Heart Rate Variability in Adolescents with Autistic Spectrum Disorder Practicing a Virtual Reality Using Two Different Interaction Devices (Concrete and Abstract): A Prospective Randomized Crossover Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Participants
2.3. Inclusion and Exclusion Criteria
2.4. Sample Characterization
2.4.1. Wechsler Intelligence Scale
2.4.2. Childhood Autism Rating Scale
2.4.3. Pediatric Evaluation of Disability Inventory
2.5. Sample Size
2.6. Abstract Activity
2.7. Heart Rate Variability
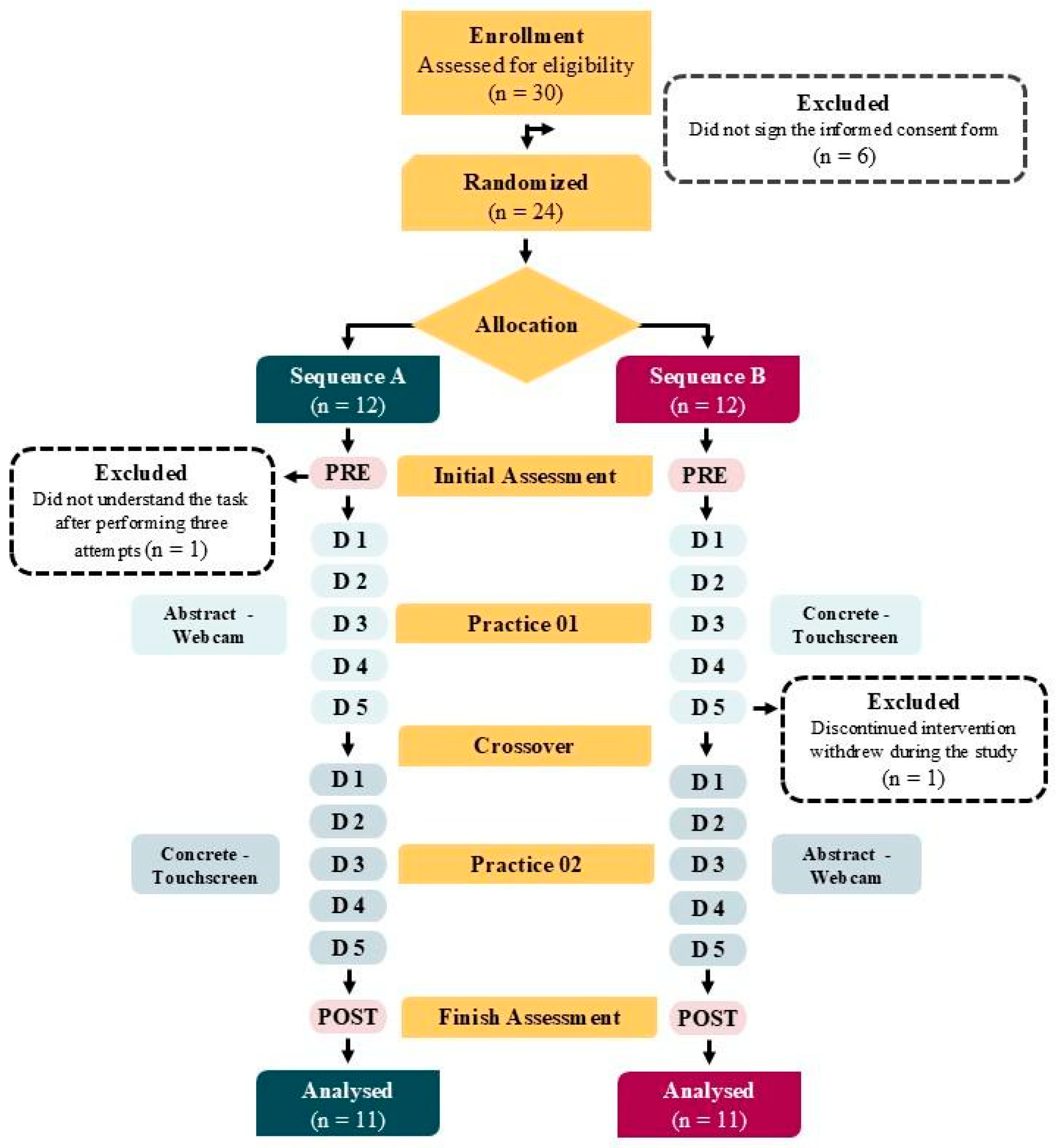
2.8. Randomization
2.9. Flow OS Participants
2.10. Data Analysis
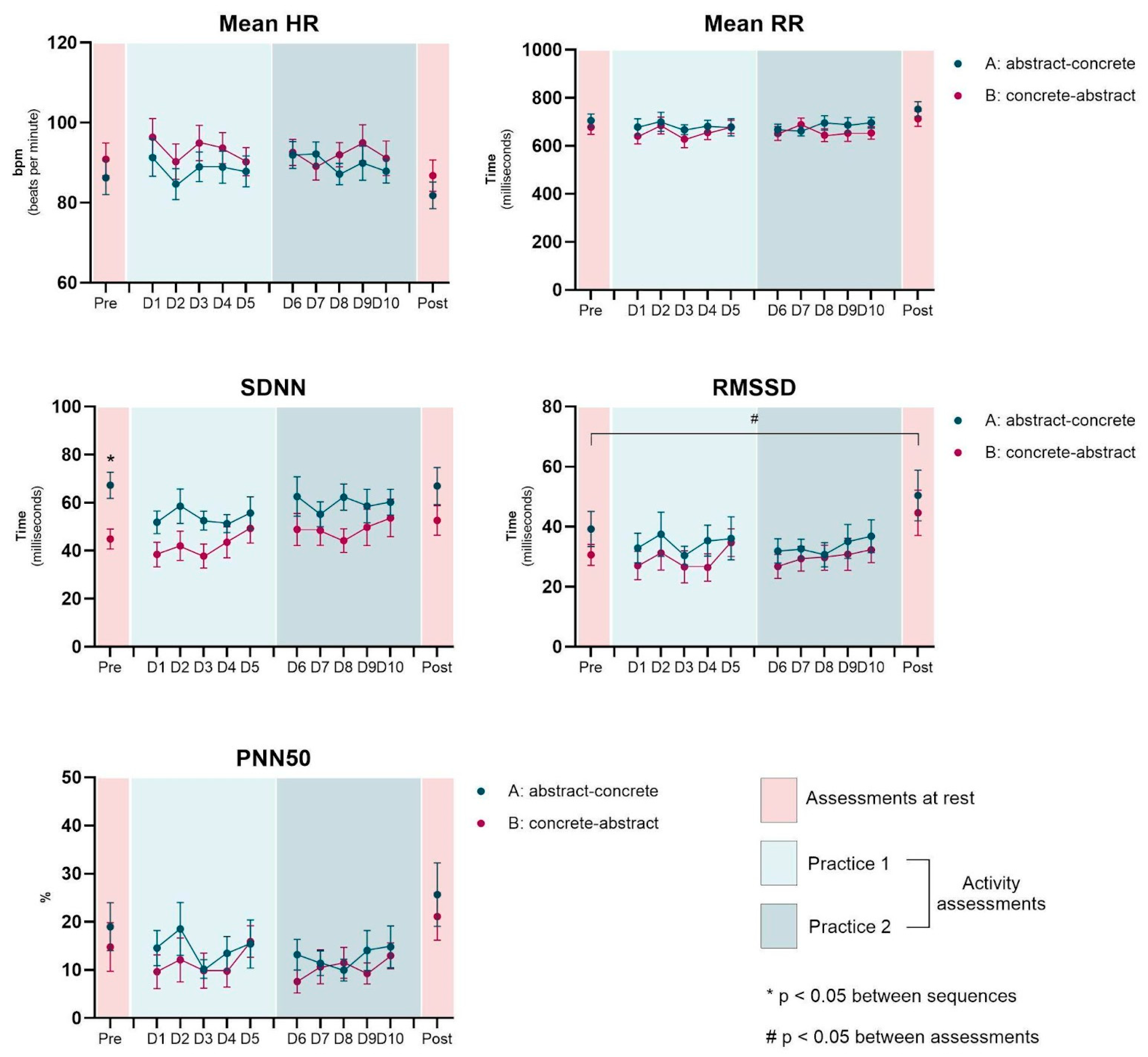
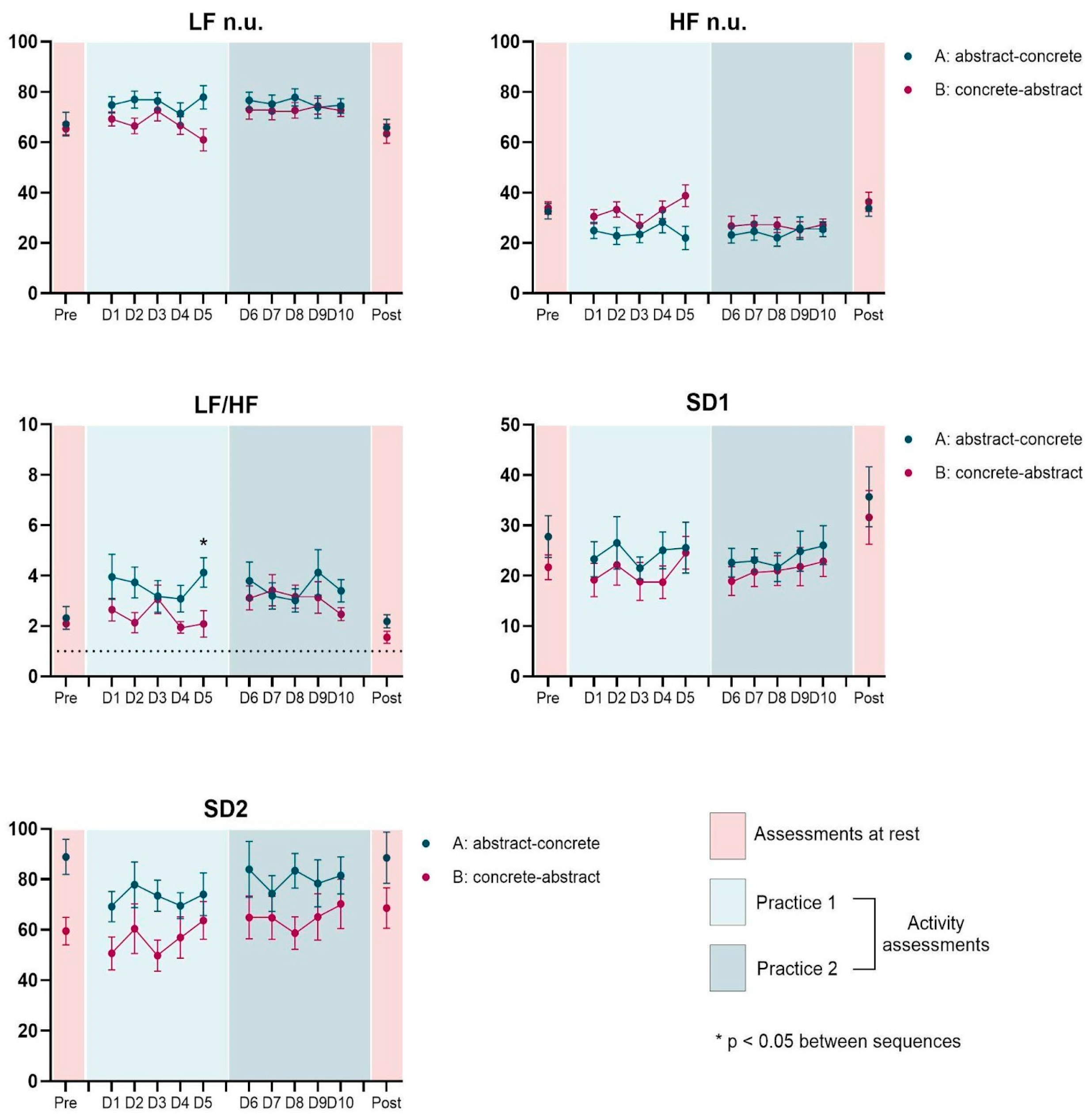
3. Results
3.1. Heart Rate Variability Measurements
3.2. Pre vs. Post Assessments
3.3. Effect of Interventions (Rest-Pre to Activity on D1)
3.4. Effect of Interventions (Concrete vs. Abstract)
3.5. Effect of Practice (D1 × D5)
3.6. Effect of Sequence (Concrete First vs. Abstract First)
3.7. Regression Analysis
4. Discussion
4.1. Improvements in HRV Indices After 10 Days of Intervention
4.2. Rest vs. First Day of Activity: Higher LF/HF Ratio in Abstract Tasks
4.3. Differences Between Concrete and Abstract Tasks
4.4. VR Tasks to ASD Population Benefits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANS | Autonomic Nervous System |
| ASD | Autism Spectrum Disorder |
| BPM | Beats Per Minute |
| CARS | Childhood Autism Rating Scale |
| GAPI | Integrated Psychopedagogical Support Group |
| HF | High Frequency |
| HRV | Heart Rate Variability |
| IQ | Intelligence Quotient |
| LF | Low Frequency |
| LSD | Least Significant Difference |
| PEDI | Pediatric Evaluation of Disability Inventory |
| pNN50 | Percentage of Normal RR Intervals Differing by More Than 50 ms |
| RMSSD | Root Mean Square of Successive Differences |
| RR | Interval between R waves of the PQRST complex |
| SDNN | Standard Deviation of Normal RR Intervals |
| VR | Virtual Reality |
| WISC | Wechsler Intelligence Scale |
References
- American Psychiatric Association. DSM-5: Manual diagnóstico e estatístico de transtornos mentais. Porto Alegre Artmed 2014, 5, 50–59. [Google Scholar]
- Ecker, C. The neuroanatomy of autism spectrum disorder: An overview of structural neuroimaging findings and their translatability to the clinical setting. Autism 2017, 21, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Rynkiewicz, A.; Schuller, B.; Marchi, E.; Piana, S.; Camurri, A.; Lassalle, A.; Baron-Cohen, A. An investigation of the ‘female camouflage effect’ in autism using a computerized ADOS-2 and a test of sex/gender differences. Mol. Autism 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Ketcheson, L.; Hauck, J.; Ulrich, D. The effects of an early motor skill intervention on motor skills, levels of physical activity, and socialization in young children with autism spectrum disorder: A pilot study. Autism 2017, 21, 481–492. [Google Scholar] [CrossRef]
- Lohse, K.; Shirzad, N.; Verster, A.; Hodges, N.; Van der Loos, H.F. Video games and rehabilitation: Using design principles to enhance engagement in physical therapy. J. Neurol. Phys. Ther. 2013, 37, 166–175. [Google Scholar] [CrossRef]
- Dias, P.; Silva, R.; Amorim, P.; Lains, J.; Roque, E.; Pereira, I.S.F.; Pereira, F.; Santos, B.S.; Potel, M. Using virtual reality to increase motivation in poststroke rehabilitation. IEEE Comput. Graph. Appl. 2019, 39, 64–70. [Google Scholar] [CrossRef]
- Silveira, A.C.; de Moraes, I.A.P.; Vidigal, G.P.; Simcsik, A.O.; Rosa, R.M.; Favero, F.M.; Fernandes, S.M.S.; Garner, D.M.; Araújo, L.V.; Massa, M.; et al. Cardiac autonomic modulation in subjects with amyotrophic lateral sclerosis (ALS) during an upper limb virtual reality task: A prospective control trial. Biomed Res. Int. 2022, 4439681. [Google Scholar] [CrossRef] [PubMed]
- Newbutt, N.; Sung, C.; Kuo, H.J.; Leahy, M.J.; Lin, C.C.; Tong, B. Brief Report: A Pilot Study of the Use of a Virtual Reality Headset in Autism Populations. J. Autism Dev. Disord. 2016, 46, 3166–3176. [Google Scholar] [CrossRef]
- Hurkmans, H.L.; Van Den Berg-Emons, R.J.; Stam, H.J. Energy expenditure in adults with cerebral palsy playing Wii Sports. Arch. Phys. Med. Rehabil. 2010, 91, 1577–1581. [Google Scholar] [CrossRef]
- Qian, J.; McDonough, D.J.; Gao, Z. The Effectiveness of Virtual Reality Exercise on Individual’s Physiological, Psychological and Rehabilitative Outcomes: A Systematic Review. Int. J. Environ. Res. Public Health 2020, 17, 4133. [Google Scholar] [CrossRef]
- Moraes, I.A.P.; Lima, J.A.; Silva, N.M.; Simcsik, A.O.; Silveira, A.C.; Menezes, L.D.C.; Araújo, L.V.; Crocetta, T.B.; Voos, M.C.; Tonks, J.; et al. Effect of Longitudinal Practice in Real and Virtual Environments on Motor Performance, Physical Activity and Enjoyment in People with Autism Spectrum Disorder: A Prospective Randomized Crossover Controlled Trial. Int. J. Environ. Res. Public Health 2022, 19, 14668. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.; Yun, J.; Agiovlasitis, S. Impact of Enjoyment on Physical Activity and Health among Children with Disabilities in Schools. Disabil. Health J. 2018, 11, 14–19. [Google Scholar] [CrossRef]
- Chung, P.J.; Vanderbilt, D.L.; Schrager, S.M.; Nguyen, E.; Fowler, E. Active Videogaming for Individuals with Severe Movement Disorders: Results from a Community Study. Games Health J. 2015, 4, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Mineo, B.A.; Ziegler, W.; Gill, S.; Salkin, D. Engagement with Electronic Screen Media Among Students with Autism Spectrum Disorders. J. Autism Dev. Disord. 2009, 39, 172–187. [Google Scholar] [CrossRef]
- Moraes, I.A.P.; Monteiro, C.B.M.; Silva, T.D.; Masseti, T.; Crocetta, T.B.; Menezes, L.C.; Rezende, G.P.; Ré, A.H.N.; Dawes, H.; Coe, S.; et al. Motor learning and transfer between real and virtual environments in young people with autism spectrum disorder: A prospective randomized cross over controlled trial. Autism Res. 2020, 13, 307–319. [Google Scholar] [PubMed]
- Cheshire, W.P. Highlights in clinical autonomic neuroscience: New insights into autonomic dysfunction in autism. Auton Neurosci. 2012, 171, 4–7. [Google Scholar] [CrossRef]
- Ming, X.; Julu, P.O.; Brimacombe, M.; Connor, S.; Daniels, M.L. Reduced cardiac parasympathetic activity in children with autism. Brain Dev. 2005, 27, 509–516. [Google Scholar] [CrossRef]
- Ming, X.; Patel, R.; Kang, V.; Chokroverty, S.; Julu, P.O. Respiratory and autonomic dysfunction in children with autism spectrum disorders. Brain Dev. 2016, 38, 225–232. [Google Scholar] [CrossRef]
- Vanderlei, L.C.; Pastre, C.M.; Hoshi, R.A.; Carvalho, T.D.; Godoy, M.F. Noções básicas de variabilidade da frequência cardíaca e sua aplicabilidade clínica. Braz. J. Cardiovasc. Surg. 2009, 24, 205–217. [Google Scholar] [CrossRef]
- Solhjoo, S.; Haigney, M.C.; McBee, E.; van Merrienboer, J.J.; Schuwirth, L.; Artino, A.R., Jr.; Durning, S.J. Heart rate and heart rate variability correlate with clinical reasoning performance and self-reported measures of cognitive load. Sci. Rep. 2019, 9, 14668. [Google Scholar] [CrossRef]
- Tornberg, J.; Ikäheimo, T.M.; Kiviniemi, A.; Pyky, R.; Hautala, A.; Mäntysaari, M.; Korpelainen, R. Physical activity is associated with cardiac autonomic function in adolescent men. PLoS ONE 2019, 14, e0222121. [Google Scholar] [CrossRef]
- Turner, L.; Shamseer, L.; Altman, D.G.; Weeks, L.; Peters, J.; Kober, T.; Dias, S.; Schulz, K.F.; Plint, A.C.; Moher, D. Consolidated standards of reporting trials (CONSORT) and the completeness of reporting of randomised controlled trials (RCTs) published in medical journals. Cochrane Database Syst. Rev. 2012, 11, MR000030. [Google Scholar] [CrossRef]
- Moher, D.; Hopewell, S.; Schulz, K.F.; Montori, V.; Gøtzsche, P.C.; Devereaux, P.J.; Elbourne, D.; Egger, M.; Altman, D.G. CONSORT 2010 Explanation and Elaboration: Updated Guidelines for Reporting Parallel Group Randomized Trials. BMJ 2010, 340, c869. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, É.; Livanos, A.; Garbin, J.A.L.; Monteiro, C.B.M.; Ré, A.; Moraes, Í.A.P.; Silva, T. Heart rate variability in adolescents with autistic spectrum disorder practicing a virtual reality using two different interaction devices. Mendeley Data 2025, V1. [Google Scholar] [CrossRef]
- Rao, V.S.; Raman, V.; Mysore, A.V. Issues Related to Obtaining Intelligence Quotient-Matched Controls in Autism Research. Indian J. Psychol. Med. 2015, 37, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.M. Internal and external validity of seven Wechsler Intelligence Scale for Children-Third Edition short forms in a sample of psychiatric inpatients. Psychol. Assess. 1998, 10, 431–434. [Google Scholar] [CrossRef]
- Yu, T.Y.; Chou, W.; Chow, J.C.; Lin, C.H.; Tung, L.C.; Chen, K.L. IQ discrepancy differentiates levels of fine motor skills and their relationship in children with autism spectrum disorders. Neuropsychiatr. Dis. Treat. 2018, 14, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Al Backer, N.B. Correlation between Autism Treatment Evaluation Checklist (ATEC) and Childhood Autism Rating Scale (CARS) in the evaluation of autism spectrum disorder. Sudan. J. Paediatr. 2016, 16, 17–22. [Google Scholar]
- Pereira, A.; Riesgo, R.S.; Wagner, M.B. Childhood autism: Translation and validation of the Childhood Autism Rating Scale for use in Brazil. J. Pediatr. 2008, 84, 487–494. [Google Scholar] [CrossRef]
- Berg, M.; Jahnsen, R.; Frøslie, K.F.; Hussain, A. Reliability of the Pediatric Evaluation of Disability Inventory (PEDI). Phys. Occup. Ther. Pediatr. 2004, 24, 61–77. [Google Scholar] [CrossRef]
- Mancini, M.C. Inventário de Avaliação Pediátrica de Incapacidade (PEDI)-Manual Da Versão Brasileira Adaptada; Editora UFMG: Belo Horizonte, Brazil, 2005. [Google Scholar]
- Silva, T.D.; Fontes, A.M.G.G.; Furlan, B.S.O.; Roque, T.T.; Lima, A., II; Souza, B.M.M.; Alberissi, C.A.O.; Silveira, A.C.; Moraes, Í.A.P.; Collett, J.; et al. Effect of Combined Therapy of Virtual Reality and Transcranial Direct Current Stimulation in Children and Adolescents With Cerebral Palsy: A Study Protocol for a Triple-Blinded Randomized Controlled Crossover Trial. Front. Neurol. 2020, 11, 953. [Google Scholar] [CrossRef] [PubMed]
- Gutin, B.; Owens, S.; Slavens, G.; Riggs, S.; Treiber, F. Effect of Physical Training on Heart-Period Variability in Obese Children. J. Pediatr. 1997, 130, 938–943. [Google Scholar] [CrossRef] [PubMed]
- Kalfiÿrt, L.; Su, C.-T.; Fu, C.P.; Lee, S.D.; Yang, A.L. Motor Skills, Heart Rate Variability, and Arterial Stiffness in Children with Autism Spectrum Disorder. Healthcare 2023, 11, 1898. [Google Scholar] [CrossRef]
- Thapa, R.; Pokorski, I.; Ambarchi, Z.; Thomas, E.; Demayo, M.; Boulton, K.; Matthews, S.; Patel, S.; Sedeli, I.; Hickie, I.B.; et al. Heart Rate Variability in Children With Autism Spectrum Disorder and Associations With Medication and Symptom Severity. Autism Res. 2021, 14, 75–85. [Google Scholar] [CrossRef]
- Healy, S.; Nacario, A.; Braithwaite, R.; Hopper, C. The effect of physical activity interventions on youth with autism spectrum disorder: A meta-analysis. Autism Res. 2018, 11, 818–833. [Google Scholar] [CrossRef]
- Rutkowski, S.; Szary, P.; Sacha, J.; Casaburi, R. Immersive Virtual Reality Influences Physiologic Responses to Submaximal Exercise: A Randomized, Crossover Trial. Front. Physiol 2021, 12, 702266. [Google Scholar] [CrossRef] [PubMed]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-regulation, Adaptation, and Health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Eggenberger, P.; Annaheim, S.; Küding, K.A.; Rossi, R.M.; Münzer, T.; Bruin, E.D. Heart Rate Variability Mainly Relates to Cognitive Executive Functions and Improves Through Exergame Training in Older Adults: A Secondary Analysis of a 6-Month Randomized Controlled Trial. Front. Aging Neurosci. 2020, 12, 197. [Google Scholar] [CrossRef]
- Marín-Morales, J.; Higuera-Trujillo, J.; Guixeres, J.; Llinares, C.; Alcañiz, M.; Valenza, G. Heart rate variability analysis for the assessment of immersive emotional arousal using virtual reality: Comparing real and virtual scenarios. PLoS ONE 2021, 16, e0254098. [Google Scholar] [CrossRef]
- Hayano, J.; Yuda, E. Assessment of autonomic function by long-term heart rate variability: Beyond the classical framework of LF and HF measurements. J. Physiol. Anthropol. 2021, 40, 21. [Google Scholar] [CrossRef]
- Held, J.; Vîslă, A.; Wolfer, C.; Messerli-Bürgy, N.; Flückiger, C. Heart rate variability change during a stressful cognitive task in individuals with anxiety and control participants. BMC Psychol. 2021, 9, 44. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Moneghetti, K.J.; Christle, J.W.; Hadley, D.; Plews, D.; Froelicher, V. Heart Rate Variability: An Old Metric with New Meaning in the Era of using mHealth Technologies for Health and Exercise Training Guidance. Part One Physiol. Methods Arrhythmia Electrophysiol. Rev. 2018, 7, 193–198. [Google Scholar]
- Wittels, H.L.; Wittels, S.H.; Wishon, M.J.; Vogl, J.; St. Onge, P.; McDonald, S.M.; Temme, L.A. Examining the Influence of Cognitive Load and Environmental Conditions on Autonomic Nervous System Response in Military Aircrew: A Hypoxia–Normoxia Study. Biology 2024, 13, 343. [Google Scholar] [CrossRef]
- Chen, A.; Li, M.; Gao, Y. Mem-Box: VR sandbox for adaptive working memory evaluation and training using physiological signals. Vis. Comput. 2024, 40, 7559–7573. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Gomes, R.D.A.; Monteiro, C.B.D.M.; Dias, R.M.; Simcsik, A.O.; Araújo, L.V.D.; Maia, L.C.P.; Oliveira, A.P.D.; Freitas, B.L.D.; Dawes, H.; et al. Heart Rate Variability during Virtual Reality Activity in Individuals after Hospitalization for COVID-19: A Cross-Sectional Control Study. Electronics 2023, 12, 1925. [Google Scholar] [CrossRef]
- Sandercock, G.R.; Bromley, P.D.; Brodie, D.A. Effects of exercise on heart rate variability: Inferences from meta-analysis. Med. Sci. Sports Exerc. 2005, 37, 433–439. [Google Scholar] [CrossRef]
- Casanova, M.F.; Hensley, M.K.; Sokhadze, E.M.; El-Baz, A.S.; Wang, Y.; Li, X.; Sears, L. Effects of weekly low-frequency rTMS on autonomic measures in children with autism spectrum disorder. Front. Hum. Neurosci. 2014, 8, 851. [Google Scholar] [CrossRef]
- Wang, Y.; Hensley, M.K.; Tasman, A.; Sears, L.; Casanova, M.F.; Sokhadze, E.M. Heart rate variability and skin conductance during repetitive TMS course in children with autism. Appl. Psychophysiol. Biofeedback 2016, 41, 47–60. [Google Scholar] [CrossRef]
- Sammito, S.; Böckelmann, I. Reference values for time- and frequency-domain heart rate variability measures. Heart Rhythm 2016, 13, 1309–1316. [Google Scholar] [CrossRef]
- Neuhaus, E.; Bernier, R.; Beauchaine, T.P. Brief report: Social skills, internalizing and externalizing symptoms, and respiratory sinus arrhythmia in autism. J. Autism Dev. Disord. 2014, 44, 730–737. [Google Scholar] [CrossRef]

| Variable | Sequence A | Sequence B | p-Value | ||
|---|---|---|---|---|---|
| Mean ± SD | CI [LL, UL] | Mean ± SD | CI [LL, UL] | ||
| Age (years) | 14.1 ± 1.7 | [12, 15] | 13.9 ± 1.9 | [12, 15] | 0.822 |
| Height (meters) | 1.65 ± 0.13 | [1.57, 1.73] | 1.67 ± 0.11 | [1.59, 1.75] | 0.693 |
| Weight (kilograms) | 61.9 ± 14.8 | [51, 72] | 63.0 ± 18.8 | [52, 73] | 0.877 |
| BMI (kg/m2) | 22.0 ± 4.0 | [19, 25] | 22.0 ± 6.0 | [19, 25] | 0.899 |
| IQ | 87.6 ± 11.3 | [79, 95] | 83.13 ± 13.6 | [75, 91] | 0.412 |
| CARS | 34.3 ± 1.3 | [33, 35] | 33.7 ± 1.7 | [32, 34] | 0.348 |
| PEDI—FS Self-Care | 89.6 ± 11.3 | [82, 96] | 91.8 ± 10.1 | [85, 98] | 0.648 |
| PEDI—FS Mobility | 66.3 ± 7.7 | [62, 69] | 67.7 ± 1.6 | [64, 71] | 0.586 |
| PEDI—FS Social Function | 77.5 ± 9.8 | [71, 83] | 77.8 ± 9.9 | [71, 84] | 0.947 |
| PEDI—CA Self-Care | 94.2 ± 9.0 | [89, 99] | 97.0 ± 6.7 | [92, 102] | 0.419 |
| PEDI—CA Mobility | 92.4 ± 15.0 | [85, 99] | 96.8 ± 5.7 | [89, 103] | 0.377 |
| PEDI—CA Social Function | 88.0 ± 11.5 | [80, 95] | 87.1 ± 12.8 | [79, 94] | 0.867 |
| Variable | S | Pre-Rest | Day 1—D1 | Day 5—D5 | Day 10—D10 | Post | p (Pre vs. Post) | p (Pre vs. D1) | p (Pre vs. D10) | p (D1 vs. D5) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | M | M × S | S | M | M × S | S | M | M × S | S | M | M × S | S | ||
| Mean RR | A | 705.5 | 91.8 | 667.2 | 76.0 | 696.3 | 75.7 | 677.2 | 117.8 | 753.1 | 101.8 | 0.058 | - | - | - | - | - | - | - | - | - | - | - |
| B | 678.2 | 101.5 | 640.6 | 109.5 | 679.0 | 89.6 | 653.7 | 85.6 | 713.1 | 104.6 | |||||||||||||
| Mean HR | A | 86.2 | 14.0 | 91.9 | 11.1 | 87.9 | 9.8 | 87.8 | 12.8 | 81.8 | 11.0 | - | - | - | - | - | - | - | - | - | - | - | - |
| B | 90.9 | 13.5 | 96.4 | 15.4 | 90.2 | 11.6 | 91.1 | 14.3 | 86.8 | 12.9 | |||||||||||||
| SDNN | A | 67.3 | 18.2 | 62.6 | 27.3 | 60.2 | 18.1 | 55.7 | 22.4 | 66.9 | 25.6 | - | - | 0.020 | - | 0.024 | - | - | 0.039 | - | - | - | - |
| B | 44.9 | 13.8 | 38.4 | 16.9 | 49.3 | 20.3 | 53.7 | 25.9 | 52.6 | 20.5 | |||||||||||||
| RMSSD | A | 39.2 | 19.5 | 31.9 | 13.3 | 36.8 | 18.0 | 36.1 | 23.7 | 50.4 | 27.9 | 0.026 | - | - | - | - | - | - | - | - | - | - | - |
| B | 30.6 | 11.6 | 27.1 | 15.5 | 34.7 | 15.3 | 32.3 | 14.2 | 44.6 | 24.9 | |||||||||||||
| pNN50 | A | 19.0 | 16.5 | 13.2 | 10.5 | 14.8 | 14.3 | 15.4 | 16.6 | 25.7 | 21.8 | - | - | - | - | - | - | - | - | - | - | - | - |
| B | 14.8 | 16.7 | 9.6 | 11.6 | 15.9 | 10.8 | 13.0 | 8.9 | 21.1 | 16.3 | |||||||||||||
| LF n.u. | A | 67.2 | 15.7 | 76.7 | 10.7 | 74.5 | 9.6 | 77.9 | 15.3 | 65.9 | 10.7 | - | - | - | - | 0.014 | - | 0.001 | - | - | - | - | - |
| B | 65.4 | 8.1 | 69.3 | 9.2 | 61.0 | 14.5 | 72.8 | 8.1 | 63.5 | 12.8 | |||||||||||||
| HF n.u. | A | 32.6 | 10.3 | 23.2 | 10.7 | 25.5 | 9.6 | 22.0 | 15.3 | 33.8 | 10.6 | - | - | - | - | 0.022 | 0.046 | 0.005 | - | - | - | - | - |
| B | 33.9 | 8.1 | 30.5 | 9.2 | 38.8 | 14.4 | 27.1 | 8.1 | 36.4 | 12.7 | |||||||||||||
| LF/HF | A | 2.3 | 1.5 | 3.8 | 2.5 | 3.4 | 1.4 | 4.1 | 1.9 | 2.2 | 0.9 | - | - | - | 0.010 | 0.009 | - | <0.001 | 0.011 | - | - | - | - |
| B | 2.1 | 0.7 | 2.7 | 1.5 | 2.1 | 1.7 | 2.5 | 0.8 | 1.6 | 0.8 | |||||||||||||
| SD1 | A | 27.8 | 13.8 | 22.6 | 9.4 | 26.1 | 12.8 | 25.6 | 16.8 | 35.7 | 19.8 | 0.026 | - | - | - | - | - | - | - | - | - | - | - |
| B | 21.7 | 8.2 | 19.2 | 11.0 | 24.6 | 10.8 | 22.9 | 10.0 | 31.6 | 17.6 | |||||||||||||
| SD2 | A | 88.9 | 23.1 | 84.0 | 36.9 | 81.5 | 24.3 | 74.1 | 28.0 | 88.6 | 33.6 | - | - | 0.017 | - | 0.030 | 0.045 | - | 0.043 | - | - | - | - |
| B | 59.5 | 18.1 | 50.7 | 21.8 | 63.7 | 24.7 | 70.3 | 32.4 | 68.6 | 26.5 | |||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, É.; Livanos, A.; Garbin, J.A.L.; Fernandes, S.M.S.; Simcsik, A.O.; Crocetta, T.B.; Dias, E.D.; Monteiro, C.B.M.; Magalhães, F.H.; Ré, A.H.N.; et al. Heart Rate Variability in Adolescents with Autistic Spectrum Disorder Practicing a Virtual Reality Using Two Different Interaction Devices (Concrete and Abstract): A Prospective Randomized Crossover Controlled Trial. Healthcare 2025, 13, 1402. https://doi.org/10.3390/healthcare13121402
Rodrigues É, Livanos A, Garbin JAL, Fernandes SMS, Simcsik AO, Crocetta TB, Dias ED, Monteiro CBM, Magalhães FH, Ré AHN, et al. Heart Rate Variability in Adolescents with Autistic Spectrum Disorder Practicing a Virtual Reality Using Two Different Interaction Devices (Concrete and Abstract): A Prospective Randomized Crossover Controlled Trial. Healthcare. 2025; 13(12):1402. https://doi.org/10.3390/healthcare13121402
Chicago/Turabian StyleRodrigues, Étria, Ariane Livanos, Joyce A. L. Garbin, Susi M. S. Fernandes, Amanda O. Simcsik, Tânia B. Crocetta, Eduardo D. Dias, Carlos B. M. Monteiro, Fernando H. Magalhães, Alessandro H. N. Ré, and et al. 2025. "Heart Rate Variability in Adolescents with Autistic Spectrum Disorder Practicing a Virtual Reality Using Two Different Interaction Devices (Concrete and Abstract): A Prospective Randomized Crossover Controlled Trial" Healthcare 13, no. 12: 1402. https://doi.org/10.3390/healthcare13121402
APA StyleRodrigues, É., Livanos, A., Garbin, J. A. L., Fernandes, S. M. S., Simcsik, A. O., Crocetta, T. B., Dias, E. D., Monteiro, C. B. M., Magalhães, F. H., Ré, A. H. N., Moraes, Í. A. P., & Silva-Magalhães, T. D. (2025). Heart Rate Variability in Adolescents with Autistic Spectrum Disorder Practicing a Virtual Reality Using Two Different Interaction Devices (Concrete and Abstract): A Prospective Randomized Crossover Controlled Trial. Healthcare, 13(12), 1402. https://doi.org/10.3390/healthcare13121402










