Abstract
Cardiopulmonary exercise testing combined with exercise stress Echocardiography (CPET-ESE) is an advanced diagnostic modality for evaluating cardiovascular disease and tailoring patient-specific treatment strategies. By integrating metabolic, ventilatory, and hemodynamic data with real-time imaging, CPET-ESE offers a comprehensive assessment of cardiovascular function under physiological stress. CPET provides detailed insights into metabolic and ventilatory performance, while ESE allows for the dynamic visualisation of cardiac structure and function during exercise. This review outlines the physiological foundations and core parameters of CPET and ESE, emphasising their complementary roles in cardiovascular diagnostics and prognostication and exploring their clinical value for evaluating unexplained dyspnoea and exercise-induced hemodynamic abnormalities. CPET-ESE plays a pivotal role in detecting subtle hemodynamic abnormalities, assessing functional capacity, and contributing to earlier diagnosis, targeted interventions, and improved clinical outcomes.
1. Introduction
Exercise intolerance results from an impaired interplay between the cardiovascular, pulmonary, and muscular systems during physical activity. Particularly, it can result from cardiac dysfunction, lung disease, or reduced peripheral oxygen extraction due to impaired muscle metabolism [1].
Cardiopulmonary exercise testing (CPET) has emerged as a pivotal tool in evaluating exercise intolerance in various pathological conditions. Although CPET allows for a detailed analysis of the physiological or pathological response to exercise, it does not distinguish its central (i.e., ventricular function) and peripheral components (i.e., oxygen delivery and extraction) unless exercise is carried out together with a concurrent invasive hemodynamic evaluation by cardiac catheterization [2] or with imaging techniques such as stress echocardiography (ESE) [1,3]. As a non-invasive method, ESE has been successfully used to gain insight into cardiovascular mechanisms underpinning effort intolerance in patients with different cardiovascular diseases [4,5].
The combined approach with CPET-ESE provides complementary yet distinct sets of parameters (Graphical Abstract) for analysing metabolic and ventilatory responses during exercise, enabling a more direct assessment of cardiovascular performance [1]. Moreover, while the earliest alterations of cardiovascular diseases become evident during physical effort, this technique can timely reveal pathologies in the initial stages before the onset of overt symptoms. All these features make this test a powerful tool to optimise decision-making, improve outcome prediction, and provide objective values for tailored treatment also in the early stages of several diseases such as heart failure (HF), cardiomyopathies, valvulopathies, coronary artery disease (CAD), pulmonary hypertension (PH), and pulmonary embolism (PE) sequelae [6]. Moreover, it represents a tool for risk stratification in heart and lung transplantation and pre-surgical evaluations [7,8,9,10,11,12,13]. Currently, a standardised protocol for combined CPET-ESE has not been established. However, adhering to general recommendations can enhance the reliability and consistency of the results [12]. This narrative review explores the clinical value and integration of the CPET-ESE protocol for evaluating unexplained dyspnoea and exercise-induced hemodynamic abnormalities.
2. Methods
This narrative review was conducted to summarise and interpret the existing literature on the integration of CPET and ESE for clinical evaluation and risk stratification, with the aim to answer a simple question: how can CPET-ESE contribute to improving the understanding and detection of exercise-induced hemodynamic abnormalities in cardiovascular diseases? To ensure a comprehensive and up-to-date review of the literature, we conducted a structured search of relevant scientific databases. Specifically, the databases PubMed and Scopus were searched for articles related to CPET, ESE, and their combined application in the evaluation of HF, cardiomyopathies, valvulopathies, CAD, and pulmonary diseases.
The search included publications from 1991 to 2025. Keywords and MeSH terms used in various combinations included “cardiopulmonary exercise testing”, “CPET”, “exercise stress echocardiography”, “ESE”, “heart failure with preserved ejection fraction”, “heart failure with reduced ejection fraction”, “HFpEF”, “HFrEF”, “pulmonary hypertension”, “valvular heart disease”, “mitral regurgitation”, “mitral stenosis”, “aortic regurgitation”, “aortic stenosis”, “hypertrophic cardiomyopathy”, “prognosis”, and “diagnostic accuracy”.
The analysis included original studies, reviews, and guidelines written in English, focusing specifically on adult populations. Eligible articles involved the use of CPET and/or ESE in clinical practice or research settings and provided data relevant to diagnosis, prognosis, or therapeutic decision-making. Studies were excluded if they involved paediatric populations, lacked sufficient methodological detail, or had unclear endpoints.
3. Physiology and Key Parameters of CPET
The use of oxygen as the primary energy resource by peripheral tissues—i.e., oxygen consumption (VO2)—depends on cardiac output (CO), on the efficient distribution of the substrate to active tissues, and finally on their ability to extract the substrate from the bloodstream. This is represented in Fick’s principle, whereby VO2 is calculated as the product between CO (i.e., heart rate [HR] x stroke volume [HR]) and the arteriovenous oxygen difference (AVO2diff), which is the difference between arterial and venous oxygen content, reflecting how much oxygen is extracted by the muscles during exercise [14].
VO2 increases up to six-fold at peak exercise in healthy adults, indicating good functional capacity and prognosis. Lower values, particularly in conditions like HF, are associated with poorer outcomes [7,15]. The VO2/work ratio reflects the efficiency of VO2 relative to the external workload and can help identify physical deconditioning or left ventricular dysfunction, particularly when myocardial ischemia is present.
The other crucial component of effort capacity is ventilatory efficiency. The minute ventilation–carbon dioxide production (VE/VCO2) slope is an index of ventilation–perfusion matching in the lung and describes the increase in minute ventilation for any given amount of CO2 generated from cellular respiration [16,17]. It is determined by CO2 production, the physiological dead space to tidal volume ratio (VD/VT), and the arterial CO2 partial pressure. End-tidal CO2 pressure (PETCO2) represents the partial pressure of CO2 in exhaled air; thus, it is an indirect marker of alveolar ventilation and ventilation–perfusion matching [18]. The breathing reserve (BR), resulting from the difference between maximal voluntary ventilation (MVV) and VE, indicates the ventilatory capacity during exertion [17,19]. Exercise Oscillatory Ventilation (EOV) is an ominous sign that refers to cyclic fluctuations in ventilation and expired gas exchange during exercise, commonly observed in individuals with advanced diseases and poor prognosis (Table 1).

Table 1.
Cardiopulmonary exercise testing: parameters and interpretation.
4. Physiology and Key Parameters of ESE
ESE allows for the evaluation of chamber geometry and volumes, left ventricle (LV) and right ventricle (RV) function, and valvular responses to exercise (Table 2).

Table 2.
Exercise stress echocardiography: parameters and interpretation.
The inability to increase the LVEF ≥ 7.5% from rest to peak exercise identifies the absence of contractile reserve, which can be related to the progressive impairment of the cardiac mechanics and/or the coronary flow reserve. Other parameters have been proposed as earlier markers of LV contractility, namely Tissue Doppler-derived early systolic velocity of the mitral annulus (TDI-S’) and Speckle tracking-derived global longitudinal strain (GLS) [7].
The diastolic function can be thoroughly assessed by utilising various techniques that provide insights into the diastolic response to exercise. Peak Tricuspid Regurgitation Velocity (TRV) evaluates the degree of regurgitant flow through the tricuspid valve. Systolic pulmonary arterial pressure (sPAP) is estimated during RV systole to assess pulmonary hemodynamics [29].
The average E/e’ ratio, which is the ratio of transmitral flow velocity to mitral annular velocity in early diastole, correlates well with left ventricular filling pressure when assessed at rest [36]. Still, doubts have been recently raised regarding the technical feasibility and reliability of this parameter at rest [52,53] and during exercise [37].
The assessment of heart valve disease relies on various echocardiographic parameters that measure the severity of regurgitation and stenosis by evaluating flow dynamics, orifice size, and pressure gradients. The vena contracta is the narrowest part of the regurgitant jet and provides a semi-quantitative estimate of its severity; its value can vary with physical exertion and is influenced by technical and flow-related factors [50]. The effective regurgitant orifice area (EROA) quantitatively measures the effective regurgitant orifice area, offering a direct indication of severity and is also affected by hemodynamic conditions and the type of regurgitation [50]. Regarding valvular stenosis, peak velocity and mean pressure gradient (MPG) measured by Doppler allow for the assessment of the degree of narrowing; both are flow-dependent and can be influenced by factors such as cardiac output, blood pressure, and vascular compliance [18,29,51]. Tricuspid annular plane systolic excursion (TAPSE), a key indicator of RV systolic performance, is based on tricuspid annular displacement during systole [11]. The TAPSE/sPAP ratio is measured to assess RV–pulmonary artery (RV-PA) coupling, representing the relationship between RV function and pulmonary vascular load [45]. The left atrium (LA) is another component of the RV and RV-PA coupling evaluation, and its dysfunction contributes to reduced pulmonary vessel compliance and abnormal right heart adaptation to exercise, as indicated by its correlation with the sPAP/TAPSE ratio [35,41]. Left atrial reservoir function (LARS) quantifies LA deformation during atrial filling [39]. Similarly, the mean pulmonary artery pressure (mPAP)/CO slope is a hemodynamic parameter that reflects the coupling between pulmonary arterial pressure and CO during exercise. It considers changes in both mPAP and CO from rest to exertion [9,47,48]. Any significant valvular abnormality identified through colour Doppler imaging should be further evaluated using semi-quantitative or quantitative methods, following current guidelines, both at rest and during peak exercise [54]. Lung ultrasound (LUS) plays an essential role in assessing pulmonary congestion, particularly in the setting of HF [13,55], and it has been recently integrated into exercise testing [1]. During exercise, the increase in B-lines, rather than their absolute number at a given moment, seems a better indicator of extravascular lung water accumulation [7].
Moreover, the assessment of congestion has been enhanced by extending the evaluation of the inferior vena cava (IVC) from rest to peak exercise. While IVC measurements were already part of the baseline evaluation, they are now systematically acquired at peak effort as well. This allows for the estimation of right atrial pressure (RAP) during stress, based on dynamic changes in IVC diameter and collapsibility. Specifically, IVC metrics obtained at peak exercise are integrated to estimate exercise-induced RAP. When a significant discrepancy between resting and peak is observed, a correction can be added to the tricuspid regurgitation (TR) pressure gradient.
5. Integrated CPET-ESE Protocol
CPET provides a global assessment of the integrative responses involving the pulmonary, cardiovascular, and muscular systems during exercise. It is especially valuable when evaluating functional capacity and uncovering hidden mechanisms of dyspnoea. On the other hand, ESE enables the dynamic visualisation of cardiac structure and function during effort. Individually, both techniques offer useful but incomplete information. CPET lacks anatomical and hemodynamic detail, while ESE may miss functional and ventilatory parameters. Combining them into a unified protocol—CPET-ESE—offers a unique opportunity to integrate gas exchange data with simultaneous echocardiographic assessment under physiological stress.
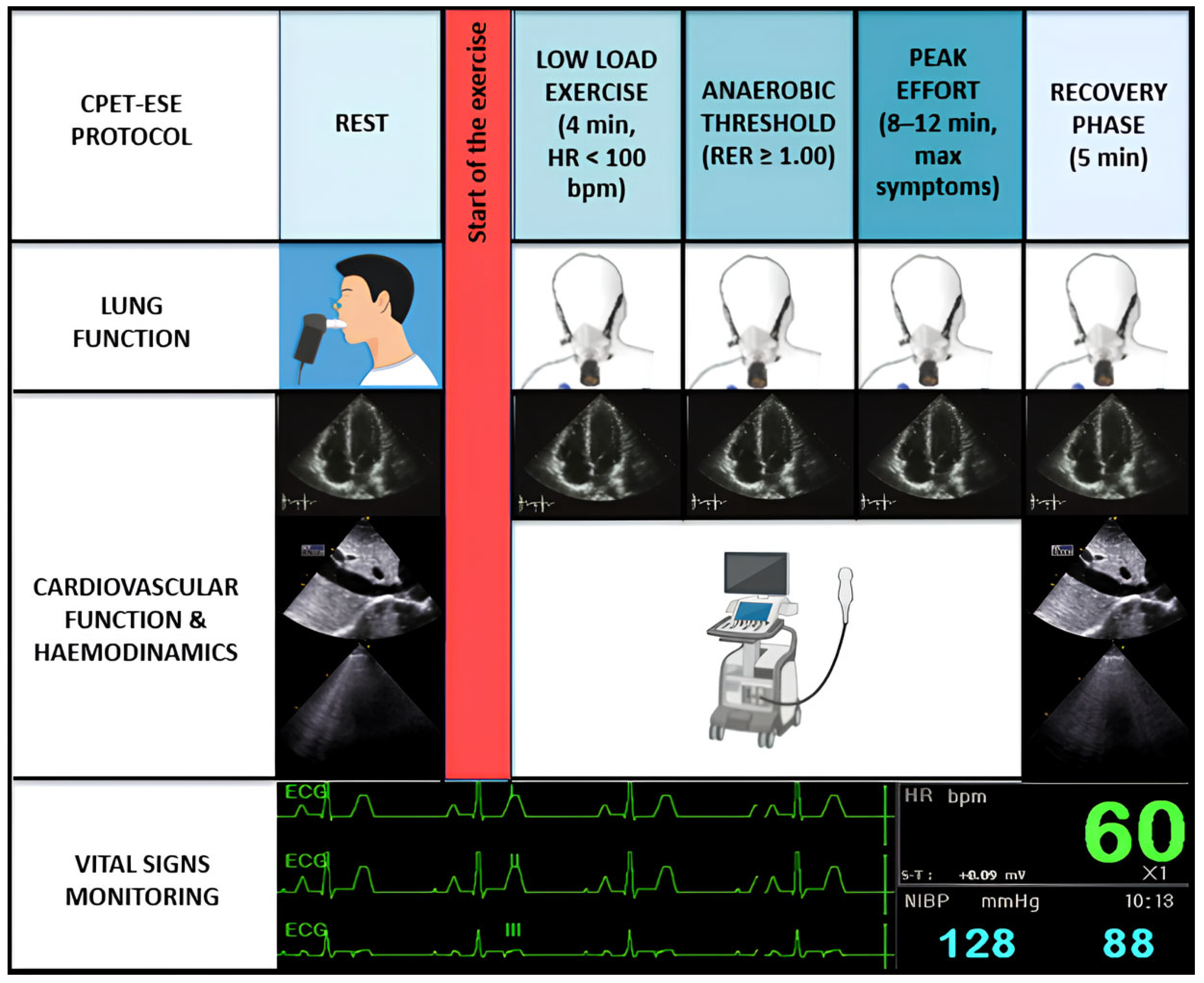
CPET-ESE offers an objective evaluation of exercise intolerance, usually measuring each parameter according to a four-stage protocol (Figure 1): (i) rest; (ii) low-load effort (typically within the first 4 min when heart rate (HR) is usually <100 bpm); (iii) anaerobic threshold (AT, i.e., when the respiratory exchange ratio (RER) [expressed by the carbon dioxide production (VCO2)/VO2 ratio] is steady ≥1.00); and (iv) peak effort, when the patient experiences effort-limiting symptoms, within 8–12 min [1,3]. A recovery phase of approximately five minutes follows the peak exercise stage. Throughout the exercise protocol a cardiac ultrasound probe is actively used to acquire real-time echocardiographic images.
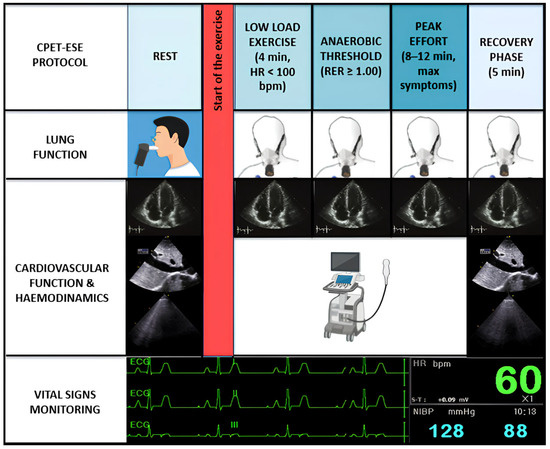
Figure 1.
Combined CPET-ESE protocol. CPET-ESE enables an objective assessment of exercise intolerance using a graded ramp protocol to ensure gradual workload increase. Briefly, we designed a structured four-stage protocol: (i) rest, (ii) low-load effort (HR < 100 bpm), (iii) anaerobic threshold (RER ≥ 1.00), and (iv) peak effort, followed by a recovery phase. The test includes continuous breath-by-breath gas exchange analysis, real-time echocardiographic imaging, ECG, BP, and HR monitoring. Preceding spirometry helps identify pulmonary abnormalities contributing to exercise limitation.
After a brief warm-up phase, a symptom-limited graded ramp test is initiated, featuring continuous breath-by-breath gas exchange analysis, along with the ongoing monitoring of blood pressure (BP) and HR. Throughout the exercise, a 12-lead electrocardiogram is performed. Likewise, a functional pulmonary evaluation using spirometry should precede CPET-ESE to identify lung abnormalities associated with exercise intolerance, particularly moderate or greater airflow obstruction (i.e., a forced expiratory volume in the first second [FEV1] to forced vital capacity [FVC] ratio < 50% of predicted), restrictive patterns (FVC < 80% of predicted), or exercise-induced bronchospasm. Incremental ramps allow for a gradual and low-intensity workload increase (8–15 W/min), different from the more abrupt increment used in the Bruce protocol stress test to diagnose CAD [7]. To collect clear echocardiographic images, patients are typically asked to exercise in a semi-reclined position, which slightly differs from standard CPET protocols using upright cycle ergometers or treadmills. However, there appears to be little to no difference in peak VO2 achieved between these methods. Despite its advantages, CPET-ESE remains underutilised due to logistical challenges and the need for trained multidisciplinary teams. Further studies are warranted to validate its prognostic value and integrate it into routine clinical algorithms.
6. CPET-ESE Role in the Diagnosis and Risk Stratification of Cardiovascular Diseases
6.1. HF Spectrum
CPET-ESE may hold a pivotal tool in the diagnosis and risk stratification of patients with HFrEF (LVEF < 40%), preserved ejection fraction (HFpEF, LVEF ≥ 50%), or mid-range LVEF (40–49%, HFmrEF).
Patients with HF often suffer from multiple comorbidities, making it difficult to rely solely on clinical data; thus, CPET-ESE helps clinicians to identify the pathophysiologic and prognostic impact of cardiovascular and ventilatory alterations contributing to exercise intolerance [7,56]. Moreover, this technique allows for the early identification of patients with initial impairment before symptoms manifest at rest. The transition from cardiometabolic conditions, such as hypertension and diabetes, to overt HF is often subtle, and in the early stages, it only becomes evident under physiological stress [21,57,58,59]. Reduced peak VO2 values, associated with decreased AVO2diff and mild signs of left ventricular systolic dysfunction, have been shown in hypertensive, asymptomatic subjects. Similarly, pulmonary congestion markers, namely a steeper VE/VCO2 slope and increased B-lines, have been observed in hypertensive patients and latent HFpEF [8]. Increased central arterial stiffness and altered BP pulsatility significantly correlate with reduced peak VO2 in a similar population of hypertensive subjects [17]. Similarly, in the evaluation of ventricular–arterial coupling through resting, Doppler-derived proximal aortic stiffness (aa-PWV) has proven feasible and reproducible across the heart failure spectrum, with the aa-PWV/GLS ratio emerging as an independent predictor of peak VO2 and functional capacity impairment [60]. Hypertensive response to exercise is also associated with impaired functional capacity across the HF spectrum [21].
In HFmrEF patients, the reduced peak VO2 observed during CPET may be primarily driven by impaired AVO2diff, suggesting a physiological behaviour closer to HFpEF than HFrEF. This diminished peripheral oxygen extraction capability—commonly observed in “poor extractors”—highlights the potential value of interventions aimed at enhancing skeletal muscle perfusion and oxygen utilisation in this subgroup. Combined CPET-ESE thus enhances phenotypic characterisation and functional assessment in HFmrEF, bridging the diagnostic and therapeutic gap between reduced and preserved ejection fraction phenotypes [61].
Pulmonary comorbidities play a crucial role in the progression and symptom burden of HF, especially in HFpEF, as follows: increased pulmonary capillary wedge pressure during exertion leads to fluid accumulation in the lungs, triggering vascular dysfunction and impaired pulmonary mechanics. This results in ventilation inefficiency and exercise-induced dyspnoea, which are common in HFpEF patients. In patients with overt or suspected HFpEF, CPET-ESE helps investigate the relationship between exercise-induced pulmonary hypertension, excess lung water accumulation, and ventilation–perfusion mismatch [8,17]. The detection of an exaggerated rise in pulmonary vascular resistance during exercise, impaired pulmonary diffusing capacity, and increased VD/VT indicate a maladaptive pulmonary hemodynamic response. Such findings correlate with disease progression and poorer prognosis, highlighting the critical role of pulmonary dysfunction in limiting functional capacity and worsening symptoms in HFpEF patients [62,63,64].
6.2. Aortic Stenosis and Regurgitation
Severe symptomatic aortic stenosis (AS) is typically an indication of aortic valve replacement. However, many patients with degenerative aortic stenosis (AS)—especially elderly individuals in Western countries—may appear asymptomatic due to activity self-limitation or may report non-specific dyspnoea stemming from a variety of extra-valvular and extracardiac comorbidities [54]. In such cases, CPET-ESE proves highly valuable by objectively quantifying exercise intolerance via peak VO2 and correlating findings with additional CPET-ESE parameters to determine dyspnoea aetiology. Furthermore, CPET-ESE helps detect the worsening of AS during exertion, impaired functional capacity, and the onset of symptoms, which can guide changes in the patient’s therapeutic approach [29]. ESE examination is critical as it evaluates the hemodynamic severity of the condition. This includes assessing peak transvalvular velocity, mean transvalvular pressure gradient, estimated aortic valve area, and the presence or absence of LV functional flow or contractile reserve (i.e., appropriate SV or LVEF increase during exercise). ESE can also identify dynamic increases in sPAP and pulmonary congestion throughout exercise, which carry negative prognostic implications [65]. Meanwhile, CPET correlates echocardiographic signs of pulmonary congestion with data on ventilation–perfusion mismatch, specifically through the VE/VCO2 slope. These combined measures significantly enhance prognostic assessment and risk stratification in AS patients [1]. Recently, mPAP/CO, a sign of pulmonary hypertension, has been proposed in AS evaluation as an early marker of pulmonary vascular and myocardial maladaptation. A high mPAP/CO slope and low peak VO2 resulted in worse outcomes, including HF hospitalisation, atrial fibrillation, and cardiovascular mortality [9]. Their integration into clinical evaluations could refine decision-making regarding aortic valve replacement and provide insights into disease progression beyond conventional severity markers such as aortic valve area and LA volume index.
Similarly, CPET-ESE plays a crucial role in assessing aortic regurgitation (AR), mainly in asymptomatic patients, allowing for the detection of early signs of LV decompensation, such as an insufficient increase in LVEF, impaired contractile reserve, or even subclinical systolic dysfunction with GLS. Additionally, ESE can evaluate exercise-induced aortic regurgitation and identify patients at risk of deterioration, aiding in optimal treatment planning and follow-up [66]. An increase in sPAP or the development of exercise-induced mitral regurgitation are indicators of progression and may necessitate early intervention [66,67,68]. In chronic AR, the LV faces a significant volume overload, which, if prolonged, results in left ventricular dilation and secondary increases in LA pressures. Elevated LV pressures are transmitted backwards into the pulmonary circulation, predisposing those affected to the development of secondary PH. Under exercise conditions, the mismatch between increased cardiac output and fixed pulmonary vascular abnormalities is expected to steepen the mPAP/CO slope. Chronic pressure overload from PH may also progressively impair RV function, lowering the TAPSE/sPAP ratio.
6.3. Mitral Regurgitation and Stenosis
In patients with asymptomatic or mildly symptomatic mitral regurgitation (MR), CPET-ESE is a valuable tool to assess functional capacity and unmask the underlying causes of any limitations. MR severity assessment at rest and peak exercise includes semi-quantitative techniques (vena contracta) and quantitative indices (effective regurgitant orifice area, regurgitant volume, and regurgitant fraction). By tracking these parameters throughout exercise, CPET-ESE unveils the progression of valvular dysfunction and its impact on functional performance [29]. ESE is particularly useful when there is a discrepancy between the severity of MR observed at rest and the presence of symptoms, as it can reveal an abnormal hemodynamic response that suggests the need for closer monitoring or early surgical intervention. A rise in LV filling pressure can be observed, subsequently affecting the pulmonary circulation, leading to increased sPAP, lung congestion, and ventilation–perfusion mismatch. An increase in sPAP beyond 60 mmHg during ESE has been linked to symptom onset, offering a valuable diagnostic tool for identifying patients at higher risk of deterioration despite appearing asymptomatic at baseline [69]. These signs are reflected in a steeper VE/VCO2 slope. In more severe cases, EOV may occur, particularly in patients with secondary MR, such as those with tethered mitral valve leaflets in a dilated and dysfunctional LV [29]. CPET-derived parameters, particularly reduced peak VO2, have been associated with increased mortality and HF hospitalisations. Moreover, the inability to achieve an adequate exercise response may indicate a higher risk of major adverse cardiovascular events. By identifying patients with poor exercise tolerance and impaired cardiopulmonary reserve, CPET-ESE helps stratify risk and optimise therapeutic strategies, reinforcing its role as a key prognostic tool in MR evaluation [70]. In MR, the regurgitant volume directly increases LA pressures even at rest, leading to chronic pulmonary venous hypertension. Exercise exacerbates this hemodynamic burden by increasing the regurgitant fraction and further elevating pulmonary artery pressures. Consequently, the limited ability of pulmonary circulation to accommodate the increased flow results in an abnormal mPAP/CO slope during exertion [71]. Simultaneously, the sustained pressure overload and remodelling of the pulmonary vasculature eventually impair RV performance, contributing to a decreased TAPSE/sPAP ratio, especially during dynamic conditions like exercise. It has been demonstrated that the mPAP/CO slope and TAPSE/sPAP slope correlate with simultaneously measured invasive catheterisation values and accurately predicted effort intolerance, independently of exercise-induced changes in MR severity or peak pulmonary artery pressures [47].
ESE is an essential tool in evaluating mitral stenosis (MS), particularly in cases where resting echocardiography may underestimate its severity. The exercise can reveal significant increases in mean transvalvular gradient and sPAP, indicating hemodynamic impairment. A marked rise in mPAP or sPAP during exertion suggests limited hemodynamic reserve and a higher likelihood of symptom progression. These findings support decision-making regarding balloon valvuloplasty or surgical intervention. ESE becomes particularly valuable in patients preparing for major surgery or pregnancy, as it predicts the likelihood of decompensation with increased cardiac demand [66]. MS creates a fixed obstruction to left ventricular filling, causing the persistent elevation of LA pressure, which is directly transmitted to the pulmonary veins and arteries. As a result, secondary PH is a common complication, often worsening under stress or exercise. The inability of the pulmonary vasculature to handle increased CO during exertion leads to a disproportionate rise in pulmonary artery pressures, manifesting as an increased mPAP/CO slope. Prolonged PH in MS also exposes the RV to chronic afterload stress, progressively leading to RV-PA uncoupling and a reduced TAPSE/sPAP ratio.
6.4. Hypertrophic Cardiomyopathy
Hypertrophic cardiomyopathy (HCM) is a multifaceted condition, with several factors that could contribute to exercise limitation, including left ventricular diastolic dysfunction, left ventricular outflow tract (LVOT) obstruction, and potential peripheral muscle alterations. Around 5% of patients with HCM develop systolic dysfunction, with ventricular wall thinning and cavity dilation, leading to exercise intolerance mechanisms similar to those seen in systolic HF. A key determinant of reduced exercise capacity in HCM is the inability to increase SV [72]. Exercise capacity seems primarily influenced by reduced SV, which correlates inversely with the time to peak left ventricular filling [73]. Further studies have confirmed that peak VO2 is closely related to peak cardiac index, itself linked to diastolic function markers such as E/e’ [74]. LVOT obstruction significantly limits SV augmentation during exercise and can exacerbate mitral regurgitation, elevate pulmonary pressures, and further impair diastolic function by prolonging systole. ESE can reveal stress-induced LVOT obstruction, worsening mitral regurgitation, and latent diastolic dysfunction. Chronotropic incompetence is another factor that contributes to exercise limitation in HCM. While its exact mechanisms are not fully understood, potential causes include sinoatrial node remodelling, altered beta-receptor function, and impaired intracellular calcium signalling [75]. Finally, many HCM patients experience deconditioning due to reduced activity levels, which can impair peripheral oxygen extraction [76]. Current HCM Guidelines suggest CPET for identifying candidates for septal reduction procedures [77]. Moreover, an independent relationship between peak VO2, VE/VCO2 slope, and HF-related outcomes (i.e., death and transplantation [78]) has been demonstrated.
Diastolic dysfunction is a hallmark feature in HCM, resulting from impaired ventricular relaxation and increased myocardial stiffness. This leads to elevated LV end-diastolic pressure and subsequent elevation of left atrial pressure. Over time, the chronic rise in pulmonary venous pressure can induce post-capillary PH. During exercise, the inability of the stiff LV to accommodate increased preload exacerbates pulmonary pressures, leading to a steeper mPAP/CO slope. Furthermore, the chronic RV afterload stress may impair RV-PA coupling, manifesting as a reduced TAPSE/sPAP ratio. It has been demonstrated that impaired TAPSE and a reduced TAPSE/sPAP ratio during exercise are independent predictors of adverse outcomes in patients with non-obstructive HCM [79].
6.5. CAD
CPET-ESE is particularly useful in evaluating patients with CAD when traditional stress testing is inconclusive, as it can detect abnormalities in oxygen delivery and utilisation before overt ischemia occurs [10]. Moreover, ESE is a valuable tool for detecting CAD by assessing regional wall motion abnormalities induced by ischemia, although CPET-ESE is less sensitive than the Bruce protocol in diagnosing CAD [80]. ESE’s high specificity makes it particularly effective in distinguishing true ischemic abnormalities from non-obstructive causes, especially in women, where microvascular dysfunction and endothelial abnormalities may contribute to ischemic symptoms despite normal coronary angiography [81]. HR response is a CPET parameter evaluated in real-time per the Fick equation. The ΔHR–WR slope (change in heart rate to work-rate slope) compares the slope of heart rate increase during the final 2 min of exercise to that during the mid-exercise phase, providing insight into cardiovascular response and potential chronotropic incompetence. Values >15% (positive ΔHR–WR slope) indicate abnormal compensatory HR acceleration in late exercise. Healthy individuals exhibit zero or negative ΔHR–WR slopes (no change or deceleration of HR response [82]). In patients with advanced CAD who cannot increase their HR (a condition present also in patients with autonomic dysfunction or chronotropic incompetence or due to HR-limiting medications), there is an abrupt plateau or decline in SV, leading to a reduction in CO [82]. This decrease is reflected in the characteristic flattened ΔVO2/ΔWR [83,84]. CPET offers valuable prognostic insights into long-term mortality in patients with established CAD. For women with CAD, each 1 mL/kg/min increase in peak VO2 is associated with a 10% reduction in mortality [85]. Moreover, a peak VO2 < 16 mL/kg/min post-myocardial infarction or post-percutaneous coronary intervention indicates an increased risk of adverse events over 2 years [82]. On the contrary, even modest improvements in peak VO2 correlate with enhanced survival, reduced cardiovascular risk, and better quality of life [86].
6.6. CPET-ESE in Pulmonary Diseases
CPET-ESE provides detailed submaximal and maximal exercise data that help identify abnormal hemodynamic responses associated with PH and RV dysfunction. By analysing pulsatile pulmonary vascular pressure–flow relationships, CPET allows for assessing RV hemodynamic load, which is crucial in understanding the disease’s impact on cardiac function [87].
During the examination, various indicators can signal RV issues, such as the flattening of the interventricular septum, which leads to a D-shaped LV, suggesting RV pressure or volume overload. Additionally, impaired RV- PA coupling, assessed through the TAPSE/sPAP ratio, worsening tricuspid regurgitation (TR), and increased B-lines all point to elevated LV filling pressures and increased ventricular interdependence [43].
Evidence demonstrates that CPET-ESE can unmask different RV contractile reserve phenotypes in heart failure patients. Importantly, RV dysfunction at rest does not necessarily indicate a poor adaptive response during exercise. By integrating CPET-derived gas exchange parameters with echocardiographic markers such as TAPSE and PASP during exercise, it is possible to non-invasively assess RV-to-pulmonary circulation coupling and contractile reserve. This has important clinical implications, as impaired RV reserve is associated with reduced ventilatory efficiency, increased ventilatory oscillations, and a higher risk of adverse events. Furthermore, exercise-induced changes in PASP and the presence of significant mitral regurgitation at rest are strong correlates of impaired RV performance. Therefore, CPET-ESE provides a powerful tool to identify RV dysfunction that is not evident at rest and may help stratify risk and guide advanced therapeutic decisions [88].
Additionally, CPET is particularly useful in differentiating the exertional limitations caused by chronic obstructive pulmonary disease or interstitial lung disease from those primarily driven by PH [89]. Blanco et al. showed that PH significantly reduces peak VO2 and oxygen pulse volume, indicating an interaction between ventilatory limitation and cardiovascular restriction [90]. Reduced peak VO2 is a strong prognostic marker for disease progression and mortality in PH patients [26,87], as well as RV strain and TAPSE/sPAP [91]. Lower TAPSE/PASP values correlate with higher disease severity and independently predict overall survival and disease severity in pulmonary arterial hypertension, performing better than other echocardiographic indices of RV function by integrating both RV contractility and pulmonary afterload [92]. Also, the mPAP/CO slope is valuable for characterising pulmonary circulation during exercise. Unlike the absolute values of mPAP, the mPAP/CO slope remains largely unaffected by variations in workload, providing a more stable and reliable measure. It is particularly relevant in pulmonary diseases, as an increased slope is associated with worse survival across various cardiopulmonary conditions [93].
Another application of CPET-ESE is the assessment of patients following an episode of pulmonary embolism (PE). CPET-ESE can provide objective insights into exercise limitation patterns, distinguishing between ventilatory inefficiency and insufficient cardiocirculatory reserve, essential for diagnosing the sequelae of PE, such as chronic thromboembolic pulmonary hypertension (CTEPH) and post-PE impairment. At three months post-PE, approximately 50% of patients exhibit some degree of cardiopulmonary exercise limitation, with ventilatory inefficiency being the predominant abnormality. This pattern persists in nearly 45% of patients at twelve months, highlighting the potential long-term impact of PE on pulmonary and cardiovascular function [26]. CPET effectively identifies these abnormalities by evaluating parameters such as VE/VCO2 and peak oxygen pulse. In patients diagnosed with CTEPH, severe ventilatory inefficiency is a consistent finding, but insufficient cardiocirculatory reserve is also commonly present. These findings underscore CPET diagnostic utility in differentiating post-PE impairment from deconditioning, particularly in cases where echocardiographic findings at rest are inconclusive [94]. Among patients without significant chronic comorbidities, those who experienced an intermediate- or high-risk PE event are significantly more likely to exhibit persistent or worsening cardiopulmonary limitation. This highlights the importance of CPET in the early identification of at-risk individuals, guiding further investigations such as ventilation–perfusion lung scans and, when indicated, right heart catheterisation. Current recommendations emphasise structured follow-up after PE, and CPET-ESE offers valuable insight for guiding risk stratification, functional recovery, and individualised rehabilitation strategies. While not yet a standard component of routine post-PE evaluation, its ability to provide objective and reproducible measurements of functional impairment suggests that it could play a greater role in the comprehensive management of PE survivors [26].
7. Conclusions and Clinical Perspectives
CPET-ESE has emerged as a crucial diagnostic and prognostic tool in assessing cardiovascular and pulmonary diseases. Its combined approach provides a comprehensive understanding of functional capacity, helping clinicians evaluate the cardiovascular system and the pulmonary and peripheral responses to exercise. Beyond diagnosis, CPET-ESE is essential for risk stratification and prognosis. It allows clinicians to identify high-risk patients who may benefit from early intervention, individualised treatment strategies, or closer follow-up. The prognostic value of CPET parameters such as peak VO2 and VE/VCO2 slope is well-established across various cardiovascular conditions, helping to predict adverse events, disease progression, and mortality [70]. Applications of CPET-ESE are increasing, as traditionally, this method was reserved for evaluating candidates for heart transplantation or mechanical support. However, recent European guidelines have expanded their use to assess exercise intolerance severity and evaluate BP responses to exercise [7,95]. Importantly, CPET-ESE is not limited to patients with overt cardiovascular disease. It is also valuable in identifying early functional impairment in asymptomatic individuals and guiding exercise-based rehabilitation programmes. The CPET-ESE protocol is not readily available in all clinical settings due to several technical and logistical limitations, including high costs, time requirements, the need for equipment and trained personnel, and the possibility that some patients may be unable to perform physical exertion. Nonetheless, integrating CPET-ESE into routine clinical practice can enhance patient outcomes by improving diagnostic accuracy, refining therapeutic decisions, and optimising risk management. Despite its clinical potential, the broader implementation of CPET-ESE is limited by heterogeneity in protocols and the need for advanced operator training.
Hence, as research advances, future developments in CPET-ESE may further expand its clinical applications, focusing on a more individualised approach to diagnosis and treatment, leading to improved patient care. Multicenter prospective studies are required to standardise CPET-ESE protocols and define clear indications. Moreover, research should focus on cost-effectiveness, training requirements, and integration into clinical workflows. By bridging the gap between cardiac and pulmonary physiology, CPET-ESE stands as a cornerstone of modern cardiopulmonary evaluation, offering a more precise and personalised approach to cardiovascular care and allowing more effective management strategies.
This review highlights the rationale, protocol, and clinical relevance of CPET-ESE and encourages the broader implementation of this powerful diagnostic strategy to fill current gaps in the evaluation of exertional symptoms. By synthesising current insights and identifying gaps in knowledge, this narrative review underscores the evolving role of integrated CPET-ESE protocols in the non-invasive assessment of functional and hemodynamic limitations—a promising field for further clinical validation and innovation.
Author Contributions
Conceptualization, N.R.P. and I.F.; methodology, V.D.F. and L.D.P.; software, N.D.B.; validation, V.D.F., L.D.P., and N.D.B.; formal analysis, V.D.F.; investigation, L.D.P. and N.D.B.; resources, S.M. and S.T.; data curation, L.D.P. and N.D.B.; writing—original draft preparation, V.D.F. and L.D.P.; writing—review and editing, J.R., M.E., and C.P.; visualisation, V.D.F.; supervision, I.F. and N.R.P.; project administration, N.R.P.; funding acquisition, S.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable. No new data were created or analysed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Del Punta, L.; De Biase, N.; Armenia, S.; Di Fiore, V.; Maremmani, D.; Gargani, L.; Mazzola, M.; De Carlo, M.; Mengozzi, A.; Lomonaco, T.; et al. Combining cardiopulmonary exercise testing with echocardiography: A multiparametric approach to the cardiovascular and cardiopulmonary systems. Eur. Heart J.—Imaging Methods Pract. 2023, 1, qyad021. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Olson, T.P.; Obokata, M.; Melenovsky, V.; Borlaug, B.A. Hemodynamic Correlates and Diagnostic Role of Cardiopulmonary Exercise Testing in Heart Failure With Preserved Ejection Fraction. JACC Heart Fail. 2018, 6, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.R.; De Biase, N.; Balletti, A.; Filidei, F.; Pieroni, A.; D’Angelo, G.; Armenia, S.; Mazzola, M.; Gargani, L.; Del Punta, L.; et al. Characterization of hemodynamic and metabolic abnormalities in the heart failure spectrum: The role of combined cardiopulmonary and exercise echocardiography stress test. Minerva Cardiol. Angiol. 2022, 70, 370–384. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, I.; Pugliese, N.R.; Galeotti, G.G.; D’Agostino, A.; Mazzola, M.; Pedrinelli, R.; Dini, F.L. The Added Value of Exercise Stress Echocardiography in Patients With Heart Failure. Am. J. Cardiol. 2019, 123, 1470–1477. [Google Scholar] [CrossRef]
- Donal, E.; Lund, L.H.; Oger, E.; Reynaud, A.; Schnell, F.; Persson, H.; Drouet, E.; Linde, C.; Daubert, C. Value of exercise echocardiography in heart failure with preserved ejection fraction: A substudy from the KaRen study. Eur Heart J Cardiovasc. Imaging 2016, 17, 106–113. [Google Scholar] [CrossRef]
- Leopold, J.A.; Loscalzo, J. Emerging Role of Precision Medicine in Cardiovascular Disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef]
- Pugliese, N.R.; De Biase, N.; Gargani, L.; Mazzola, M.; Conte, L.; Fabiani, I.; Natali, A.; Dini, F.L.; Frumento, P.; Rosada, J.; et al. Predicting the transition to and progression of heart failure with preserved ejection fraction: A weighted risk score using bio-humoural, cardiopulmonary, and echocardiographic stress testing. Eur. J. Prev. Cardiol. 2021, 28, 1650–1661. [Google Scholar] [CrossRef]
- De Biase, N.; Mazzola, M.; Del Punta, L.; Di Fiore, V.; De Carlo, M.; Giannini, C.; Costa, G.; Paneni, F.; Mengozzi, A.; Nesti, L.; et al. Haemodynamic and metabolic phenotyping of patients with aortic stenosis and preserved ejection fraction: A specific phenotype of heart failure with preserved ejection fraction? Eur. J. Heart Fail. 2023, 25, 1947–1958. [Google Scholar] [CrossRef]
- Hoedemakers, S.; Pugliese, N.R.; Stassen, J.; Vanoppen, A.; Claessens, J.; Gojevic, T.; Bekhuis, Y.; Falter, M.; Ferreira, S.M. mPAP/CO Slope and Oxygen Uptake Add Prognostic Value in Aortic Stenosis. Circulation 2024, 149, 1172–1182. [Google Scholar] [CrossRef]
- Conraads, V.M.; Pattyn, N.; De Maeyer, C.; Beckers, P.J.; Coeckelberghs, E.; Cornelissen, V.A.; Denollet, J.; Frederix, G.; Goetschalckx, K.; Hoymans, V.Y.; et al. Aerobic interval training and continuous training equally improve aerobic exercise capacity in patients with coronary artery disease: The SAINTEX-CAD study. Int. J. Cardiol. 2015, 179, 203–210. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for cardiac chamber quantification by echocardiography in adults: An update from the American society of echocardiography and the European association of cardiovascular imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef] [PubMed]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 focused update: Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2016, 133, e694–e711. [Google Scholar] [CrossRef] [PubMed]
- Di Fiore, V.; Del Punta, L.; De Biase, N.; Pellicori, P.; Gargani, L.; Dini, F.L.; Armenia, S.; Li Vigni, M.; Maremmani, D.; Masi, S.; et al. Integrative assessment of congestion in heart failure using ultrasound imaging. Intern. Emerg. Med. 2024, 20, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Dhakal, B.P.; Malhotra, R.; Murphy, R.M.; Pappagianopoulos, P.P.; Baggish, A.L.; Weiner, R.B.; Houstis, N.E.; Eisman, A.S.; Hough, S.S.; Lewis, G.D. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circ. Heart Fail. 2015, 8, 286–294. [Google Scholar] [CrossRef]
- Mancini, D.M.; Eisen, H.; Kussmaul, W.; Mull, R.; Edmonds, L.H.; Wilson, J.R. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation 1991, 83, 778–786. [Google Scholar] [CrossRef]
- Tumminello, G.; Guazzi, M.; Lancellotti, P.; Piérard, L.A. Exercise ventilation inefficiency in heart failure: Pathophysiological and clinical significance. Eur. Heart J. 2007, 28, 673–678. [Google Scholar] [CrossRef]
- Guazzi, M.; Wilhelm, M.; Halle, M.; Van, E.; Kemps, H.; de Boer, R.A.; Coats, A.J.S.; Lund, L.; Mancini, D.; Borlaug, B.; et al. Exercise testing in heart failure with preserved ejection fraction : An appraisal through diagnosis, pathophysiology and therapy—A clinical consensus statement of the Heart Failure Association and European Association of Preventive Cardiology of the European Society of Cardiology. Eur. J. Hear. Fail. 2022, 24, 1327–1345. [Google Scholar]
- Guazzi, M.; Adams, V.; Conraads, V.; Halle, M.; Mezzani, A.; Vanhees, L.; Arena, R.; Fletcher, G.F.; Forman, D.E.; Kitzman, D.W.; et al. Clinical recommendations for cardiopulmonary exercise testing data assessment in specific patient populations. Circulation 2012, 126, 2261–2274. [Google Scholar] [CrossRef]
- Laveneziana, P.; Di Paolo, M.; Palange, P. The clinical value of cardiopulmonary exercise testing in the modern era. Eur. Respir. Rev. 2021, 30, 200187. [Google Scholar] [CrossRef]
- Brubaker, P.H.; Kitzman, D.W. Chronotropic incompetence: Causes, consequences, and management. Circulation 2011, 123, 1010–1020. [Google Scholar] [CrossRef]
- Pugliese, N.R.; De Biase, N.; Del Punta, L.; Balletti, A.; Armenia, S.; Buralli, S.; Mengozzi, A.; Taddei, S.; Metra, M.; Pagnesi, M.; et al. Deep phenotype characterization of hypertensive response to exercise: Implications on functional capacity and prognosis across the heart failure spectrum. Eur. J. Heart Fail. 2023, 25, 497–509. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, W.T.; Qureshi, W.T.; Blaha, M.J.; Keteyian, S.J.; Brawner, C.A.; Al-mallah, M.H. Systolic Blood Pressure Response During Exercise Stress Testing: The Henry Ford ExercIse Testing (FIT) Project. J. Am. Heart Assoc. 2015, 4, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pugliese, N.R.; Mazzola, M.; Fabiani, I.; Gargani, L.; De Biase, N.; Pedrinelli, R.; Natali, A.; Dini, F.L. Haemodynamic and metabolic phenotyping of hypertensive patients with and without heart failure by combining cardiopulmonary and echocardiographic stress test. Eur. J. Heart Fail. 2020, 22, 458–468. [Google Scholar] [CrossRef]
- Hansen, J.E.; Sue, D.Y.; Wasserman, K. Predicted values for clinical exercise testing. Am. Rev. Respir. Dis. 1984, 129, S49–S55. [Google Scholar] [CrossRef] [PubMed]
- Weber, K.T.; Kinasewitz, G.T.; Janicki, J.S.; Fishman, A.P. Oxygen utilization and ventilation during exercise in patients with chronic cardiac failure. Circulation 1982, 65, 1213–1223. [Google Scholar] [CrossRef]
- Farmakis, I.T.; Valerio, L.; Barco, S.; Alsheimer, E.; Ewert, R.; Giannakoulas, G.; Hobohm, L.; Keller, K.; Mavromanoli, A.C.; Rosenkranz, S.; et al. Cardiopulmonary exercise testing during follow-up after acute pulmonary embolism. Eur. Respir. J. 2023, 61, 2300059. [Google Scholar] [CrossRef]
- Guazzi, M.; Boracchi, P.; Labate, V.; Arena, R.; Reina, G. Exercise Oscillatory Breathing and NT-proBNP Levels in Stable Heart Failure Provide the Strongest Prediction of Cardiac Outcome When Combining Biomarkers With Cardiopulmonary Exercise Testing. J. Card. Fail. 2012, 18, 313–320. [Google Scholar] [CrossRef]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Lancellotti, P.; Pellikka, P.A.; Budts, W.; Chaudhry, F.A.; Donal, E.; Dulgheru, R.; Edvardsen, T.; Garbi, M.; Ha, J.-W.; Kane, G.C.; et al. The clinical use of stress echocardiography in non-ischaemic heart disease: Recommendations from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. Eur. Heart J.—Cardiovasc. Imaging 2016, 17, 1191–1229. [Google Scholar] [CrossRef]
- Eek, C.; Brunvand, H.; Aakhus, S.; Endresen, K.; Hol, P.K.; Smiseth, O.A.; Edvardsen, T.; Skulstad, H. Strain echocardiography and wall motion score index predicts final Infarct size in patients with non-ST-segment-elevation myocardial infarction. Circ. Cardiovasc. Imaging 2010, 3, 187–194. [Google Scholar] [CrossRef]
- Patel, H.N.; Miyoshi, T.; Addetia, K.; Henry, M.P.; Citro, R.; Daimon, M.; Fajardo, P.G.; Kasliwal, R.R.; Kirkpatrick, J.N.; Monaghan, M.J.; et al. Normal Values of Cardiac Output and Stroke Volume According to Measurement Technique, Age, Sex, and Ethnicity: Results of the World Alliance of Societies of Echocardiography Study. J. Am. Soc. Echocardiogr. 2021, 34, 1077–1085.e1. [Google Scholar] [CrossRef] [PubMed]
- Charoenpanichkit, C.; Little, W.C.; Mandapaka, S.; Dall’ARmellina, E.; Morgan, T.M.; Hamilton, C.A.; Hundley, W.G. Impaired left ventricular stroke volume reserve during clinical dobutamine stress predicts future episodes of pulmonary Edema. J. Am. Coll. Cardiol. 2011, 57, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Daros, C.B.; Ciampi, Q.; Cortigiani, L.; Gaibazzi, N.; Rigo, F.; Wierzbowska-Drabik, K.; Kasprzak, J.D.; Dodi, C.; Villari, B.; Antonini-Canterin, F.; et al. Coronary flow, left ventricular contractile and heart rate reserve in non-ischemic heart failure. J. Clin. Med. 2021, 10, 3405. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Sun, Y.-P.; Divakaran, S.; Bajaj, N.S.; Gupta, A.; Chandra, A.; Morgan, V.; Barrett, L.; Martell, L.; Bibbo, C.F.; et al. Association of Myocardial Blood Flow Reserve With Adverse Left Ventricular Remodeling in Patients With Aortic Stenosis: The Microvascular Disease in Aortic Stenosis (MIDAS) Study. JAMA Cardiol. 2022, 7, 93–99. [Google Scholar] [CrossRef]
- Pugliese, N.R.; De Biase, N.; Conte, L.; Gargani, L.; Mazzola, M.; Fabiani, I.; Natali, A.; Dini, F.L.; Frumento, P.; Rosada, J.; et al. Cardiac Reserve and Exercise Capacity: Insights from Combined Cardiopulmonary and Exercise Echocardiography Stress Testing. J. Am. Soc. Echocardiogr. 2021, 34, 38–50. [Google Scholar] [CrossRef]
- Nagueh, S.F.; Smiseth, O.A.; Appleton, C.P.; Byrd, B.F., 3rd; Dokainish, H.; Edvardsen, T.; Flachskampf, F.A.; Gillebert, T.C.; Klein, A.L.; Lancellotti, P.; et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Echocardiogr. 2016, 29, 277–314. [Google Scholar] [CrossRef]
- Sharifov, O.F.; Gupta, H. What Is the Evidence That the Tissue Doppler Index E/e′ Reflects Left Ventricular Filling Pressure Changes After Exercise or Pharmacological Intervention for Evaluating Diastolic Function? A Systematic Review. J. Am. Heart Assoc. 2017, 6, e004766. [Google Scholar] [CrossRef]
- Yingchoncharoen, T.; Agarwal, S.; Popović, Z.; Marwick, T. Normal Ranges of Left Ventricular Strain: A Meta-Analysis. J. Am. Soc. Echocardiogr. 2013, 26, 185–191. [Google Scholar] [CrossRef]
- Reddy, Y.N.; Obokata, M.; Egbe, A.; Yang, J.H.; Pislaru, S.; Lin, G.; Carter, R.; Borlaug, B.A. Left atrial strain and compliance in the diagnostic evaluation of heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2019, 21, 891–900. [Google Scholar] [CrossRef]
- Pathan, F.; D’ELia, N.; Nolan, M.T.; Marwick, T.H.; Negishi, K. Normal Ranges of Left Atrial Strain by Speckle-Tracking Echocardiography: A Systematic Review and Meta-Analysis. J. Am. Soc. Echocardiogr. 2017, 30, 59–70.e8. [Google Scholar] [CrossRef]
- Sugimoto, T.; Bandera, F.; Generati, G.; Alfonzetti, E.; Bussadori, C.; Guazzi, M. Left Atrial Function Dynamics During Exercise in Heart Failure: Pathophysiological Implications on the Right Heart and Exercise Ventilation Inefficiency. JACC Cardiovasc. Imaging 2017, 10, 1253–1264. [Google Scholar] [CrossRef] [PubMed]
- Reddy, Y.N.V.; Obokata, M.; Verbrugge, F.H.; Lin, G.; Borlaug, B.A. Atrial Dysfunction in Patients with Heart Failure with Preserved Ejection Fraction and Atrial Fibrillation. J. Am. Coll. Cardiol. 2020, 76, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Ricci, F.; Bufano, G.; Galusko, V.; Sekar, B.; Benedetto, U.; Awad, W.I.; Di Mauro, M.; Gallina, S.; Ionescu, A.; Badano, L.; et al. Tricuspid regurgitation management: A systematic review of clinical practice guidelines and recommendations. Eur. Heart J.—Qual. Care Clin. Outcomes 2022, 8, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Topyła-Putowska, W.; Tomaszewski, M.; Wojtkowska, A.; Styczeń, A.; Wysokiński, A. Tricuspid Regurgitation Velocity/Tricuspid Annular Plane Systolic Excursion (TRV/TAPSE) Ratio as a Novel Indicator of Disease Severity and Prognosis in Patients with Precapillary Pulmonary Hypertension. Diseases 2023, 11, 117. [Google Scholar] [CrossRef]
- Guazzi, M.; Bandera, F.; Pelissero, G.; Castelvecchio, S.; Menicanti, L.; Ghio, S.; Temporelli, P.L.; Arena, R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: An index of right ventricular contractile function and prognosis. Am. J. Physiol.—Heart Circ. Physiol. 2013, 305, H1373–H1381. [Google Scholar] [CrossRef]
- Gargani, L.; Pugliese, N.R.; De Biase, N.; Mazzola, M.; Agoston, G.; Arcopinto, M.; Argiento, P.; Armstrong, W.F.; Bandera, F.; Cademartiri, F.; et al. Exercise Stress Echocardiography of the Right Ventricle and Pulmonary Circulation. J Am Coll Cardiol 2023, 82, 1973–1985. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Hidaka, T.; Susawa, H.; Izumi, K.; Harada, Y.; Kinoshita, M.; Itakura, K.; Masada, K.; Kihara, Y. Exercise-Stress Echocardiography and Effort Intolerance in Asymptomatic/Minimally Symptomatic Patients with Degenerative Mitral Regurgitation Combined Invasive-Noninvasive Hemodynamic Monitoring. Circ. Cardiovasc. Imaging 2018, 11, e007282. [Google Scholar] [CrossRef]
- Zeder, K.; Banfi, C.; Steinrisser-Allex, G.; Maron, B.A.; Humbert, M.; Lewis, G.D.; Berghold, A.; Olschewski, H.; Kovacs, G. Diagnostic, prognostic and differential-diagnostic relevance of pulmonary haemodynamic parameters during exercise : A systematic review. Eur. Respir. J. 2022, 60, 2103181. [Google Scholar] [CrossRef]
- Chemla, D.; Castelain, V.; Humbert, M.; Heébert, J.-L.; Simonneau, G.; Lecarpentier, Y.; Herveé, P. New Formula for Predicting Mean Pulmonary Artery Pressure Using Systolic Pulmonary Artery Pressure. Chest 2004, 126, 1313–1317. [Google Scholar] [CrossRef]
- Zoghbi, W.A.; Adams, D.; Bonow, R.O.; Enriquez-Sarano, M.; Foster, E.; Grayburn, P.A.; Hahn, R.T.; Han, Y.; Hung, J.; Lang, R.M.; et al. Recommendations for Noninvasive Evaluation of Native Valvular Regurgitation: A Report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J. Am. Soc. Echocardiogr. 2017, 30, 303–371. [Google Scholar] [CrossRef]
- Baumgartner, H.; Hung, J.; Bermejo, J.; Chambers, J.B.; Evangelista, A.; Griffin, B.P.; Iung, B.; Otto, C.M.; Pellikka, P.A.; Quiñones, M. Echocardiographic Assessment of Valve Stenosis: EAE/ASE Recommendations for Clinical Practice. J. Am. Soc. Echocardiogr. 2009, 22, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Sharifov, O.F.; Schiros, C.G.; Aban, I.; Denney, T.S.; Gupta, H. Diagnostic accuracy of tissue Doppler index E/e’ for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: A systematic review and meta-analysis. J Am Heart Assoc 2016, 5, e002530. [Google Scholar] [CrossRef]
- Chubuchny, V.; Pugliese, N.R.; Taddei, C.; Poggianti, E.; Spini, V.; Barison, A.; Formichi, B.; Airò, E.; Bauleo, C.; Prediletto, R.; et al. A novel echocardiographic method for estimation of pulmonary artery wedge pressure and pulmonary vascular resistance. ESC Heart Fail. 2021, 8, 1216–1229. [Google Scholar] [CrossRef]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2022, 43, 561–632. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Pellicori, P.; Filidei, F.; Del Punta, L.; De Biase, N.; Balletti, A.; Di Fiore, V.; Mengozzi, A.; Taddei, S.; Gargani, L.; et al. The incremental value of multi-organ assessment of congestion using ultrasound in outpatients with heart failure. Eur Heart J Cardiovasc Imaging 2023, 24, 961–971. [Google Scholar] [CrossRef]
- Motiwala, S.R.; Nayor, M. Risk Stratification in Advanced Heart Failure. Can Simple Hemodynamic Indices Replace Comprehensive CPET? J. Am. Coll. Cardiol. 2024, 12, 272–274. [Google Scholar]
- Nesti, L.; Pugliese, N.R.; Chiriacò, M.; Trico, D.; Baldi, S.; Natali, A. Epicardial adipose tissue thickness is associated with reduced peak oxygen consumption and systolic reserve in patients with type 2 diabetes and normal heart function. Diabetes Obes. Metab. 2022, 25, 177–188. [Google Scholar] [CrossRef]
- Nesti, L.; Pugliese, N.R.; Santoni, L.; Armenia, S.; Chiriacò, M.; Sacchetta, L.; De Biase, N.; Del Punta, L.; Masi, S.; Tricò, D.; et al. Distinct effects of type 2 diabetes and obesity on cardiopulmonary performance. Diabetes, Obes. Metab. 2024, 26, 351–361. [Google Scholar] [CrossRef]
- Nesti, L.; Pugliese, N.R.; Sciuto, P.; De Biase, N.; Mazzola, M.; Fabiani, I.; Trico, D.; Masi, S.; Natali, A. Mechanisms of reduced peak oxygen consumption in subjects with uncomplicated type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 1–13. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Balletti, A.; Armenia, S.; De Biase, N.; Faita, F.; Mengozzi, A.; Paneni, F.; Ruschitzka, F.; Virdis, A.; Ghiadoni, L.; et al. Ventricular-Arterial Coupling Derived From Proximal Aortic Stiffness and Aerobic Capacity Across the Heart Failure Spectrum. JACC Cardiovasc. Imaging 2022, 15, 1545–1559. [Google Scholar] [CrossRef]
- Pugliese, N.R.; Fabiani, I.; Santini, C.; Rovai, I.; Pedrinelli, R.; Natali, A.; Dini, F.L. Value of combined cardiopulmonary and echocardiography stress test to characterize the haemodynamic and metabolic responses of patients with heart failure and mid-range ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 828–836. [Google Scholar] [CrossRef] [PubMed]
- Shimiaie, J.; Sherez, J.; Aviram, G.; Megidish, R.; Viskin, S.; Halkin, A.; Ingbir, M.; Nesher, N.; Biner, S.; Keren, G.; et al. Determinants of Effort Intolerance in Patients With Heart Failure: Combined Echocardiography and Cardiopulmonary Stress Protocol. JACC Heart Fail. 2015, 3, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Gorter, T.M.; Obokata, M.; Reddy, Y.N.V.; Melenovsky, V.; Borlaug, B.A. Exercise unmasks distinct pathophysiologic features in heart failure with preserved ejection fraction and pulmonary vascular disease. Eur. Heart J. 2018, 39, 2825–2835. [Google Scholar] [CrossRef] [PubMed]
- Parasuraman, S.K.; Loudon, B.L.; Lowery, C.; Cameron, D.; Singh, S.; Schwarz, K.; Gollop, N.D.; Rudd, A.; McKiddie, F.; Phillips, J.J.; et al. Diastolic ventricular interaction in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Szabó, I.A.; Gargani, L.; Morvai-illés, B.; Polestyuk-németh, N. Prognostic Value of Lung Ultrasound in Aortic Stenosis. Front. Physiol. 2022, 13, 838479. [Google Scholar] [CrossRef]
- Lancellotti, P.; Dulgheru, R.; Go, Y.Y.; Sugimoto, T.; Marchetta, S.; Oury, C.; Garbi, M. Stress echocardiography in patients with native valvular heart disease. Heart 2017, 104, 807–813. [Google Scholar] [CrossRef]
- Magne, J.; Lancellotti, P.; Pie, L.A. Exercise Pulmonary Hypertension in Asymptomatic Degenerative Mitral Regurgitation. Circulation 2010, 122, 33–41. [Google Scholar] [CrossRef]
- Magne, J.; Donal, E.; Mahjoub, H.; Miltner, B.; Dulgheru, R.; Thebault, C.; Pierard, L.A.; Pibarot, P.; Lancellotti, P. Impact of exercise pulmonary hypertension on postoperative outcome in primary mitral regurgitation. Heart 2014, 101, 391–396. [Google Scholar] [CrossRef]
- Kadoglou, N.P.E.; Papadopoulos, C.H.; Krommydas, A. The prognostic value of exercise-induced pulmonary hypertension in asymptomatic patients with primary mitral regurgitation. J. Cardiol. 2022, 79, 306–310. [Google Scholar] [CrossRef]
- Suzuki, T.; Izumo, M.; Suzuki, K.; Koto, D.; Tsukahara, M.; Teramoto, K. Prognostic value of exercise stress echocardiography in patients with secondary mitral regurgitation: A long-term follow-up study. J. Echocardiogr. 2018, 17, 147–156. [Google Scholar] [CrossRef]
- Kempton, H.; Hungerford, S.; Muller, D.W.; Hayward, C.S. Pulmonary arterial compliance as a measure of right ventricular loading in mitral regurgitation. IJC Heart Vasc. 2024, 53, 101472. [Google Scholar] [CrossRef] [PubMed]
- Magrì, D.; Santolamazza, C. Cardiopulmonary Exercise Test in Hypertrophic Cardiomyopathy. Ann. Am. Thorac. Soc. 2017, 14, S102–S109. [Google Scholar] [CrossRef] [PubMed]
- Lele, S.S.; Thomson, H.L.; Seo, H.; Belenkie, I.; Mckenna, W.J.; Frenneaux, M.P. Exercise capacity in hypertrophic cardiomyopathy: Role of stroke volume limitation, heart rate, and diastolic filling characteristics. Circulation 1995, 92, 2886–2894. [Google Scholar] [CrossRef] [PubMed]
- Finocchiaro, G.; Haddad, F.; Knowles, J.; Caleshu, C.; Pavlovic, A.; Homburger, J.; Shmargad, Y.; Sinagra, G. Cardiopulmonary Responses and Prognosis in Hypertrophic Cardiomyopathy. JACC Heart Fail. 2015, 3, 408–418. [Google Scholar] [CrossRef]
- Agostoni, P.; Maria, F.; Beatrice, C.; Magrı, D.; Egidy, G.; Carlo, A.; De Cecco, N.; Muscogiuri, G.; Maruotti, A. Determinants of peak oxygen uptake in patients with hypertrophic cardiomyopathy: A single-center study. Intern. Emerg. Med. 2012, 9, 293–302. [Google Scholar]
- Magrì, D.; Palermo, P.; Cauti, F.M.; Contini, M.; Farina, S.; Cattadori, G.; Apostolo, A.; Salvioni, E.; Magini, A.; Vignati, C.; et al. Chronotropic Incompentence and Functional Capacity in Chronic Heart Failure: No Role of β-Blockers and β-Blocker Dose. Circ. Heart Fail. 2012, 30, 100–108. [Google Scholar] [CrossRef]
- Elliott, P.M.; Uk, C.; Anastasakis, A.; Germany, M.A.B.; Germany, M.B.; Cecchi, F.; France, P.C.; Alain, A.; France, H.; Lafont, A.; et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy The Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 2014, 35, 2733–2779. [Google Scholar]
- Coats, C.J.; Rantell, K.; Bartnik, A.; Patel, A.; Mist, B.; Mckenna, W.J.; Elliott, P.M. Cardiopulmonary Exercise Testing and Prognosis in Hypertrophic Cardiomyopathy. Circ. Heart Fail. 2015, 8, 1022–1031. [Google Scholar] [CrossRef]
- Hirasawa, K.; Izumo, M.; Mizukoshi, K.; Nishikawa, H.; Sato, Y.; Watanabe, M.; Kamijima, R.; Akashi, Y.J. Prognostic significance of right ventricular function during exercise in asymptomatic/minimally symptomatic patients with nonobstructive hypertrophic cardiomyopathy. Echocardiography 2021, 38, 916–923. [Google Scholar] [CrossRef]
- Bruce, R.A.; Blackmon, J.R.; Jones, J.W.; Strait, G. Exercising Testing in Adult Normal Subjects and Cardiac Patients. Ann. Noninvasive Electrocardiol. 2004, 9, 291–303. [Google Scholar] [CrossRef]
- Padang, R.; Pellikka, P.A. The role of stress echocardiography in the evaluation of coronary artery disease and myocardial ischemia in women. J. Nucl. Cardiol. 2016, 23, 1023–1035. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.; Arena, R.; Bhatt, D.L.; Verma, S. A practical clinical approach to utilize cardiopulmonary exercise testing in the evaluation and management of coronary artery disease: A primer for cardiologists. Curr. Opin. Cardiol. 2018, 33, 168–177. [Google Scholar] [CrossRef] [PubMed]
- Belardinelli, R.; Lacalaprice, F.; Tiano, L.; Muçai, A.; Piero, G. Cardiopulmonary exercise testing is more accurate than ECG-stress testing in diagnosing myocardial ischemia in subjects with chest pain. Int. J. Cardiol. 2014, 174, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Pinkstaff, S.; Peberdy, M.A.; Kontos, M.C.; Fabiato, A.; Finucane, S.; Arena, R. Usefulness of Decrease in Oxygen Uptake Efficiency Slope to Identify Myocardial Perfusion Defects in Men Undergoing Myocardial Ischemic Evaluation. Am. J. Cardiol. 2010, 106, 1534–1539. [Google Scholar] [CrossRef]
- Kavanagh, T.; Mertens, D.J.; Ms, C.; Hamm, L.F.; Beyene, J.; Kennedy, J.; Corey, P.; Shephard, R.J. Peak Oxygen Intake and Cardiac Mortality in Women Referred for Cardiac Rehabilitation. J. Am. Coll. Cardiol. 2003, 42, 2139–2143. [Google Scholar] [CrossRef]
- Myers, J.; Prakash, M.; Froelicher, V.; Do, D.; Partignon, S.; Atwood, J.E. Exercise capacity and mortality among men referred from exercise testing. N. Engl. J. Med. 2002, 346, 793–801. [Google Scholar] [CrossRef]
- Song, Y.; Jia, H.; Ma, Q.; Zhang, L.; Lai, X. The causes of pulmonary hypertension and the benefits of aerobic exercise for pulmonary hypertension from an integrated perspective. Front. Physiol. 2024, 5, 1461519. [Google Scholar] [CrossRef]
- Gargani, L.; Pugliese, N.R.; De Biase, N.; Mazzola, M.; Agoston, G.; Arcopinto, M.; Argiento, P.; Armstrong, W.F.; Bandera, F.; Cademartiri, F.; et al. Right Ventricular Contractile Reserve and Pulmonary Circulation Uncoupling During Exercise Challenge in Heart Failure: Pathophysiology and Clinical Phenotypes. JACC Heart Fail. 2016, 4, 625–635. [Google Scholar]
- Sherman, A.E.; Saggar, R. Cardiopulmonary Exercise Testing in Pulmonary Arterial Hypertension. Heart Fail. Clin. 2023, 19, 35–43. [Google Scholar] [CrossRef]
- Blanco, I.; Valeiro, B.; Torres-castro, R.; Barberán-garcía, A.; Torralba, Y.; Moisés, J.; Sebastián, L.; Osorio, J.; Rios, J.; Gimeno-santos, E.; et al. Effects of Pulmonary Hypertension on Exercise Capacity in Patients With Chronic Obstructive Pulmonary Disease. Arch Bronconeumol. 2020, 56, 499–505. [Google Scholar] [CrossRef]
- Mukherjee, M.; Rudski, L.G.; Addetia, K.; Afilalo, J.; D’Alto, M.; Freed, B.H.; Friend, L.B.; Gargani, L.; Grapsa, J.; Hassoun, P.M.; et al. Guidelines for the Echocardiographic Assessment of the Right Heart in Adults and Special Considerations in Pulmonary Hypertension: Recommendations from the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2025, 38, 141–186. [Google Scholar] [CrossRef] [PubMed]
- Tello, K.; Axmann, J.; Ghofrani, H.A.; Naeije, R.; Narcin, N.; Rieth, A.; Seeger, W.; Gall, H.; Richter, M.J. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int. J. Cardiol. 2018, 266, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Finlay, M.; Middleton, H.C.; Peake, M.D.; Howard, P. Cardiac output, pulmonary hypertension, hypoxaemia and survival in patients with chronic obstructive airways disease. Eur. J. Respir. Dis. 1983, 64, 252–263. [Google Scholar] [PubMed]
- Fernandes, T.M.; Alotaibi, M.; Strozza, D.; Stringer, W.; Faulkner, G.; Castro, C.; Tran, D.; Morris, T.A. Dyspnea postpulmonary embolism from physiological dead space proportion and stroke volume defects during exercise. Chest 2019, 157, 936–944. [Google Scholar] [CrossRef]
- Maron, B.J.; Maron, M.S. Hypertrophic cardiomyopathy. Lancet 2013, 381, 242–255. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).