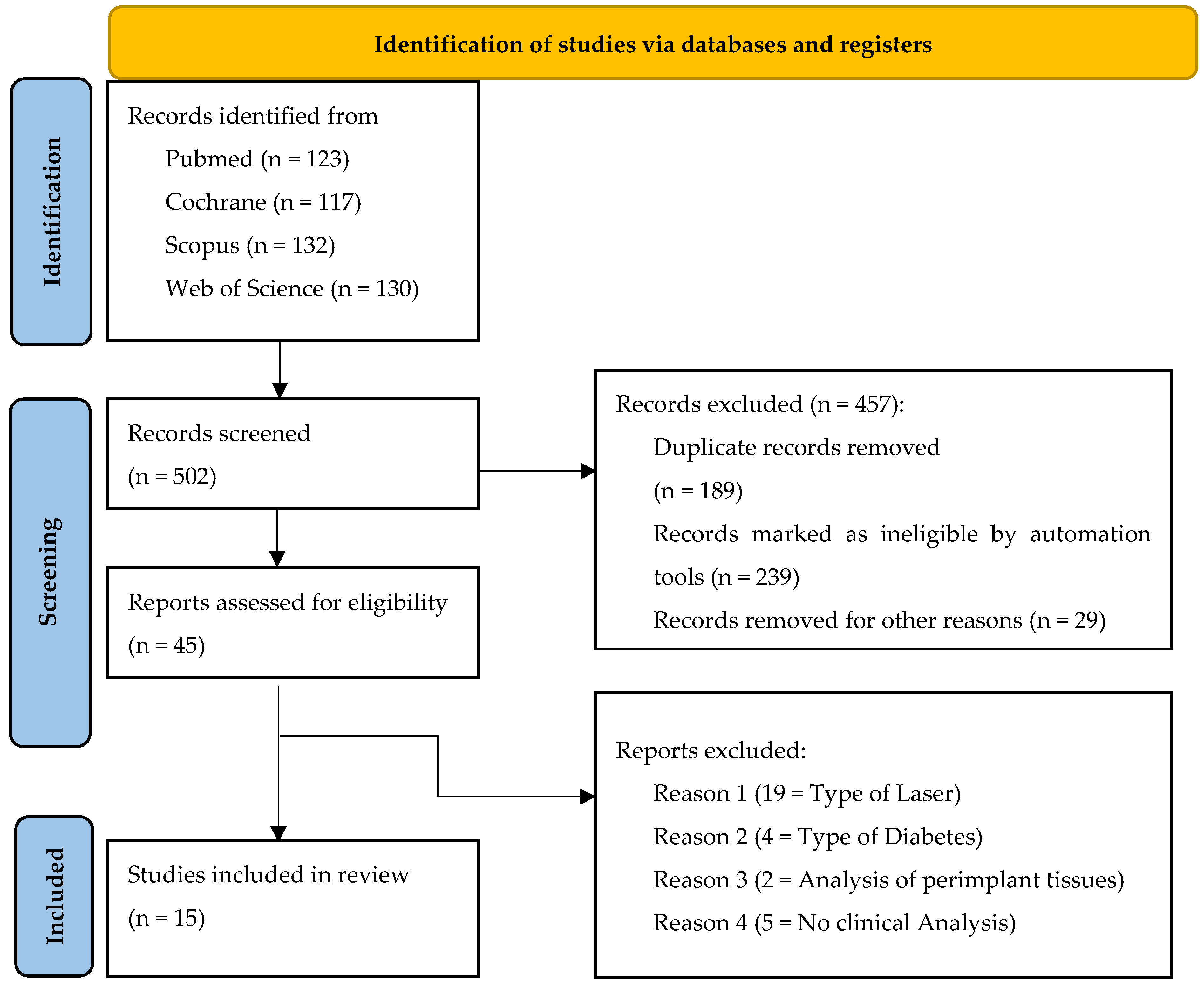
3.1. Study Selection
3.2. Study Characteristics
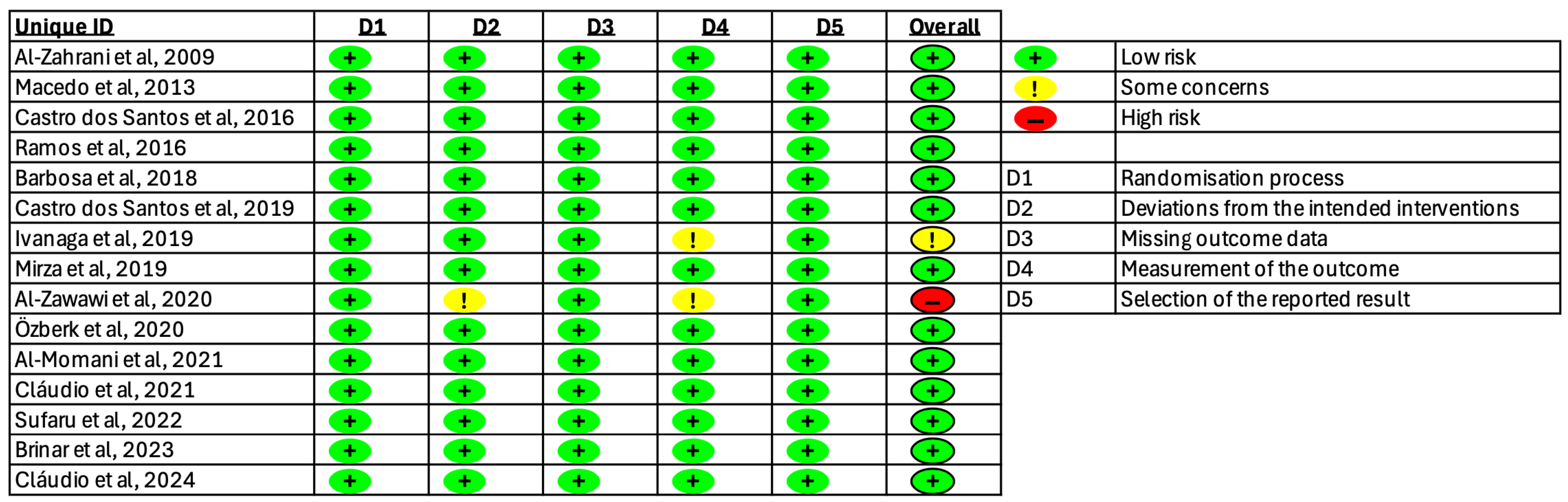
3.3. Risk of Bias
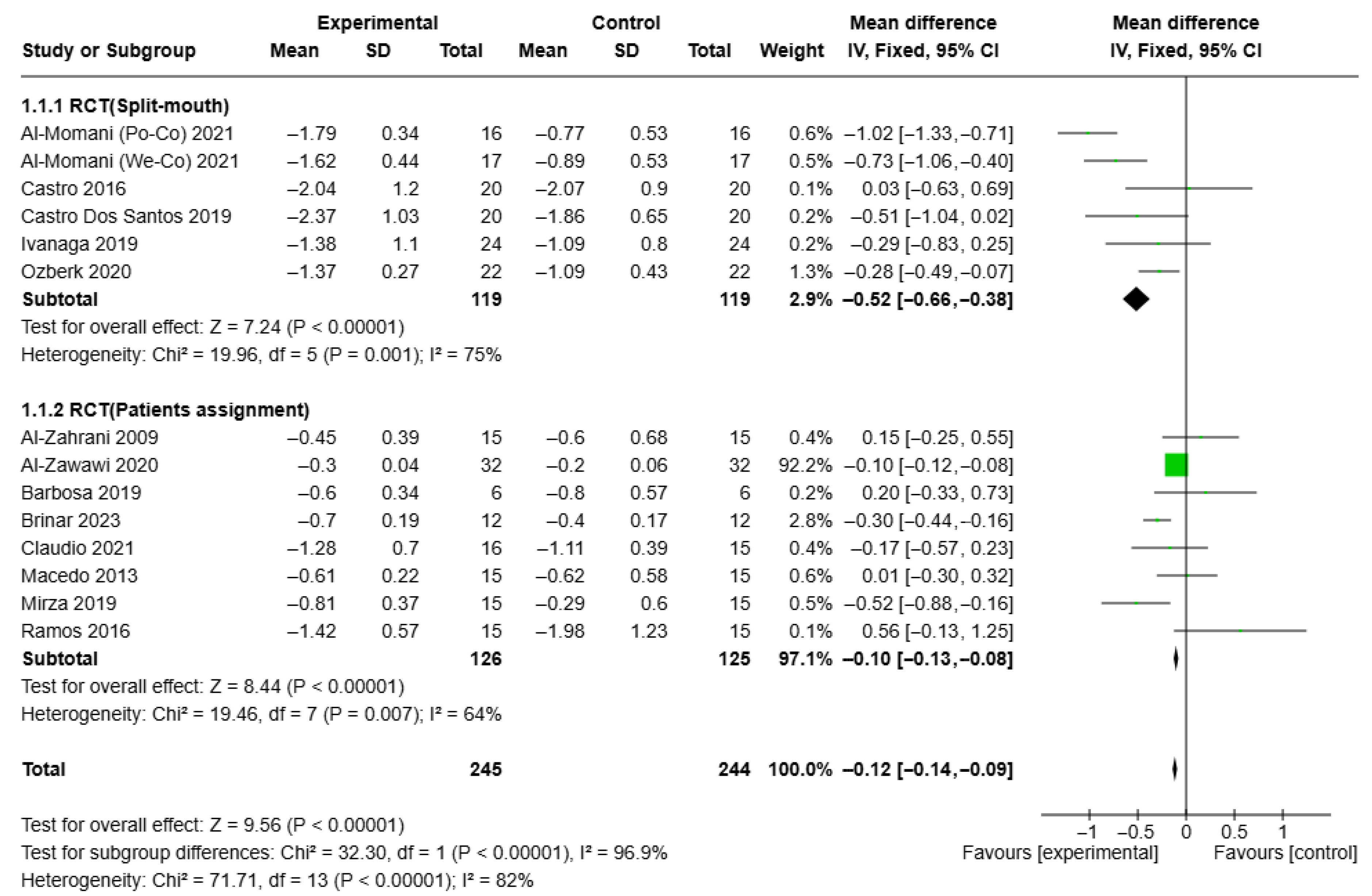
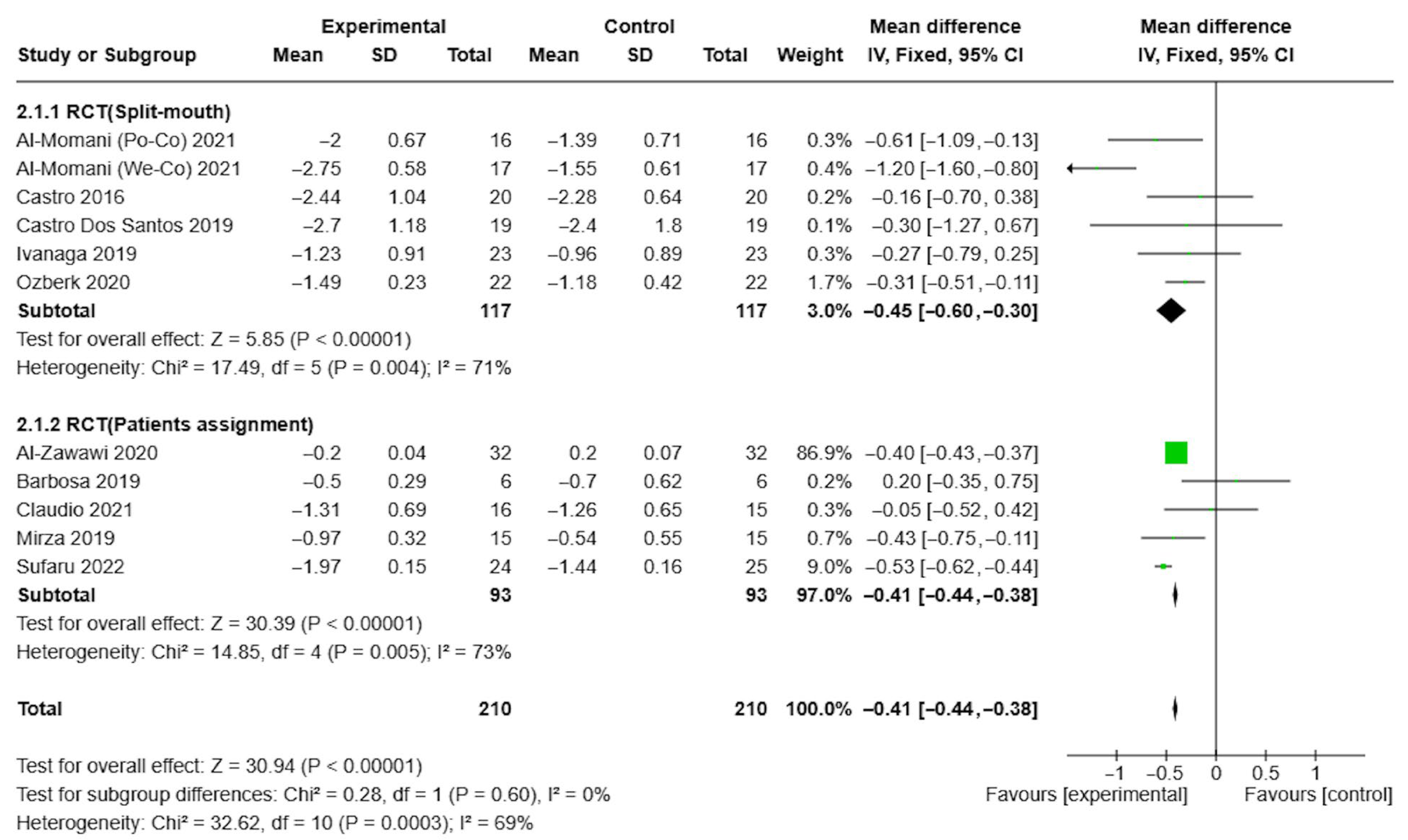
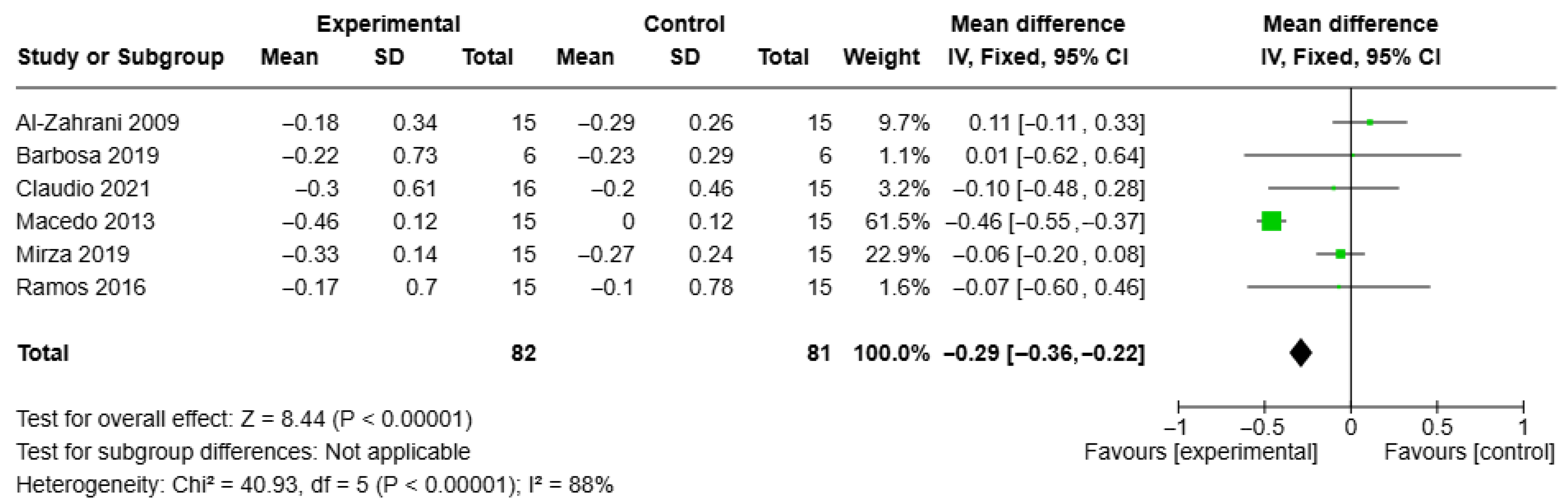
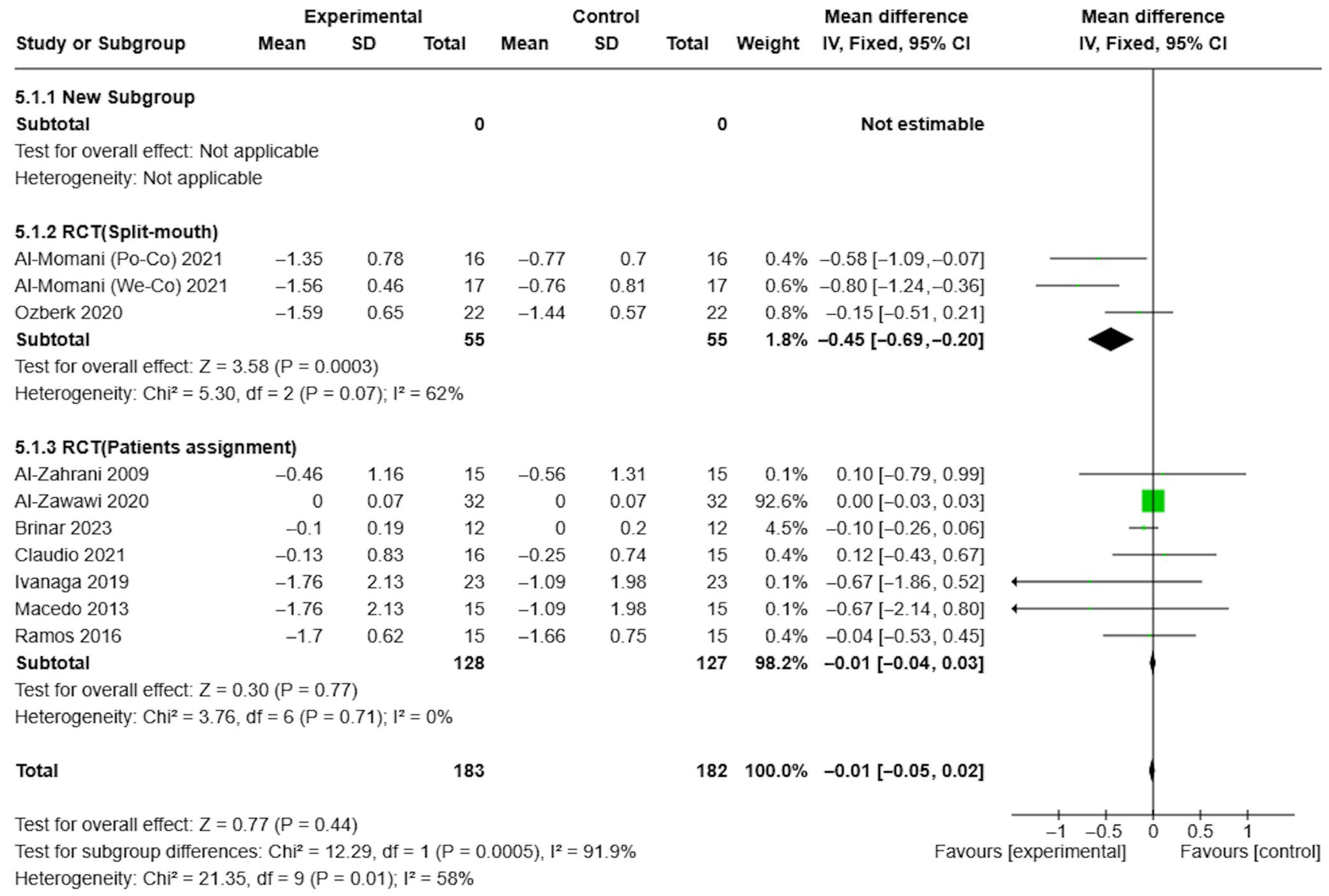
3.4. Results of Syntheses
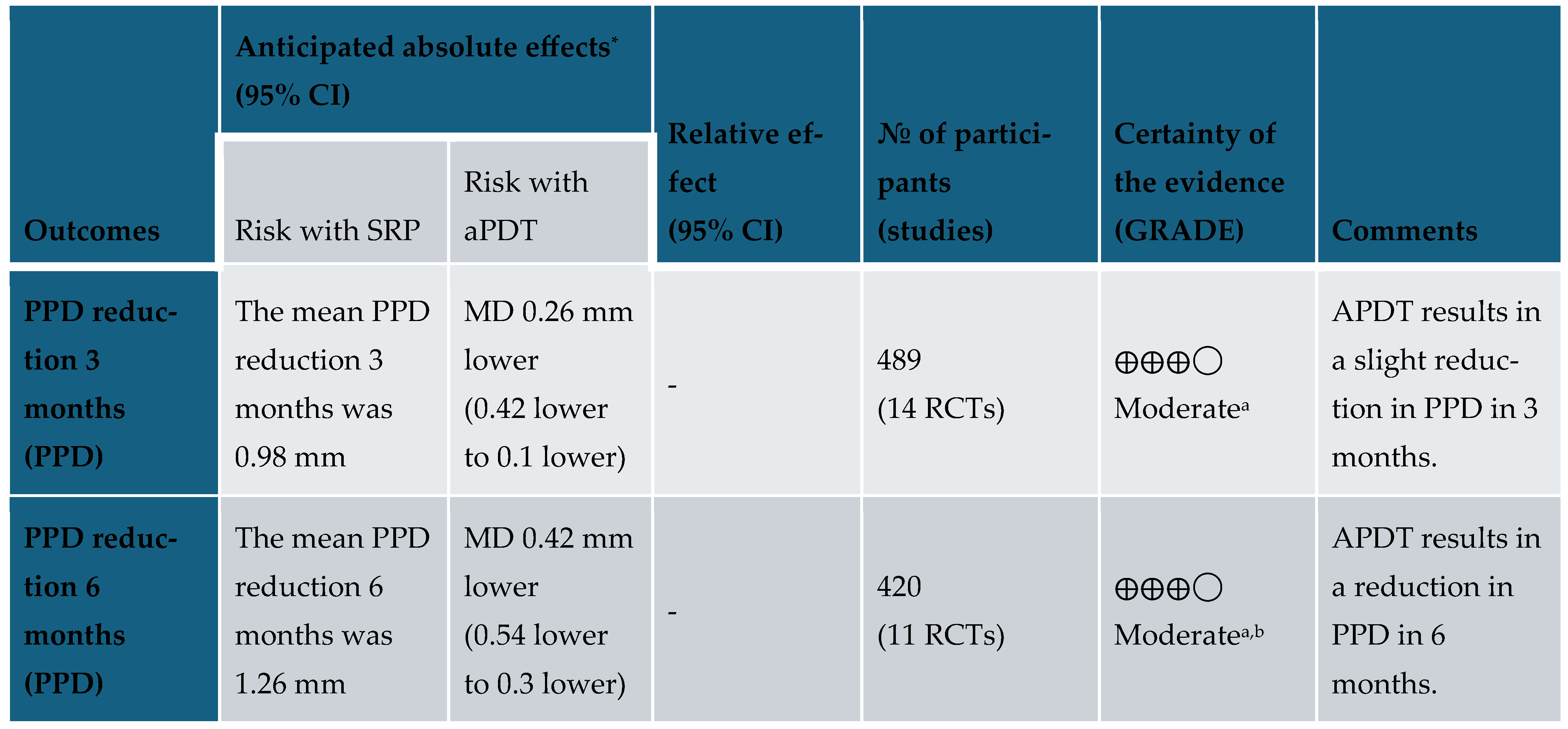
3.5. Certainty of Evidence
4. Discussion
Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Joseph, S.; Curtis, M.A. Microbial transitions from health to disease. Periodontol. 2000 2021, 86, 201–209. [Google Scholar] [CrossRef]
- Curtis, M.A.; Diaz, P.I.; Van Dyke, T.E. The role of the microbiota in periodontal disease. Periodontol. 2000 2020, 83, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.-Y.; Xiao, E.; Graves, D.T. Diabetes mellitus related bone metabolism and periodontal disease. Int. J. Oral. Sci. 2015, 7, 63–72. [Google Scholar] [CrossRef] [PubMed]
- Puppalwar, G.; Sawant, S.; Silgiri, B.; Shukla, K.; Barkate, H. Evaluation of Safety and Efficacy of Glaritus® versus Lantus® in Combination with Insulin Lispro among Adults with Type 1 Diabetes Mellitus-Phase IV Study. Open J. Endocr. Metab. Dis. 2017, 7, 111–125. [Google Scholar] [CrossRef]
- Graves, D.T.; Liu, R.; Oates, T.W. Diabetes-enhanced inflammation and apoptosis—Impact on periodontal pathosis. Periodontol. 2000 2007, 45, 128–137. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Diabetes as a potential risk for periodontitis: Association studies. Periodontol. 2000 2020, 83, 40–45. [Google Scholar] [CrossRef]
- Isola, G.; Matarese, G.; Ramaglia, L.; Pedullà, E.; Rapisarda, E.; Iorio-Siciliano, V. Association between periodontitis and glycosylated haemoglobin before diabetes onset: A cross-sectional study. Clin. Oral. Investig. 2020, 24, 2799–2808. [Google Scholar] [CrossRef]
- Polak, D.; Sanui, T.; Nishimura, F.; Shapira, L. Diabetes as a risk factor for periodontal disease—Plausible mechanisms. Periodontol. 2000 2020, 83, 46–58. [Google Scholar] [CrossRef]
- Alikhani, Z.; Alikhani, M.; Boyd, C.M.; Nagao, K.; Trackman, P.C.; Graves, D.T. Advanced Glycation End Products Enhance Expression of Pro-apoptotic Genes and Stimulate Fibroblast Apoptosis through Cytoplasmic and Mitochondrial Pathways. J. Biol. Chem. 2005, 280, 12087–12095. [Google Scholar] [CrossRef]
- Lalla, E.; Lamster, I.B.; Feit, M.; Huang, L.; Spessot, A.; Qu, W.; Kislinger, T.; Lu, Y.; Stern, D.M.; Schmidt, A.M. Blockade of RAGE suppresses periodontitis-associated bone loss in diabetic mice. J. Clin. Investig. 2000, 105, 1117–1124. [Google Scholar] [CrossRef]
- Liu, R.; Bal, H.; Desta, T.; Krothapalli, N.; Alyassi, M.; Luan, Q.; Graves, D. Diabetes Enhances Periodontal Bone Loss through Enhanced Resorption and Diminished Bone Formation. J. Dent. Res. 2006, 85, 510–514. [Google Scholar] [CrossRef]
- Olszewska-Czyz, I.; Michalak, E.; Dudzik, A. A Three-Month Clinical Trial on the Efficacy of Hyaluronic Acid Adjunctive Non-Surgical Therapy for Periodontitis in Patients with Type 2 Diabetes Mellitus. Biomedicines 2024, 12, 2516. [Google Scholar] [CrossRef]
- Monzavi, A.; Chinipardaz, Z.; Mousavi, M.; Fekrazad, R.; Moslemi, N.; Azaripour, A.; Bagherpasand, O.; Chiniforush, N. Antimicrobial photodynamic therapy using diode laser activated indocyanine green as an adjunct in the treatment of chronic periodontitis: A randomized clinical trial. Photodiagnosis Photodyn. Ther. 2016, 14, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.M. Clinical significance of non-surgical periodontal therapy: An evidence-based perspective of scaling and root planing. J. Clin. Periodontol. 2002, 29 (Suppl. S2), 6–16. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, S.; Kirchberger, S. Antimicrobial effects of dental ultrasound on periodontitis-associated bacteria. Int. J. Dent. Res. 1998, 77, 796. [Google Scholar]
- Nocini, R.; Lippi, G. Periodontal disease: The portrait of an epidemic. J. Public. Health Emerg. 2020, 4, 10. [Google Scholar] [CrossRef]
- Herrera, D.; Alonso, B.; León, R.; Roldán, S.; Sanz, M. Antimicrobial therapy in periodontitis: The use of systemic antimicrobials against the subgingival biofilm. J. Clin. Periodontol. 2008, 35, 45–66. [Google Scholar] [CrossRef]
- Cionca, N.; Giannopoulou, C.; Ugolotti, G.; Mombelli, A. Amoxicillin and Metronidazole as an Adjunct to Full-Mouth Scaling and Root Planing of Chronic Periodontitis. J. Periodontol. 2009, 80, 364–371. [Google Scholar] [CrossRef]
- Jepsen, K.; Jepsen, S. Antibiotics/antimicrobials: Systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol. 2000 2016, 71, 82–112. [Google Scholar] [CrossRef]
- Nagahara, A.; Mitani, A.; Fukuda, M.; Yamamoto, H.; Tahara, K.; Morita, I.; Ting, C.; Watanabe, T.; Fujimura, T.; Osawa, K.; et al. Antimicrobial photodynamic therapy using a diode laser with a potential new photosensitizer, indocyanine green-loaded nanospheres, may be effective for the clearance of Porphyromonas gingivalis. J. Periodontal Res. 2013, 48, 591–599. [Google Scholar] [CrossRef]
- Sukumar, K.; Tadepalli, A.; Parthasarathy, H.; Ponnaiyan, D. Evaluation of combined efficacy of photodynamic therapy using indocyanine green photosensitizer and non-surgical periodontal therapy on clinical and microbial parameters in the management of chronic periodontitis subjects: A randomized split-mouth design. Photodiagnosis Photodyn. Ther. 2020, 31, 101949. [Google Scholar] [CrossRef] [PubMed]
- Harmouche, L.; Courval, A.; Mathieu, A.; Petit, C.; Huck, O.; Severac, F.; Davideau, J.-L. Impact of tooth-related factors on photodynamic therapy effectiveness during active periodontal therapy: A 6-months split-mouth randomized clinical trial. Photodiagnosis Photodyn. Ther. 2019, 27, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Andersen, R.; Loebel, N.; Hammond, D.; Wilson, M. Treatment of periodontal disease by photodisinfection compared to scaling and root planing. J. Clin. Dent. 2007, 18, 34–38. [Google Scholar] [PubMed]
- Braun, A.; Dehn, C.; Krause, F.; Jepsen, S. Short-term clinical effects of adjunctive antimicrobial photodynamic therapy in periodontal treatment: A randomized clinical trial. J. Clin. Periodontol. 2008, 35, 877–884. [Google Scholar] [CrossRef]
- Lulic, M.; Görög, I.L.; Salvi, G.E.; Ramseier, C.A.; Mattheos, N.; Lang, N.P. One-year outcomes of repeated adjunctive photodynamic therapy during periodontal maintenance: A proof-of-principle randomized-controlled clinical trial. J. Clin. Periodontol. 2009, 36, 661–666. [Google Scholar] [CrossRef]
- Corrêa, M.G.; Oliveira, D.H.; Saraceni, C.H.C.; Ribeiro, F.V.; Pimentel, S.P.; Cirano, F.R.; Casarin, R.C.V. Short-term microbiological effects of photodynamic therapy in non-surgical periodontal treatment of residual pockets: A split-mouth RCT. Lasers Surg. Med. 2016, 48, 944–950. [Google Scholar] [CrossRef]
- Shingnapurkar, S.; Mitra, D.; Kadav, M.; Shah, R.; Rodrigues, S.; Prithyani, S. The effect of indocyanine green-mediated photodynamic therapy as an adjunct to scaling and root planing in the treatment of chronic periodontitis: A comparative split-mouth randomized clinical trial. Indian. J. Dent. Res. 2016, 27, 609. [Google Scholar] [CrossRef]
- Gandhi, K.; Pavaskar, R.; Cappetta, E.; Drew, H. Effectiveness of Adjunctive Use of Low-Level Laser Therapy and Photodynamic Therapy After Scaling and Root Planing in Patients with Chronic Periodontitis. Int. J. Periodontics Restor. Dent. 2019, 39, 837–843. [Google Scholar] [CrossRef]
- Azarpazhooh, A.; Shah, P.S.; Tenenbaum, H.C.; Goldberg, M.B. The Effect of Photodynamic Therapy for Periodontitis: A Systematic Review and Meta-Analysis. J. Periodontol. 2010, 81, 4–14. [Google Scholar] [CrossRef]
- Christodoulides, N.; Nikolidakis, D.; Chondros, P.; Becker, J.; Schwarz, F.; Rössler, R.; Sculean, A. Photodynamic Therapy as an Adjunct to Non-Surgical Periodontal Treatment: A Randomized, Controlled Clinical Trial. J. Periodontol. 2008, 79, 1638–1644. [Google Scholar] [CrossRef]
- Pourabbas, R.; Kashefimehr, A.; Rahmanpour, N.; Babaloo, Z.; Kishen, A.; Tenenbaum, H.C.; Azarpazhooh, A. Effects of Photodynamic Therapy on Clinical and Gingival Crevicular Fluid Inflammatory Biomarkers in Chronic Periodontitis: A Split-Mouth Randomized Clinical Trial. J. Periodontol. 2014, 85, 1222–1229. [Google Scholar] [CrossRef] [PubMed]
- Pulikkotil, S.; Toh, C.; Mohandas, K.; Leong, K. Effect of photodynamic therapy adjunct to scaling and root planing in periodontitis patients: A randomized clinical trial. Aust. Dent. J. 2016, 61, 440–445. [Google Scholar] [CrossRef] [PubMed]
- Segarra-Vidal, M.; Guerra-Ojeda, S.; Vallés, L.S.; López-Roldán, A.; Mauricio, M.D.; Aldasoro, M.; Alpiste-Illueca, F.; Vila, J.M. Effects of photodynamic therapy in periodontal treatment: A randomized, controlled clinical trial. J. Clin. Periodontol. 2017, 44, 915–925. [Google Scholar] [CrossRef]
- Theodoro, L.H.; Silva, S.P.; Pires, J.R.; Soares, G.H.G.; Pontes, A.E.F.; Zuza, E.P.; Spolidório, D.M.P.; de Toledo, B.E.C.; Garcia, V.G. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med. Sci. 2012, 27, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Thomas, J. Cochrane Handbook for Systematic Reviews of Interventions Version 6.5; John Wiley & Sons: Chichester, UK, 2024; Available online: https://training.cochrane.org/handbook (accessed on 1 January 2025).
- RoB 2: A Revised Cochrane Risk-Of-Bias Tool for Randomized Trials|Cochrane Bias (n.d.). Available online: https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials (accessed on 22 November 2023).
- RevMan Web. The Cochrane Collaboration. 2023. Available online: https://revman.cochrane.org (accessed on 1 January 2025).
- McMaster University and Evidence Prime, GRADEpro GDT: GRADEpro Guideline Development Tool. 2023. Available online: https://gradepro.org (accessed on 1 January 2025).
- Al-Zahrani, M.S.; Bamshmous, S.O.; Alhassani, A.A.; Al-Sherbini, M.M. Short-Term Effects of Photodynamic Therapy on Periodontal Status and Glycemic Control of Patients With Diabetes. J. Periodontol. 2009, 80, 1568–1573. [Google Scholar] [CrossRef]
- de Oliveira Macedo, G.; Novaes, A.B.; Souza, S.L.S.; Taba, M.; Palioto, D.B.; Grisi, M.F.M. Additional effects of aPDT on nonsurgical periodontal treatment with doxycycline in type II diabetes: A randomized, controlled clinical trial. Lasers Med. Sci. 2014, 29, 881–886. [Google Scholar] [CrossRef]
- dos Santos, N.C.C.; Andere, N.M.R.B.; Araujo, C.F.; de Marco, A.C.; dos Santos, L.M.; Jardini, M.A.N.; Santamaria, M.P. Local adjunct effect of antimicrobial photodynamic therapy for the treatment of chronic periodontitis in type 2 diabetics: Split-mouth double-blind randomized controlled clinical trial. Lasers Med. Sci. 2016, 31, 1633–1640. [Google Scholar] [CrossRef]
- Ramos, U.D.; Ayub, L.G.; Reino, D.M.; Grisi, M.F.; Taba, M.; Souza, S.L.; Palioto, D.B.; Novaes, A.B. Antimicrobial photodynamic therapy as an alternative to systemic antibiotics: Results from a double-blind, randomized, placebo-controlled, clinical study on type 2 diabetics. J. Clin. Periodontol. 2016, 43, 147–155. [Google Scholar] [CrossRef]
- Barbosa, F.I.; Araújo, P.V.; Machado, L.J.C.; Magalhães, C.S.; Guimarães, M.M.M.; Moreira, A.N. Effect of photodynamic therapy as an adjuvant to non-surgical periodontal therapy: Periodontal and metabolic evaluation in patients with type 2 diabetes mellitus. Photodiagnosis Photodyn. Ther. 2018, 22, 245–250. [Google Scholar] [CrossRef]
- dos Santos, N.C.; Andere, N.M.R.B.; Miguel, M.M.V.; dos Santos, L.M.; Santamaria, M.; Mathias, I.F.; Jardini, M.A.N.; Santamaria, M.P. Photobiomodulation for the treatment of periodontal pockets in patients with type 2 diabetes: 1-year results of a randomized clinical trial. Lasers Med. Sci. 2019, 34, 1897–1904. [Google Scholar] [CrossRef]
- Ivanaga, C.A.; Miessi, D.M.J.; Nuernberg, M.A.A.; Claudio, M.M.; Garcia, V.G.; Theodoro, L.H. Antimicrobial photodynamic therapy (aPDT) with curcumin and LED, as an enhancement to scaling and root planing in the treatment of residual pockets in diabetic patients: A randomized and controlled split-mouth clinical trial. Photodiagnosis Photodyn. Ther. 2019, 27, 388–395. [Google Scholar] [CrossRef]
- Mirza, S.; Khan, A.A.; Al-Kheraif, A.A.; Khan, S.Z.; Shafqat, S.S. Efficacy of adjunctive photodynamic therapy on the clinical periodontal, HbA1c and advanced glycation end product levels among mild to moderate chronic periodontal disease patients with type 2 diabetes mellitus: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2019, 28, 177–182. [Google Scholar] [CrossRef] [PubMed]
- Al-Zawawi, A.S.; Bukhari, I.A.; Bello-Correa, F.O.; Sheikh, S.A.; Albaijan, R.; Vohra, F. Influence of root debridement with adjunct photodynamic therapy on periodontal parameters and gingival crevicular fluid cortisol levels among patients with and without type-2 diabetes mellitus. Photodiagnosis Photodyn. Ther. 2020, 32, 102076. [Google Scholar] [CrossRef]
- Özberk, S.S.; Gündoğar, H.; Özkaya, M.; Taner, İ.L.; Erciyas, K. The effect of photobiomodulation therapy on nonsurgical periodontal treatment in patients with type 2 diabetes mellitus: A randomized controlled, single-blind, split-mouth clinical trial. Lasers Med. Sci. 2020, 35, 497–504. [Google Scholar] [CrossRef]
- Al-Momani, M.M. Indocyanine-mediated antimicrobial photodynamic therapy promotes superior clinical effects in stage III and grade C chronic periodontitis among controlled and uncontrolled diabetes mellitus: A randomized controlled clinical trial. Photodiagnosis Photodyn. Ther. 2021, 35, 102379. [Google Scholar] [CrossRef]
- Cláudio, M.M.; Nuernberg, M.A.A.; Rodrigues, J.V.S.; Belizário, L.C.G.; Batista, J.A.; Duque, C.; Garcia, V.G.; Theodoro, L.H. Effects of multiple sessions of antimicrobial photodynamic therapy (aPDT) in the treatment of periodontitis in patients with uncompensated type 2 diabetes: A randomized controlled clinical study. Photodiagnosis Photodyn. Ther. 2021, 35, 102451. [Google Scholar] [CrossRef]
- Sufaru, I.-G.; Martu, M.-A.; Luchian, I.; Stoleriu, S.; Diaconu-Popa, D.; Martu, C.; Teslaru, S.; Pasarin, L.; Solomon, S.M. The Effects of 810 nm Diode Laser and Indocyanine Green on Periodontal Parameters and HbA1c in Patients with Periodontitis and Type II Diabetes Mellitus: A Randomized Controlled Study. Diagnostics 2022, 12, 1614. [Google Scholar] [CrossRef]
- Brinar, S.; Skvarča, A.; Gašpirc, B.; Schara, R. The effect of antimicrobial photodynamic therapy on periodontal disease and glycemic control in patients with type 2 diabetes mellitus. Clin. Oral. Investig. 2023, 27, 6235–6244. [Google Scholar] [CrossRef]
- Cláudio, M.M.; Garcia, V.G.; Freitas, R.M.; Rodrigues, J.V.S.; Wainwright, M.; Casarin, R.C.V.; Duque, C.; Theodoro, L.H. Association of active oxygen-releasing gel and photodynamic therapy in the treatment of residual periodontal pockets in type 2 diabetic patients: A randomized controlled clinical study. J. Periodontol. 2024, 95, 360–371. [Google Scholar] [CrossRef]
- Mauri-Obradors, E.; Merlos, A.; Estrugo-Devesa, A.; Jané-Salas, E.; López-López, J.; Viñas, M. Benefits of non-surgical periodontal treatment in patients with type 2 diabetes mellitus and chronic periodontitis: A randomized controlled trial. J. Clin. Periodontol. 2018, 45, 345–353. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Khader, Y.S.; Dauod, A.S.; El-Qaderi, S.S.; Alkafajei, A.; Batayha, W.Q. Periodontal status of diabetics compared with nondiabetics: A meta-analysis. J. Diabetes Complicat. 2006, 20, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Moore, P.A.; Weyant, R.J.; Mongelluzzo, M.B.; Myers, D.E.; Rossie, K.; Guggenheimer, J.; Block, H.M.; Huber, H.; Orchard, T. Type 1 Diabetes Mellitus and Oral Health: Assessment of Periodontal Disease. J. Periodontol. 1999, 70, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Lalla, E.; Lamster, I.B.; Drury, S.; Fu, C.; Schmidt, A.M. Hyperglycemia, glycoxidation and receptor for advanced glycation endproducts: Potential mechanisms underlying diabetic complications, including diabetes-associated periodontitis. Periodontol. 2000 2000, 23, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Assuma, R.; Oates, T.; Cochran, D.; Amar, S.; Graves, D.T. IL-1 and TNF antagonists inhibit the inflammatory response and bone loss in experimental periodontitis. J. Immunol. 1998, 160, 403–409. [Google Scholar] [CrossRef]
- Nocini, R.; Favaloro, E.J.; Sanchis-Gomar, F.; Lippi, G. Periodontitis, coronary heart disease and myocardial infarction: Treat one, benefit all. Blood Coagul. Fibrinolysis 2020, 31, 339–345. [Google Scholar] [CrossRef]
- Pardo, A.; Barilli, A.; Signoriello, A.; Gualtieri, M.; Brancato, G.; Colapinto, G.; Lombardo, G.; Albanese, M. Oral health conditions and hygiene procedures in patients with Parkinson’s disease: A systematic review. Explor. Med. 2024, 5, 852–869. [Google Scholar] [CrossRef]
- Taylor, G.W. Bidirectional Interrelationships Between Diabetes and Periodontal Diseases: An Epidemiologic Perspective. Ann. Periodontol. 2001, 6, 99–112. [Google Scholar] [CrossRef]
- Tervonen, T.; Karjalainen, K. Periodontal disease related to diabetic status. J. Clin. Periodontol. 1997, 24, 505–510. [Google Scholar] [CrossRef]
- Taylor, G.W.; Burt, B.A.; Becker, M.P.; Genco, R.J.; Shlossman, M. Glycemic Control and Alveolar Bone Loss Progression in Type 2 Diabetes. Ann. Periodontol. 1998, 3, 30–39. [Google Scholar] [CrossRef]
- Brownlee, M. The Pathobiology of Diabetic Complications. Diabetes 2005, 54, 1615–1625. [Google Scholar] [CrossRef]
- Kato, H.; Taguchi, Y.; Tominaga, K.; Umeda, M.; Tanaka, A. Porphyromonas gingivalis LPS inhibits osteoblastic differentiation and promotes pro-inflammatory cytokine production in human periodontal ligament stem cells. Arch. Oral. Biol. 2014, 59, 167–175. [Google Scholar] [CrossRef]
- Sgolastra, F.; Severino, M.; Pietropaoli, D.; Gatto, R.; Monaco, A. Effectiveness of Periodontal Treatment to Improve Metabolic Control in Patients With Chronic Periodontitis and Type 2 Diabetes: A Meta-Analysis of Randomized Clinical Trials. J. Periodontol. 2013, 84, 958–973. [Google Scholar] [CrossRef]
- Graziani, F.; Gennai, S.; Solini, A.; Petrini, M. A systematic review and meta-analysis of epidemiologic observational evidence on the effect of periodontitis on diabetes An update of the EFP-AAP review. J. Clin. Periodontol. 2018, 45, 167–187. [Google Scholar] [CrossRef] [PubMed]
- Kıran, M.; Arpak, N.; Ünsal, E.; Erdoğan, M.F. The effect of improved periodontal health on metabolic control in type 2 diabetes mellitus. J. Clin. Periodontol. 2005, 32, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Yun, F.; Firkova, E.I.; Jun-Qi, L.; Xun, H. Effect of non-surgical periodontal therapy on patients with type 2 diabetes mellitus. Folia Med. 2007, 49, 32–36. [Google Scholar]
- Simpson, T.C.; Clarkson, J.E.; Worthington, H.V.; MacDonald, L.; Weldon, J.C.; Needleman, I.; Iheozor-Ejiofor, Z.; Wild, S.H.; Qureshi, A.; Walker, A.; et al. Treatment of periodontitis for glycaemic control in people with diabetes mellitus. Cochrane Database Syst. Rev. 2022, 2022, CD004714. [Google Scholar] [CrossRef]
- Teshome, A.; Yitayeh, A. The effect of periodontal therapy on glycemic control and fasting plasma glucose level in type 2 diabetic patients: Systematic review and meta-analysis. BMC Oral. Health 2017, 17, 31. [Google Scholar] [CrossRef]
- Lee, J.Y.; Choi, Y.Y.; Choi, Y.; Jin, B.H. Efficacy of non-surgical treatment accompanied by professional toothbrushing in the treatment of chronic periodontitis in patients with type 2 diabetes mellitus: A randomized controlled clinical trial. J. Periodontal Implant. Sci. 2020, 50, 83. [Google Scholar] [CrossRef]
- Carvalho, V.F.; Andrade, P.V.C.; Rodrigues, M.F.; Hirata, M.H.; Hirata, R.D.C.; Pannuti, C.M.; De Micheli, G.; Conde, M.C. Antimicrobial photodynamic effect to treat residual pockets in periodontal patients: A randomized controlled clinical trial. J. Clin. Periodontol. 2015, 42, 440–447. [Google Scholar] [CrossRef]
- Hopp, M.; Biffar, R. Photodynamic therapies–blue versus green. Int. Mag. Laser Dent. 2013, 5, 10–25. [Google Scholar]
- Boehm, T.K.; Ciancio, S.G. Diode laser activated indocyanine green selectively kills bacteria. J. Int. Acad. Periodontol. 2011, 13, 58–63. [Google Scholar]
- Abduljabbar, T.; Vohra, F.; Javed, F.; Akram, Z. Antimicrobial photodynamic therapy adjuvant to non-surgical periodontal therapy in patients with diabetes mellitus: A meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 17, 138–146. [Google Scholar] [CrossRef]
- Al-Hamoudi, N. Is antimicrobial photodynamic therapy an effective treatment for chronic periodontitis in diabetes mellitus and cigarette smokers: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2017, 19, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Pardo, A.; Butera, A.; Giordano, A.; Gallo, S.; Pascadopoli, M.; Scribante, A.; Albanese, M. Photodynamic Therapy in Non-Surgical Treatment of Periodontitis: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13 (Suppl. S2), 1086. [Google Scholar] [CrossRef]
- Lombardo, G.; Pardo, A.; Signoretto, C.; Signoriello, A.; Simeoni, E.; Rovera, A.; Nocini, P.F. Hyperbaric oxygen therapy for the treatment of moderate to severe periodontitis: A clinical pilot study. Undersea Hyperb. Med. 2020, 47, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Souza, E.Q.M.; da Rocha, T.E.; Toro, L.F.; Guiati, I.Z.; Ervolino, E.; Garcia, V.G.; Wainwright, M.; Theodoro, L.H. Antimicrobial photodynamic therapy compared to systemic antibiotic therapy in non-surgical treatment of periodontitis: Systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2020, 31, 101808. [Google Scholar] [CrossRef]
- Zhao, Y.; Pu, R.; Qian, Y.; Shi, J.; Si, M. Antimicrobial photodynamic therapy versus antibiotics as an adjunct in the treatment of periodontitis and peri-implantitis: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2021, 34, 102231. [Google Scholar] [CrossRef]
- Polansky, R.; Haas, M.; Heschl, A.; Wimmer, G. Clinical effectiveness of photodynamic therapy in the treatment of periodontitis. J. Clin. Periodontol. 2009, 36, 575–580. [Google Scholar] [CrossRef]
- Pardo, A.; Bonfante, L.; Signoriello, A.; Benetti, A.; Barillari, M.; Zanutto, P.; Lombardo, G. Treatment of Intrabony Defects with Non-Surgical Subgingival Debridement: A Radiographic Evaluation of Bone Gain Using an Experimental Digital Software “Bone Defect Analysis (BDA)”. J. Clin. Med. 2024, 13, 4315. [Google Scholar] [CrossRef]
- Akram, Z.; Raffat, M.A.; Shafqat, S.S.; Mirza, S.; Ikram, S. Clinical efficacy of photodynamic therapy as an adjunct to scaling and root planing in the treatment of chronic periodontitis among cigarette smokers: A systematic review and meta-analysis. Photodiagnosis Photodyn. Ther. 2019, 26, 334–341. [Google Scholar] [CrossRef]
- Radvar, M.; MacFarlane, T.W.; MacKenzie, D.; Whitters, C.J.; Payne, A.P.; Kinane, D.F. An evaluation of the Nd:YAG laser in periodontal pocket therapy. Br. Dent. J. 1996, 180, 57–62. [Google Scholar] [CrossRef]
- Sterne, A.C.; Sutton, A.J.; Ioannidis, J.P.A.; Terrin, N.; Jones, D.R.; Lau, J.; Carpenter, J.; Rücker, G.; Harbord, R.M.; Schmid, C.H.; et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 2011, 343, d4002. [Google Scholar] [CrossRef]
| Authors | Country | Study Design | HbA1c | Groups in the Study | PPD | Parameters | Follow-Up (Months) | |
|---|---|---|---|---|---|---|---|---|
| Test (n) | Control (n) | |||||||
| Al-Zahrani et al. 2009 [39] | Saudi Arabia | RCT, patient assignment | SRP + aPDT (15) aPDT: non-thermal Diode Laser 670 nm, 0.01% Methylene blue for 60 s, SRP in 4 sessions. | SRP (15) SRP + doxycycline (15) | SRP only Baseline 3.24 ± 0.66; 3 months 2.64 ± 0.70; p = 0.001 SRP + doxycycline Baseline 3.26 ± 0.45; 3 months 2.82 ± 0.23; p = 0.001 SRP + aPDT Baseline 3.00 ± 0.46; 3 months 2.55 ± 0.33; p = 0.001 | BoP Baseline (%): test 0.72 ± 0.24 control 0.72 ± 0.24 3 months test 0.54 ± 0.39 control 0.43 ± 0.28 PI Plaque Index (%) Baseline: test 0.90 ± 0.19 control 0.86 ± 0.14 3 months test 0.56 ± 0.33 control 0.59 ± 0.17 CAL Baseline (mm): test 4.33 ± 1.15 control 4.66 ± 1.32 3 months test 3.87 ± 1.16 control 4.10 ± 1.30 | 3 | |
| Macedo et al. 2013 [40] | Brazil | RCT, patient assignment | >7% | SRP + aPDT (15) aPDT: Diode Laser 660 nm with Chloride Photosensitizer in a concentration of 10 mg/mL. Exposition 10 s each site. A total of 60 mW of power and power density of 28 mW/cm2 with an optic fiber with a diameter of 0.6 mm. | SRP (15) | Baseline Test: 2.43 ± 0.27 Control: 2.54 ± 0.53 p = 0.05 3 months Test: 1.82 ± 0.19 Control: 1.92 ± 0.62 p = 0.05 | BoP Baseline (%): test 60.42 ± 12.2 control 64.5 ± 12.5 3 months test 14.7 ± 12 control 64.5 ± 12.5 PI Plaque Index (%) Baseline: test 50.24 ± 19.41 control 63.72 ± 16.92 3 months test 33.03 ± 43.52 control 45.11 ± 42.14 6 months test 29.78 ± 41.18 control 38.75 ± 40.22 CAL Baseline (mm): test 6.71 ± 1.85 control 6.63 ± 1.66 3 months test 4.95 ± 2.33 control 5.54 ± 2.19 | 3 |
| Castro dos Santos et al. 2016 [41] | Brazil | RCT, split mouth | from 6.5 to 11% | SRP + aPDT (20) aPDT: 0.005% Methylene blue for 60 s. Diode Laser irradiation for 60 s with a fiber optic of 0.6 mm. Power of 60 mW, irradiance of 2.15 W/cm2. Total energy delivered of 3.6 J and fluency of 1 J/cm2. | SRP (20) | Baseline Test: 6.15 ± 1.27 Control: 5.75 ± 0.91 p = 0.25 1 month Test: 4.11 ± 1.40 Control: 3.68 ± 0.89 p = 0.10 3 months Test: 3.78 ± 1.61 Control: 3.89 ± 0.99 p = 0.74 6 months Test: 3.71 ± 1.63 Control: 3.47 ± 0.97 p = 0.25 | GI Baseline: - CAL Baseline: test 4.61 ± 1.92 control 6.35 ± 1.27 6 months test 35.4 ± 3.2 control 4.26 ± 1.30 | 1–3–6 |
| Ramos et al. 2016 [42] | Brazil | RCT, patient assignment | >7% | SRP + aPDT (15) aPDT: Phenothiazine Chloride solution 10 mg/mL for 5 min. Red Laser HELBO for 10 s for each site. A total of 70 mW of power and a power density of 28 mW/cm2. Optic fiber with 0.06 mm diameter, delivering a total energy of 2.79 J/cm2 per site. | SRP + doxycycline (15) | Baseline Test: 4.89 ± 0.68 Control: 4.86 ± 1.39 p = 0.05 3 months Test: 3.47 ± 0.58 Control: 2.88 ± 0.45 p = 0.05 | BoP Baseline: test 28.13 ± 7.79 control 23.53 ± 9.33 3 months test 11.50 ± 5.41 control 13.47 ± 4.62 Plaque Index: NR CAL baseline: test 5.60 ± 0.68 control 5.53 ± 0.86 3 months test 3.90 ± 0.52 control 3.87 ± 0.52 | 1–3 |
| Barbosa et al. 2018 [43] | Brazil | RCT, patient assignment | Values below 7% measured no more than 90 days prior to selection | SRP + aPDT (6) aPDT: Methylene blue ta 10 mg/mL for 5 min. Red Diode laser 660 nm (TwinFlex). Application for 2 min. The power was 40 mW; the total energy delivered per tooth was 4.8 J. | SRP (6) | Baseline Test: 2.3 ± 0.4 Control: 2.9 ± 0.5 p = 0.1 1 months Test: 1.6 ± 0.2 Control: 2.0 ± 0.6 p = 0.1 3 months Test: 1.7 ± 0.2 Control: 2.1 ± 0.7 p = 0.2 6 months Test: 1.8 ± 0.3 Control: 2.2 ± 0.7 p = 0.3 | BoP Baseline: test 43.9 ± 16.9 control 55.5 ± 33.1 3 months test 22.4 ± 8.2 control 31.7 ± 13.9 6 months test 18.7 ± 1.5 control 32.1 ± 11.2 Plaque Index Baseline: test 31.4 ± 3 control 32.4 ± 2.2 3 months test 35.4 ± 3.2 control 36.1 ± 4.6 6 months test 31.5 ± 6.2 control 35.4 ± 4.7 | 1–3–6 |
| Castro Dos Santos et al. 2019 [44] | Brazil | RCT, split mouth | from 6.5% to 11% | SRP + aPDT (19) aPDT: GaALAs (Gallium Aluminium Arsenide) Diode Laser of 809 nm; power of 1 W; for 10 s; Optic fiber of 0.06 mm | SRP (19) | Baseline Test: 6.6 ± 1.6 Control: 6.5 ± 2.0 p = 0.36 3 months Test: 4.4 ± 1.2 Control: 4.7 ± 1.9 p = 0.65 6 months Test: 3.9 ± 1.4 Control: 4.1 ± 1.5 p = 0.65 12 months Test: 3.6 ± 1.5 Control: 3.8 ± 1.6 p = 0.49 | BoP: NR Plaque Index: NR CAL baseline: Δ12 months control 2.0 ± 1.8 test 2.5 ± 2.1 | 3–6–12 |
| Ivanaga et al. 2019 [45] | Brazil | RCT, split mouth | ≥6.5% | SRP + aPDT (24) aPDT: Wave Diode Laser with a fiber optic of 0.06 mm in diameter, a wavelength of 600 nm, power of 0.03 W, fluency of 22 J/cm2, an area of 0.028 cm2, irradiation of 1.1 W/cm2, total energy of 0.6 J. Photosensitizer: NS. | SRP (24) | Baseline Test: 5.71 ± 0.92 Control: 5.67 ± 0.78 p > 0.05 3 months Test: 4.33 ± 1.78 Control: 4.58 ± 1.24 p > 0.05 6 months Test: 4.47 ± 1.40 Control: 4.70 ± 1.37 p > 0.05 | BoP Baseline (%): test 100 control 100 3 months test 42.60 ± 44.23 control 48.26 ± 38.53 6 months test 34.99 ± 40.33 control 30.64 ± 34.50 PI Plaque Index (%) Baseline: test 68.24 ± 38.23 control 75.61 ± 32.22 3 months test 33.03 ± 43.52 control 45.11 ± 42.14 6 months test 29.78 ± 41.18 control 38.75 ± 40.22 CAL Baseline (mm): test 6.71 ± 1.85 control 6.63 ± 1.66 3 months test 4.95 ± 2.33 control 5.54 ± 2.19 6 months test 34.99 ± 40.33 control 30.64 ± 34.50 | 3–6 |
| Mirza et al. 2019 [46] | Pakistan, Saudi Arabia, Japan | RCT, patient assignment | ≥6.5% | SRP + aPDT (15) aPDT: Diode Laser HELBO 670 nm, power of 150 mW, fluency of 22 J/cm2, and density of 1.1 W/cm2. Methylene blue with 0.005% concentration. | SRP (15) | Baseline Test: 3.53 ± 0.42 Control: 3.23 ± 0.61 3 months Test: 2.72 ± 0.38 (p value: 0.01 between baseline and 3 months) Control: 2.94 ± 0.59 (p value: 0.05 between baseline and 3 months) 6 months Test: 2.56 ± 0.41 (p value: 0.05 between baseline and 6 months) Control: 2.69 ± 0.46 (p value: 0.05 between baseline and 6 months) | BoP Baseline (%): test 0.82 ± 0.12 control 0.75 ± 0.22 3 months test 0.49 ± 0.16 control 0.48 ± 0.25 6 months test 0.41 ± 0.20 control 0.39 ± 0.29 PI Plaque Index (%) Baseline: test 0.91 ± 0.17 control 0.89 ± 0.16 3 months test 0.66 ± 0.19 control 0.59 ± 0.18 6 months test 0.56 ± 0.15 control 0.51 ± 0.19 CAL: NR | 3–6 |
| Al-Zawawi et al. 2020 [47] | Saudi Arabia | RCT, patient assignment | Test Group Values at Baseline 4.3 ± 0.04 Test Group Values at 6 month’s follow-up 4.2 ± 0.1 Control Group Values at Baseline 4.3 ± 0.05 Control Group Values at 6 months’ follow up 4.2 ± 0.05 | SRP + aPDT (32) aPDT: Methylene blue 0.005%. Diode Laser 660 nm and 150 mW, irradiation was for 60 s with a fiber optic of 0.03 mm diameter. | SRP (32) | Baseline Test: 5.2 ± 0.06 Control: 4.7 ± 0.1 mm 3 months Test: 4.9 ± 0.03 mm Control: 4.5 ± 0.07 mm 6 months Test: 5.0 ± 0.05 mm Control: 4.9 ± 0.1 mm | PI: Baseline Test 3.2 ± 0.08 Control 3.6 ± 0.05 3 months: Test 2.8 ± 0.1 Control 3.4 ± 0.02 6 months: Test 3.0 ± 0.05 Control 3.2 ± 0.03 GI: Baseline, Test 3.6 ± 0.02 Control 3.5 ± 0.04 3 months: Test 3.2 ± 0.04 Control 3.3 ± 0.06 6 months: Test 3.5 ± 0.05 mm Control 3.4 ± 0.04 CAL: Baseline Test 4.2 ± 0.06 mm Control 4.5 ± 0.05 mm 3 months: Test 4.2 ± 0.08 mm Control 4.5 ± 0.08 mm 6 months: Test 5.0 ± 0.05 mm Control 4.4 ± 0.006 mm | 3–6 |
| Özberk et al. 2020 [48] | Turkey | RCT, split mouth | ≥7% | SRP + aPDT (22) aPDT: GaAlAs Diode Laser at the 980 nm wavelength (CHEESETM GIGAA). Used for 15 s continuously at 0.4 W power. Energy density 0.5 J/cm2. | SRP (22) | Baseline Test: 4.09 ± 0.29 Control: 4.08 ± 0.44 1 months Test: 2.94 ± 0.29 Control: 3.11 ± 0.48 p = 0.05 3 months Test: 2.72 ± 0.42 Control: 2.99 ± 0.42 p = 0.05 6 months Test: 2.60 ± 0.30 Control: 2.90 ± 0.40 p = 0.05 | GI Baseline (%): test 1.92 ± 0.17 control 1.91 ± 0.14 3 months test 0.40 ± 0.15 control 0.52 ± 0.17 6 months test 0.38 ± 0.10 control 0.48 ± 0.16 PI Plaque Index (%) Baseline: test 2.88 ± 0.68 control 2.89 ± 0.60 3 months test 0.54 ± 0.15 control 0.59 ± 0.16 6 months test 0.52 ± 0.12 control 0.54 ± 0.15 CAL Baseline (mm): test 4.52 ± 0.75 control 4.50 ± 0.65 3 months test 2.93 ± 0.36 control 3.06 ± 0.41 6 months test 2.79 ± 0.32 control 3.00 ± 0.39 | 1–3–6 |
| Al-Momani. 2021 [49] | Saudi Arabia | RCT, split mouth | DM Controlled 6–10% DM Uncontrolled >10% | SRP + aPDT DM Controlled (17) DM Uncontrolled (16) aPDT: ICG solution at a concentration of 0.5 mg/mL. Diode laser 810 nm (GmbH). Power 200 mW, total energy was 4 J for 10 s at each site. | SRP DM Controlled (17) DM Uncontrolled (16) | DM Controlled Baseline Test: 6.39 ± 0.59 Control: 6.47 ± 0.62 p > 0.05 3 months Test: 4.77 ± 0.68 Control: 5.58 ± 0.83 p < 0.05 6 months Test: 3.64 ± 0.84 Control: 4.92 ± 0.95 p < 0.05 DM Uncontrolled Baseline Test: 6.51 ± 0.35 Control: 6.65 ± 0.42 p > 0.05 3 months Test: 5.02 ± 0.52 Control: 5.79 ± 0.78 p < 0.05 6 months Test: 4.51 ± 0.90 Control: 5.17 ± 0.98 p > 0.05 | BoP Baseline (%): test 46 ± 21.3 control 45.2 ± 22.7 3 months test 12.4 ± 5.5 control 23.3 ± 12.8 6 months test 11.8 ± 4.9 control 19.9 ± 7.9 PI Plaque Index (%) Baseline: test 56.1 ± 23.7 control 53.8 ± 26.8 3 months test 13.4 ± 6.3 control 11.4 ± 5.3 6 months test 12.0 ± 3.1 control 14.9 ± 6.7 CAL Baseline (mm): test 6.49 ± 0.39 control 6.62 ± 0.75 3 months test 4.93 ± 0.51 control 5.86 ± 0.86 6 months test 3.94 ± 0.74 control 5.04 ± 0.97 | 3–6 |
| Cláudio et al. 2021 [50] | Brazil | RCT, patient assignment | >7% | SRP + aPDT (16) aPDT: Indium Gallium Aluminium Phosphorus Diode laser 660 nm(InGaAP) for 50 s, totaling 157 J/cm2, energy of 4.7 J, 100 mW power, and an optical fiber with 0.03 cm2 diameter. | SRP (15) | Baseline Test: 5.22 ± 0.17 Control: 5.36 ± 0.18 p = 0.12 3 months Test: 4.04 ± 0.78 Control: 4.21 ± 0.46 p = 0.58 6 months Test: 3.92 ± 0.59 Control: 4.06 ± 0.67 p = 0.50 | BoP Baseline (%): test 7.25 ± 7.01 control 6.33 ± 5.35 3 months test 3.90 ± 4.10 control 3.66 ± 3.86 6 months test 3.04 ± 3.65 control 3.44 ± 4.71 PI Plaque Index (%) Baseline: test 38.27 ± 14.44 control 35.55 ± 14.58 3 months test 33.36 ± 16.89 control 29.80 ± 17.52 6 months test 34.25 ± 19.24 control 30.78 ± 15.93 CAL Baseline (mm): test 3.61 ± 0.88 control 3.52 ± 0.71 3 months test 3.48 ± 0.76 control 3.27 ± 0.76 6 months test 3.51 ± 0.69 control 3.40 ± 0.80 | 3–6 |
| Sufaru et al. 2022 [51] | Romania | RCT, patient assignment | >6% | SRP + aPDT (24) aPDT: Diode Laser 810 nm (A.R.C) Indocyanine Green Powder. Laser applied continuously with a power of 0.2 W and a total energy of 12 J, around the tooth for 60 s. | SRP (25) | Baseline Test: 5.53 ± 0.24 Control: 5.54 ± 0.24 p > 0.05 6 months Test: 3.56 ± 0.19 Control: 4.10 ± 0.22 p < 0.05 | BoP Baseline (%): test 68.67 ± 6.1067 control 67.76 ± 6.57 6 months test 4.21 ± 3.85 control 8.08 ± 5.09 PI Plaque Index (%) Baseline: test 80.04 ± 5.90 control 79.44 ± 6.31 6 months test 17.08 ± 5.14 control 17.72 ± 6.38 CAL Baseline (mm): test 4.50 ± 0.22 control 4.51 ± 0.20 6 months test 2.58 ± 0.19 control 3.15 ± 0.17 | 6 |
| Brinar et al. 2023 [52] | Slovenia | RCT, patient assignment | >7% | SRP + aPDT (12) aPDT: Diode Laser (Fotona) with a wavelength of 810 nm, a power of 250 mW, and the photosensitizing agent Indocyanine Green at a concentration of 1 mg/mL. A total of 10 s of irradiations at each site. | SRP (12) | Baseline Test: 3.3 ± 0.2 Control: 3.1 ± 0.2 3 months Test: 2.6 ± 0.2 Control: 2.7 ± 0.1 p = 0.001 | FMBS Baseline (%): test 21.8 ± 4.2 control 26.6 ± 4.2 3 months test 7.1 ± 3.1 control 19.1 ± 3.1 FMPS Plaque Index (%) Baseline: test 48.4 ± 7.3 control 29.6 ± 8.5 3 months test 11.2 ± 5.2 control 15.9 ± 5.2 CAL Baseline (mm): test 3.7 ± 0.02 control 3.2 ± 0.2 3 months test 3.6 ± 0.2 control 3.2 ± 0.2 | 3 |
| Cláudio et al. 2024 [53] | Brazil | RCT, patient assignment | ≥7% | SRP + aPDT + BM (15) aPDT: Methylene blue 0.1 mg/mL. InGaAlP Diode Laser 660 nm for 50 s with a total of 166 J/cm2, an energy of 5 J, a power of 100 mW, and an optical fiber with a 0.03 cm2 of diameter. | SRP (15) SRP + BM (15) | Baseline Test: 4.47 ± 0.39 Control (SRP): 4.8 ± 0.79 Control (BM): 4.70 ± 0.54 p = 0.4748 3 months Test: 3.54 ± 0.57 Control (SRP): 3.86 ± 1.18 Control (BM): 3.76 ± 1.00 6 months Test: 3.55 ± 0.15 Control (SRP): 3.81 ± 0.93 Control (BM): 3.87 ± 0.93 | BoP Baseline (%): test 36.8 ± 10.56 control 35.6 ± 20.97 3 months test 23.33 ± 11.64 control 23.4 ± 10.62 6 months test 23.27 ± 12.94 control 20.8 ± 12.11 PI Plaque Index (%) Baseline: test 36.67 ± 16.06 control 34.2 ± 18.25 3 months test 23.8 ± 10.14 control 28.13 ± 19.42 6 months test 26.47 ± 11.96 control 19.53 ± 14.02 CAL Baseline (mm): test 5.4 ± 0.92 control 5.59 ± 1.52 3 months test 4.19 ± 1.10 control 4.71 ± 1.83 6 months test 4.17 ± 1.04 control 4.6 ± 1.52 | 3–6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardo, A.; Signoriello, A.; Messina, E.; Stilo, E.; De’ Manzoni Casarola, R.; Ferrara, E.; Lombardo, G.; Albanese, M. The Adjunctive Role of Antimicrobial Photodynamic Therapy to Non-Surgical Treatment in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Healthcare 2025, 13, 1703. https://doi.org/10.3390/healthcare13141703
Pardo A, Signoriello A, Messina E, Stilo E, De’ Manzoni Casarola R, Ferrara E, Lombardo G, Albanese M. The Adjunctive Role of Antimicrobial Photodynamic Therapy to Non-Surgical Treatment in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Healthcare. 2025; 13(14):1703. https://doi.org/10.3390/healthcare13141703
Chicago/Turabian StylePardo, Alessia, Annarita Signoriello, Elena Messina, Elia Stilo, Rachele De’ Manzoni Casarola, Elisabetta Ferrara, Giorgio Lombardo, and Massimo Albanese. 2025. "The Adjunctive Role of Antimicrobial Photodynamic Therapy to Non-Surgical Treatment in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis" Healthcare 13, no. 14: 1703. https://doi.org/10.3390/healthcare13141703
APA StylePardo, A., Signoriello, A., Messina, E., Stilo, E., De’ Manzoni Casarola, R., Ferrara, E., Lombardo, G., & Albanese, M. (2025). The Adjunctive Role of Antimicrobial Photodynamic Therapy to Non-Surgical Treatment in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Healthcare, 13(14), 1703. https://doi.org/10.3390/healthcare13141703







