Multidisciplinary Care in a Public University Family Medicine Group in Québec (Canada): Data on Patients’ Follow-Up and Cardiometabolic Risk Management
Abstract
1. Introduction
2. Methods
2.1. Study Population
2.2. Clinic Follow-Up
2.3. Collection of Clinical Data
2.4. Data Extraction and Computation
2.5. Statistical Analyses
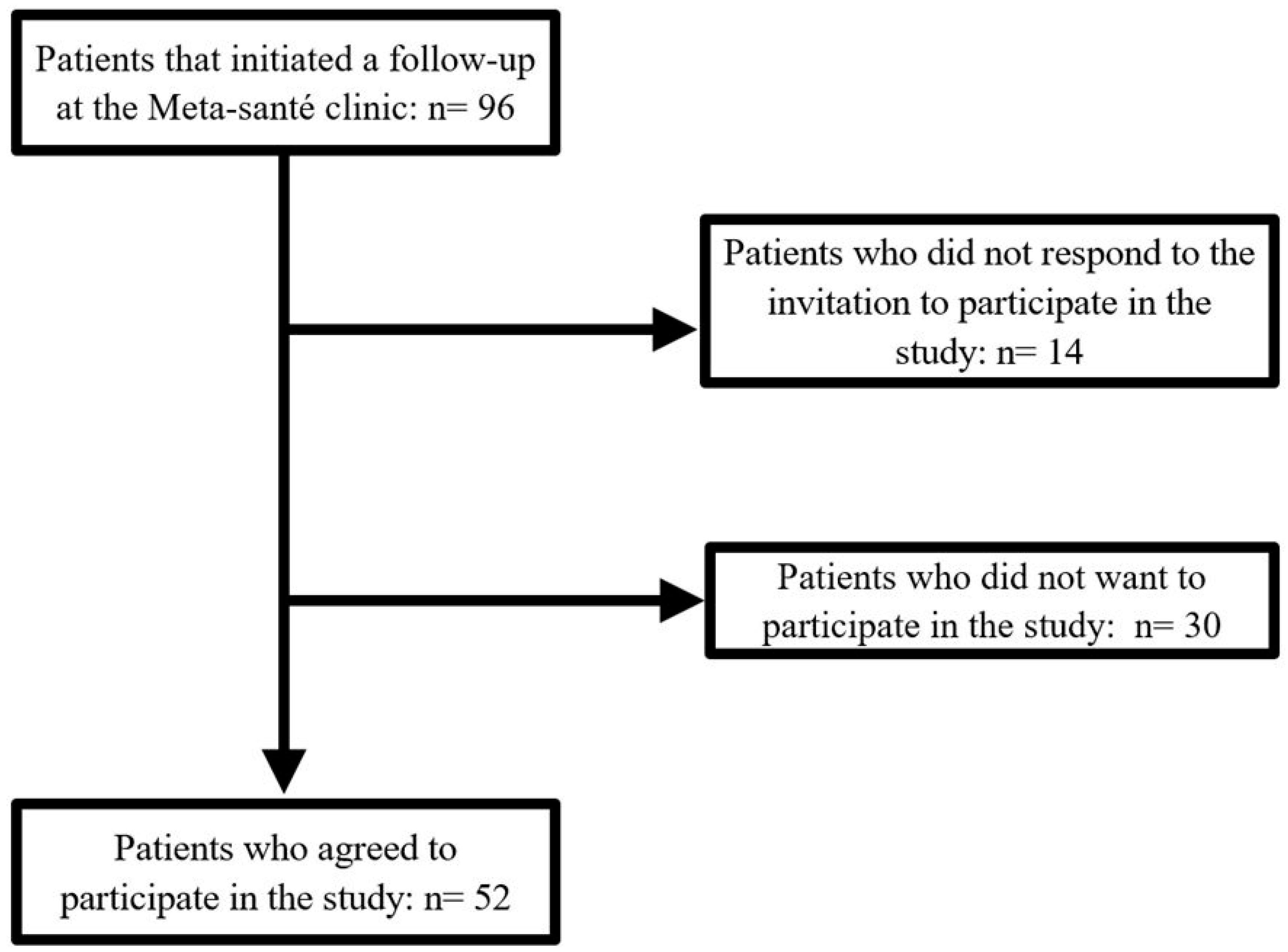
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Després, J.P. Is visceral obesity the cause of the metabolic syndrome? Ann. Med. 2006, 38, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Les Maladies du Coeur au Canada; Agence de la Santé Publique du Canada: Ottawa, ON, Canada, 2017.
- Guembe, M.J.; Fernandez-Lazaro, C.I.; Sayon-Orea, C.; Toledo, E.; Moreno-Iribas, C.; for the RIVANA Study Investigators; Cosials, J.B.; Reyero, J.B.; Martínez, J.D.; Diego, P.G.; et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020, 19, 195. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Harris, S.B.; Leiter, L.A.; Fitchett, D.H.; Teoh, H.; Bhattacharyya, O.K. Managing cardiometabolic risk in primary care: Summary of the 2011 consensus statement. Can. Fam. Physician 2012, 58, 389–393, e196–e201. [Google Scholar] [PubMed]
- Guide Priorité Santé. 2015: Santemontreal.qc.ca. Available online: https://fmf.cfpc.ca/wp-content/uploads/2017/10/S128_Le-Guide-Priorit%C3%A9-Sant%C3%A9_outil-et-mod%C3%A8le-de-pratique-collaborative-en-pr%C3%A9vention-clinique.pdf (accessed on 1 March 2025).
- Han, T.S.; Lean, M.E. A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovasc. Dis. 2016, 5, 2048004016633371. [Google Scholar] [CrossRef] [PubMed]
- Leblay, L.; Bélanger, A.; Desjardins, C.; Filiatrault, M.; Paquette, J.-S.; Drouin-Chartier, J.-P. Relationship Between Diet Quality and Antihypertensive Medication Intensity Among Adults With Metabolic Syndrome-Associated High Blood Pressure. CJC Open 2024, 6, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Desjardins, C.; Leblay, L.; Bélanger, A.; Filiatrault, M.; Barbier, O.; Guénette, L.; Leclerc, J.; Lefebvre, J.; Zongo, A.; Drouin-Chartier, J.-P. Relationship Between Diet Quality and Glucose-Lowering Medication Intensity Among Adults With Type 2 Diabetes: Results From the CARTaGENE Cohort. CJC Open 2024, 6, 20–29. [Google Scholar] [CrossRef] [PubMed]
- Bélanger, A.; Desjardins, C.; Leblay, L.; Filiatrault, M.; Barbier, O.; Gangloff, A.; Leclerc, J.; Lefebvre, J.; Zongo, A.; Drouin-Chartier, J.-P. Relationship Between Diet Quality and Statin Use Among Adults With Metabolic Syndrome From the CARTaGENE Cohort. CJC Open 2024, 6, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Leblay, L.; Lessard-Lord, J.; Paquette, J.-S.; Guénette, L.; Drouin-Chartier, J.-P. Gender differences in the association between adherence to healthy diet principles and adherence to cardiopreventive medication among adults from Québec (Canada). Br. J. Nutr. 2025, 133, 422–432. [Google Scholar] [CrossRef] [PubMed]
- Teoh, H.; Després, J.; Dufour, R.; Fitchett, D.H.; Goldin, L.; Goodman, S.G.; Harris, S.B.; Langer, A.; Lau, D.C.W.; Lonn, E.M.; et al. A comparison of the assessment and management of cardiometabolic risk in patients with and without type 2 diabetes mellitus in Canadian primary care. Diabetes Obes. Metab. 2013, 15, 1093–1100. [Google Scholar] [CrossRef] [PubMed]
- Soltani, I.; Beaulieu, M.-C.; Sestier, M.; Shen, H.C.; Hillani, A.; Matteau, A.; Mansour, S.; Potter, B.J. Adherence to Cardiovascular Prevention Guidelines in an Academic Centre. CJC Open 2023, 5, 530–536. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Lin, S.; Hyun, K.; Hafiz, N.; Manandi, D.; Koh, A.S.; Redfern, J. The effects of multidisciplinary collaborative care on cardiovascular risk factors among patients with diabetes in primary care settings: A systematic review and meta-analysis. Prim. Care Diabetes 2024, 18, 381–392. [Google Scholar] [CrossRef] [PubMed]
- Nieto-Martínez, R.; Velásquez-Rodríguez, A.; Neira, C.; Mou, X.; Neira, A.; Garcia, G.; Velásquez-Rodríguez, P.; Levy, M.; Mechanick, J.I.; Velásquez-Mieyer, P.A. Impact of a Multidisciplinary Approach on Cardiometabolic Risk Reduction in a Multiracial Cohort of Adults: A 1-Year Pilot Study. Nutrients 2022, 14, 3391. [Google Scholar] [CrossRef] [PubMed]
- Korylchuk, N.; Pelykh, V.; Nemyrovych, Y.; Didyk, N.; Martsyniak, S. Challenges and Benefits of a Multidisciplinary Approach to Treatment in Clinical Medicine. J. Pioneer. Med Sci. 2024, 13, 1–9. [Google Scholar] [CrossRef]
- Leach, B.; Morgan, P.; de Oliveira, J.S.; Hull, S.; Østbye, T.; Everett, C. Primary care multidisciplinary teams in practice: A qualitative study. BMC Fam. Pract. 2017, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Seckler, E.; Regauer, V.; Rotter, T.; Bauer, P.; Müller, M. Barriers to and facilitators of the implementation of multi-disciplinary care pathways in primary care: A systematic review. BMC Fam. Pract. 2020, 21, 113. [Google Scholar] [CrossRef] [PubMed]
- Fetters, M.D.; Guetterman, T.C. Discovering and doing family medicine and community health research. Fam. Med. Community Health 2019, 7, e000084. [Google Scholar] [CrossRef] [PubMed]
- Bonfim, D.; Belotti, L.; de Almeida, L.Y.; Eshriqui, I.; Velasco, S.R.M.; Monteiro, C.N.; Jantsch, A.G. Challenges and strategies for conducting research in primary health care practice: An integrative review. BMC Health Serv. Res. 2023, 23, 1380. [Google Scholar] [CrossRef] [PubMed]
- Bouzo, V.; Plourde, H.; Beckenstein, H.; Cohen, T.R.; Cahill, N. Evaluation of the Diet Tracking Smartphone Application Keenoa™: A Qualitative Analysis. Can. J. Diet. Pract. Res. 2022, 83, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Moyen, A.; Rappaport, A.I.; Fleurent-Grégoire, C.; Tessier, A.-J.; Brazeau, A.-S.; Chevalier, S. Relative Validation of an Artificial Intelligence–Enhanced, Image-Assisted Mobile App for Dietary Assessment in Adults: Randomized Crossover Study. J. Med. Internet Res. 2022, 24, e40449. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.K.; Magnus, D.; Constantine, M.; Lee, S.S.; Kelley, M.; Alessi, S.; Korngiebel, D.; James, C.; Kuwana, E.; Gallagher, T.H.; et al. Attitudes Toward Risk and Informed Consent for Research on Medical Practices: A Cross-sectional Survey. Ann. Intern. Med. 2015, 162, 690–696. [Google Scholar] [CrossRef] [PubMed]
- Howe, N.; Giles, E.; Newbury-Birch, D.; McColl, E. Systematic review of participants’ attitudes towards data sharing: A thematic synthesis. J. Health Serv. Res. Policy 2018, 23, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Hammack-Aviran, C.M.; Brelsford, K.M.; McKenna, K.C.; Graham, R.D.; Lampron, Z.M.; Beskow, L.M. Research Use of Electronic Health Records: Patients’ Views on Alternative Approaches to Permission. AJOB Empir. Bioeth. 2020, 11, 172–186. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.K.; Joseph, J.G.; Ohno-Machado, L. Comparison of consumers’ views on electronic data sharing for healthcare and research. J. Am. Med. Inform. Assoc. 2015, 22, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Beskow, L.M.; Brelsford, K.M.; Hammack, C.M. Patient perspectives on use of electronic health records for research recruitment. BMC Med. Res. Methodol. 2019, 19, 42. [Google Scholar] [CrossRef] [PubMed]
- Hutchinson, J.M.; Dodd, K.W.; Guenther, P.M.; Lamarche, B.; Haines, J.; Wallace, A.; Perreault, M.; Williams, T.E.; Louzada, M.L.d.C.; Jessri, M.; et al. The Canadian Food Intake Screener for assessing alignment of adults’ dietary intake with the 2019 Canada’s Food Guide healthy food choices recommendations: Scoring system and construct validity. Appl. Physiol. Nutr. Metab. 2023, 48, 620–633. [Google Scholar] [CrossRef] [PubMed]
- DeJonckheere, M.; Vaughn, L.M. Semistructured interviewing in primary care research: A balance of relationship and rigour. Fam. Med. Community Health 2019, 7, e000057. [Google Scholar] [CrossRef] [PubMed]
- Halcomb, E.; Ashley, C.; Middleton, R.; Lucas, E.; Robinson, K.; Harvey, S.; Charlton, K.; McInnes, S.; Lucas, L. Understanding perceptions of health, lifestyle risks and chronic disease in middle age. J. Clin. Nurs. 2021, 30, 2279–2286. [Google Scholar] [CrossRef] [PubMed]
- Toth-Capelli, K.M.; Brawer, R.; Plumb, J.; Daskalakis, C. Stage of change and other predictors of participant retention in a behavioral weight management program in primary care. Health Promot. Pract. 2013, 14, 441–450. [Google Scholar] [CrossRef] [PubMed]
- Ruelas, A.L.; Contreras, T.d.J.M.; Romero, J.E.; Zavala, R.G.D.; Plata, M.d.C.C.; Hingle, M.; Guirado, B.A.; Haby, M.M. Factors influencing adults to drop out of intensive lifestyle interventions for weight loss. Transl. Behav. Med. 2023, 13, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Frumkin, A.; Bi, W.G.; Magrill, J.; Newton, C. Biopsy of Canada’s family physician shortage. Fam. Med. Community Health 2023, 11, e002236. [Google Scholar] [CrossRef] [PubMed]
- Misfeldt, R.; Suter, E.; Mallinson, S.; Boakye, O.; Wong, S.; Nasmith, L. Exploring Context and the Factors Shaping Team-Based Primary Healthcare Policies in Three Canadian Provinces: A Comparative Analysis. Healthc. Policy 2017, 13, 74–93. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Breton, M.; Lévesque, J.F.; Pineault, R.; Hogg, W. Primary Care Reform: Can Quebec’s Family Medicine Group Model Benefit from the Experience of Ontario’s Family Health Teams? Healthc. Policy 2011, 7, e122–e135. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.Q.M.; Chin, Y.H.; Ng, C.H.; Liow, Y.; Devi, M.K.; Khoo, C.M.; Goh, L.H. Multidisciplinary team approach to diabetes. An outlook on providers’ and patients’ perspectives. Prim. Care Diabetes 2020, 14, 545–551. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Mean ± SD or N (%) |
|---|---|
| Age, years | 50.3 ± 15.2 |
| Sex | |
| Female | 38 (73.1) |
| Male | 14 (27.0) |
| Alcohol consumption | |
| Never | 14 (26.9) |
| Monthly frequency | 22 (42.3) |
| Weekly frequency | 12 (23.1) |
| Daily frequency | 4 (7.7) |
| Smoking status | |
| Never | 28 (53.9) |
| Former | 18 (34.6) |
| Current | 6 (11.5) |
| Illicit drug use | |
| Never | 49 (94.2) |
| Former | 0 |
| Current | 3 (5.8) |
| Personal history of cancer | |
| No | 48 (92.3) |
| Yes | 4 (7.7) |
| Personal history of cardiovascular disease | |
| No | 48 (92.3) |
| Yes | 4 (7.7) |
| Type 2 diabetes | |
| No | 40 (76.9) |
| Yes | 12 (23.1) |
| High blood pressure | |
| No | 31 (59.6) |
| Yes | 21 (40.4) |
| Unmedicated | 4 (19.0) |
| Medicated | 17 (81.0) |
| High blood cholesterol | |
| No | 37 (71.2) |
| Yes | 15 (28.9) |
| Unmedicated | 5 (33.3) |
| Medicated | 10 (66.7) |
| Weight, kg | 113 ± 28 |
| Waist circumference, cm 1 | 126 ± 17 |
| Body mass index, kg/m2 | 42.1 ± 8.1 |
| Systolic blood pressure, mm Hg 1 | 130 ± 14 |
| Diastolic blood pressure, mm Hg 1 | 79 ± 10 |
| HbA1c, % 2 | 5.96 ± 0.85 |
| Triglycerides, mmol/L 3 | 2.45 ± 2.81 |
| HDL-cholesterol, mmol/L 4 | 1.09 ± 0.24 |
| LDL-cholesterol, mmol/L 5 | 2.84 ± 1.04 |
| Data | All Patients (n = 52) | Patients Who Completed Their Follow-Up (n = 36) |
|---|---|---|
| Anthropometry | ||
| Patients with BMI calculated at entry, n (%) | 52 (100.0) | 36 (100.0) |
| Patients with BMI calculated at entry and at least 1 time during follow-up, n (%) | 30 (57.7) | 25 (69.4) |
| Patients with waist circumference measured at entry | 48 (92.3) | 34 (94.4) |
| Patients with waist circumference measured at entry and at least 1 time during follow-up | 25 (48.1) | 22 (61.1) |
| Blood pressure | ||
| Patients with blood pressure measured at entry | 48 (92.3) | 34 (94.4) |
| Patients with blood pressure measured at entry and at least 1 time during follow-up | 26 (50.0) | 23 (63.9) |
| Biochemical analyses | ||
| Patients with a lipid profile measured on or before entry | 26 (50.0) | 16 (44.4) |
| Patients with a lipid profile measured on or before entry and at least 1 time during follow-up | 5 (9.6) | 4 (11.1) |
| Patients with glucose homeostasis measured on or before entry | 41 (78.9) | 28 (77.8) |
| Patients with glucose homeostasis measured on or before entry and at least 1 time during follow-up | 13 (25.0) | 12 (33.3) |
| Dietary assessments | ||
| Patients who completed at least 1 dietary diary at entry, n (%) | 28 (53.9) | 22 (61.1) |
| Patients who completed dietary diaries at entry and during follow-up, n (%) | 9 (17.3) | 8 (22.2) |
| Characteristics | Patients Who Completed the Program (n = 36) | Patients Who Dropped Out (n = 16) | p-Value * |
|---|---|---|---|
| Age, years | 50.8 ± 13.9 | 48.9 ± 18.4 | 0.72 |
| Sex | 0.07 | ||
| Women | 29 (80.6) | 9 (56.3) | |
| Men | 7 (19.4) | 7 (43.8) | |
| Alcohol consumption | 0.34 | ||
| Never | 10 (27.8) | 4 (25.0) | |
| Monthly frequency | 12 (33.3) | 10 (62.5) | |
| Weekly frequency | 11 (30.6) | 1 (6.3) | |
| Daily frequency | 3 (8.3) | 1 (6.3) | |
| Smoking status | 0.23 | ||
| Never | 20 (55.6) | 8 (50.0) | |
| Former | 14 (38.9) | 4 (25.0) | |
| Current | 2 (5.6) | 4 (25.0) | |
| Illicit drug use | 0.17 | ||
| Never | 35 (94.2) | 14 (87.5) | |
| Current | 1 (2.8) | 2 (12.5) | |
| Personal history of cancer | 0.79 | ||
| No | 33 (91.7) | 15 (93.8) | |
| Yes | 3 (8.3) | 1 (6.3) | |
| Personal history of cardiovascular disease | 0.79 | ||
| No | 33 (91.7) | 15 (93.8) | |
| Yes | 3 (8.3) | 1 (6.3) | |
| Cardiometabolic risk factors | |||
| Type 2 diabetes | 0.83 | ||
| No | 28 (77.8) | 12 (75.0) | |
| Yes | 8 (22.2) | 4 (25.0) | |
| High blood pressure | 0.12 | ||
| No | 24 (66.7) | 7 (43.8) | |
| Yes | 12 (33.3) | 9 (56.3) | |
| High blood cholesterol | 0.02 | ||
| No | 29 (80.6) | 8 (50.0) | |
| Yes | 7 (19.4) | 8 (50.0) | |
| Weight, kg | 109 ± 25 | 123 ± 31 | 0.11 |
| Waist circumference, cm 1 | 124 ± 15 | 132 ± 19 | 0.21 |
| Body mass index, kg/m2 | 41.1 ± 8.0 | 44.3 ± 7.9 | 0.18 |
| Systolic blood pressure, mm Hg 1 | 129 ± 12 | 134 ± 17 | 0.34 |
| Diastolic blood pressure, mm Hg 1 | 79 ± 8 | 79 ± 13 | 0.93 |
| HbA1c, % 2 | 5.88 ± 0.67 | 6.15 ± 1.17 | 0.44 |
| Triglycerides, mmol/L 3 | 1.89 ± 0.76 | 3.17 ± 4.15 | 0.33 |
| HDL-cholesterol, mmol/L 4 | 1.12 ± 0.20 | 1.05 ± 0.27 | 0.49 |
| LDL-cholesterol, mmol/L 5 | 2.74 ± 1.07 | 2.98 ± 1.04 | 0.57 |
| Implication in the program | |||
| Attended the baseline group session | 0.78 | ||
| No | 15 (41.7) | 6 (37.5) | |
| Yes | 21 (58.3) | 10 (62.5) | |
| Duration of follow-up, days | 287 ± 90 | 158 ± 88 | <0.0001 |
| Total appointments, n | 16 ± 9 | 9 ± 6 | 0.002 |
| Appointments with the registered dietitian, n | 8 ± 3 | 5 ± 4 | 0.007 |
| Appointments with the clinical nurse, n | 6 ± 6 | 3 ± 2 | 0.01 |
| Appointments with the practician nurse and/or the medical doctor, n | 2 ± 1 | 1 ± 1 | 0.13 |
| Pharmaceutical interventions, n | 1 ± 5 | 0 ± 1 | 0.21 |
| β (95% CI) per Each Appointment at the Clinic | p-Value | β (95% CI) per Each Appointment with the Registered Dietitian | p-Value | β (95% CI) per Each Pharmaceutical Intervention | p-Value | |
|---|---|---|---|---|---|---|
| Weight, kg (N = 25) | ||||||
| Model 1 | 0.08 (−0.15, 0.30) | 0.49 | 0.47 (−0.21, 1.15) | 0.17 | −0.04 (−0.39, 0.31) | 0.82 |
| Model 2 | 0.04 (−0.35, 0.42) | 0.84 | 0.50 (−0.69, 1.69) | 0.38 | −0.24 (−0.72, 0.24) | 0.30 |
| Model 3 | 0.03 (−0.37, 0.43) | 0.87 | 0.53 (−0.71, 1.77) | 0.37 | −0.24 (−0.74, 0.25) | 0.31 |
| Waist circumference, cm (N = 24) | ||||||
| Model 1 | −0.33 (−0.64, −0.03) | 0.03 | −0.92 (−1.89, 0.06) | 0.06 | −0.66 (−1.09, −0.22) | 0.01 |
| Model 2 | −0.30 (−0.72, 0.13) | 0.15 | −0.31 (−1.76, 1.14) | 0.65 | −0.51 (−1.03, 0.01) | 0.05 |
| Model 3 | −0.28 (−0.71, 0.15) | 0.18 | −0.36 (−1.83, 1.11) | 0.60 | −0.51 (−1.03, 0.02) | 0.06 |
| BMI, kg/m2 (N = 25) | ||||||
| Model 1 | 0.04 (−0.05, 0.13) | 0.34 | 0.16 (−0.11, 0.43) | 0.23 | 0.00 (−0.14, 0.14) | 0.87 2 |
| Model 2 | 0.03 (−0.12, 0.17) | 0.72 | 0.13 (−0.34, 0.60) | 0.57 | −0.08 (−0.26, 0.11) | 0.40 |
| Model 3 | 0.02 (−0.13, 0.18) | 0.76 | 0.14 (−0.34, 0.62) | 0.54 | −0.08 (−0.27, 0.12) | 0.41 |
| Systolic blood pressure, mm Hg (N = 24) | ||||||
| Model 1 | −0.32 (−1.19, 0.54) | 0.44 | −0.08 (−2.85, 2.69) | 0.95 | −0.27 (−1.63, 1.09) | 0.68 |
| Model 2 | −0.25 (−1.65, 1.16) | 0.71 | 1.51 (−2.33, 5.35) | 0.41 | −0.55 (−2.66, 1.55) | 0.58 |
| Model 3 | −0.17 (−1.53, 1.19) | 0.80 | 1.57 (−2.09, 5.24) | 0.37 | −0.45 (−2.50, 1.61) | 0.65 |
| Diastolic blood pressure, mm Hg (N = 24) | ||||||
| Model 1 | −0.24 (−0.69, 0.20) | 0.27 | 0.28 (−1.16, 1.73) | 0.69 | −0.53 (−1.20, 0.15) | 0.12 |
| Model 2 | −0.38 (−1.22, 0.46) | 0.35 | 0.43 (−1.99, 2.84) | 0.71 | −1.47 (−2.51, −0.43) | 0.01 |
| Model 3 | −0.40 (−1.27, 0.47) | 0.34 | 0.41 (−2.10, 2.92) | 0.73 | −1.49 (−2.56, −0.43) | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leblay, L.; Pelland, L.D.; Gagnon, J.; Guay, V.; Desroches, S.; Drouin-Chartier, J.-P.; Paquette, J.-S. Multidisciplinary Care in a Public University Family Medicine Group in Québec (Canada): Data on Patients’ Follow-Up and Cardiometabolic Risk Management. Healthcare 2025, 13, 1704. https://doi.org/10.3390/healthcare13141704
Leblay L, Pelland LD, Gagnon J, Guay V, Desroches S, Drouin-Chartier J-P, Paquette J-S. Multidisciplinary Care in a Public University Family Medicine Group in Québec (Canada): Data on Patients’ Follow-Up and Cardiometabolic Risk Management. Healthcare. 2025; 13(14):1704. https://doi.org/10.3390/healthcare13141704
Chicago/Turabian StyleLeblay, Lise, Léanne Day Pelland, Josée Gagnon, Valérie Guay, Sophie Desroches, Jean-Philippe Drouin-Chartier, and Jean-Sébastien Paquette. 2025. "Multidisciplinary Care in a Public University Family Medicine Group in Québec (Canada): Data on Patients’ Follow-Up and Cardiometabolic Risk Management" Healthcare 13, no. 14: 1704. https://doi.org/10.3390/healthcare13141704
APA StyleLeblay, L., Pelland, L. D., Gagnon, J., Guay, V., Desroches, S., Drouin-Chartier, J.-P., & Paquette, J.-S. (2025). Multidisciplinary Care in a Public University Family Medicine Group in Québec (Canada): Data on Patients’ Follow-Up and Cardiometabolic Risk Management. Healthcare, 13(14), 1704. https://doi.org/10.3390/healthcare13141704







