Association Between Physical Activity Timing and Metabolic Syndrome in Korea: A Functional Principal Component Approach
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Source and Study Design
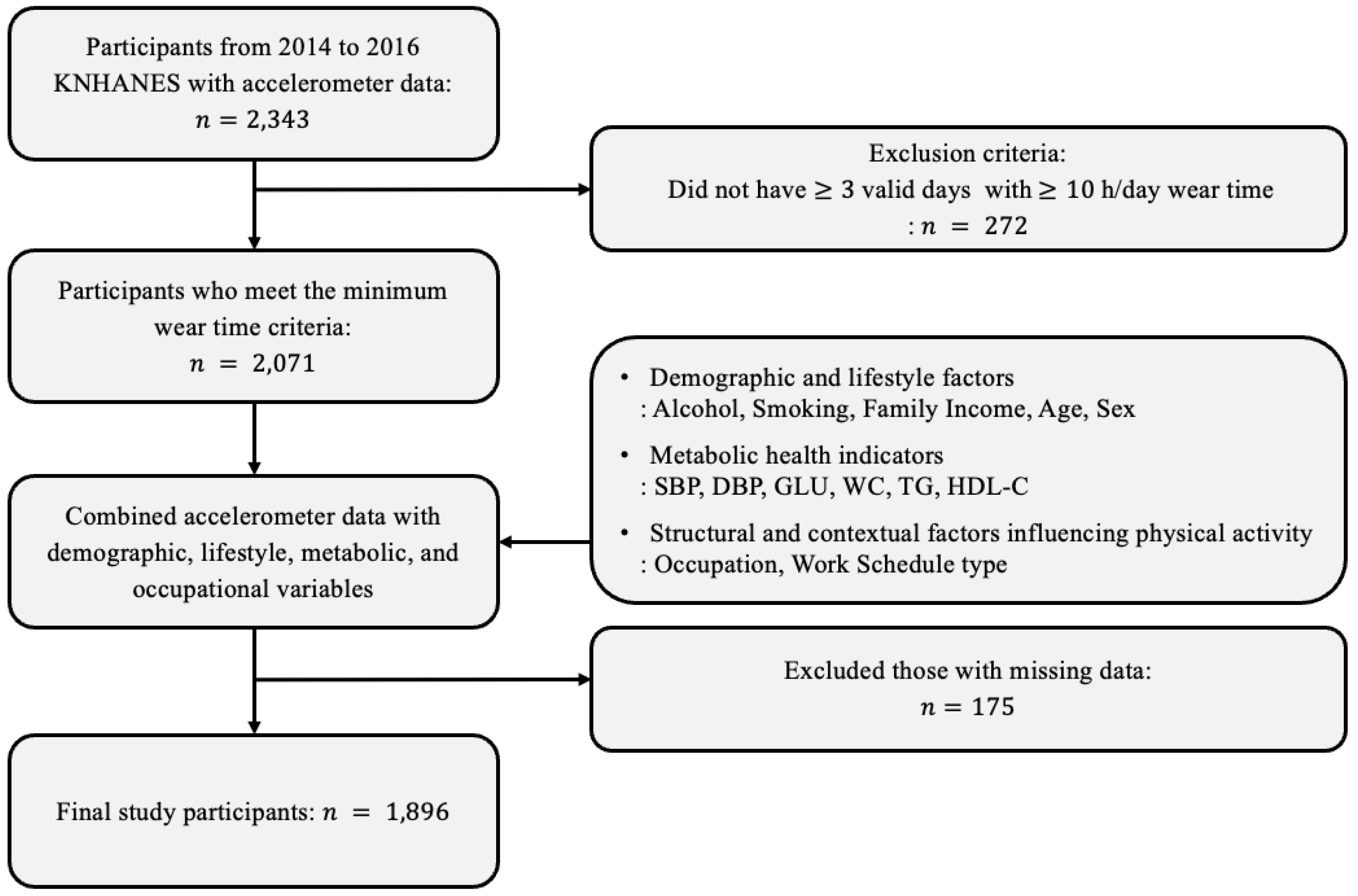
2.2. Study Participants
2.3. Physical Activity
- (1)
- Inactive: 0 min/week;
- (2)
- Insufficiently active: 1–149 min/week;
- (3)
- Active: 150–299 min/week;
- (4)
- Highly active: ≥300 min/week.
2.4. Diagnostic Criteria for Metabolic Syndrome
2.5. Confounding Factors
3. Statistical Analysis
3.1. Participant Characteristics by Metabolic Syndrome Status
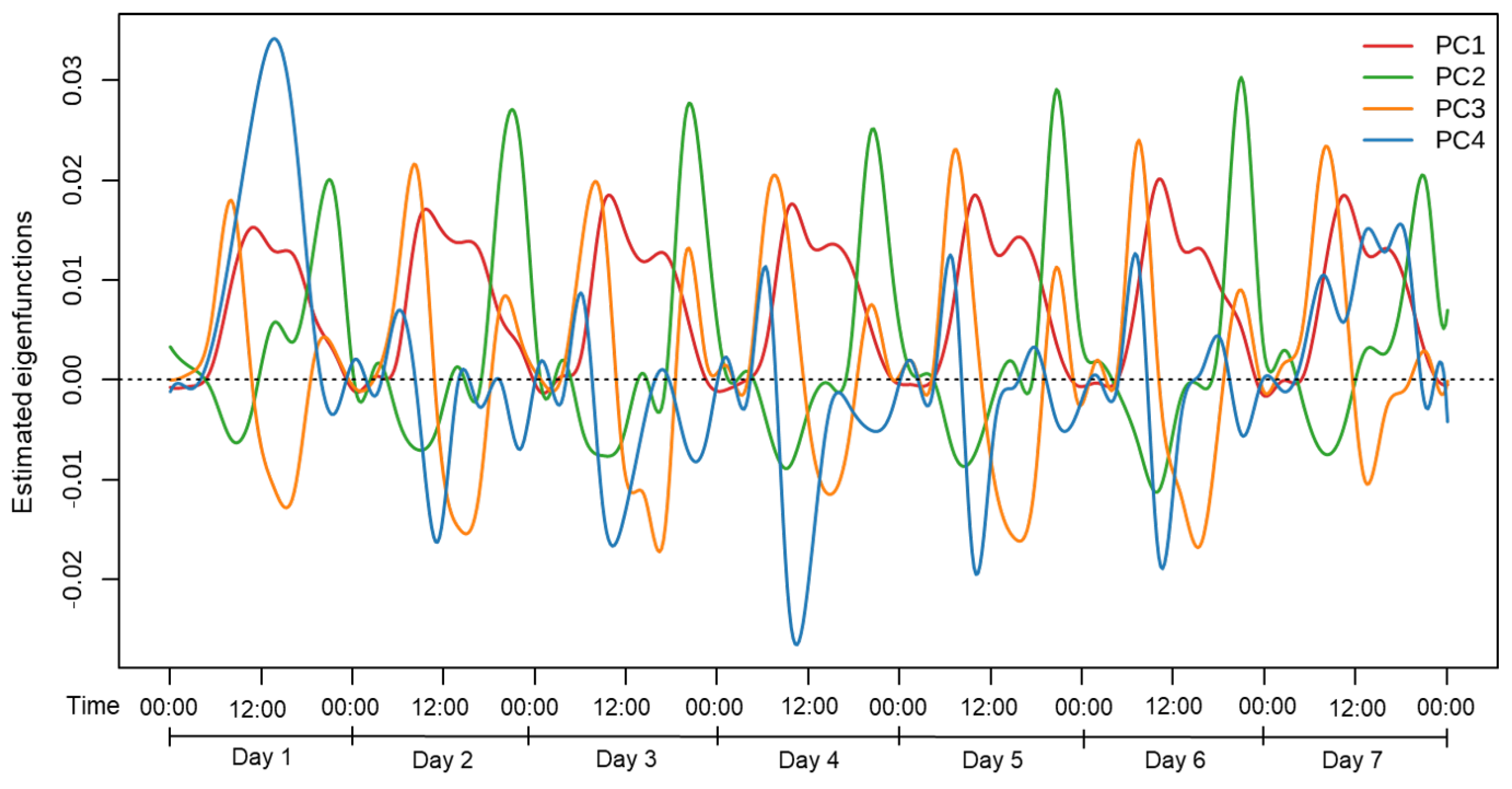
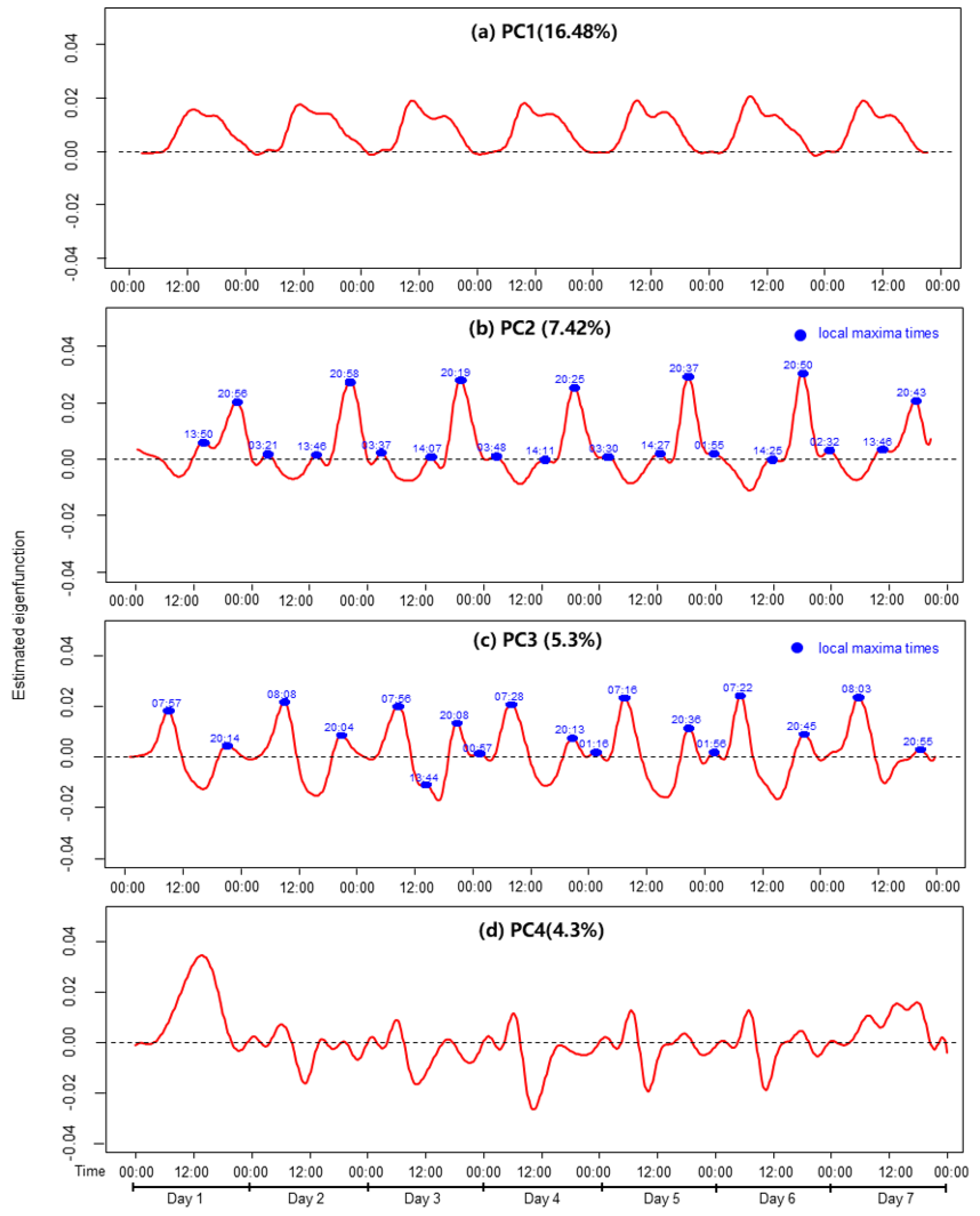
3.2. Functional Principal Component Analysis of Physical Activity
3.3. Association Between Functional Principal Component Scores and Metabolic Syndrome
4. Results
4.1. Participant Characteristics
4.2. Temporal Variation in Physical Activity
4.3. Association Between Principal Component Scores and Metabolic Syndrome
- (1)
- Model 1: A simple logistic regression model including only the FPCA scores (PC1–PC4) as independent variables to estimate unadjusted odds ratios.
- (2)
- Model 2: Adjusted for key demographic confounding variables—age, sex, family income, alcohol consumption, and smoking status.
- (3)
- Model 3: Further included occupation type and work schedule type—in addition to model 2.
- (4)
- Model 4: Additionally included MVPA-based activity groups (i.e., inactive, insufficiently active, active, and highly active) to account for physical activity volume.
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korea Disease Control and Prevention Agency. Korea National Health and Nutrition Examination Survey: 2023 Guidelines. Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2023; Available online: https://knhanes.kdca.go.kr (accessed on 2 January 2025).
- Pereira-Payo, D.; Mendoza-Muñoz, M.; Denche-Zamorano, A.; Rubio-de la Osa, A.; Moreno-Quintanilla, M.; Pastor-Cisneros, R. Physical activity is associated with the incidence of depression in United States adults from the NHANES 2013–18: A cross-sectional study. Healthcare 2024, 12, 552. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Health and Welfare. Korean Physical Activity Guidelines: Revised Edition. Ministry of Health and Welfare: Sejong, Republic of Korea, 2023; Available online: https://www.khealth.or.kr (accessed on 3 January 2025).
- An, K.Y. Physical activity level in Korean adults: The Korea National health and nutrition examination survey 2017. Epidemiol. Health 2019, 41, e2019047. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, R.; Doherty, A.; Smith-Byrne, K.; Rahimi, K.; Bennett, D.; Woodward, M.; Walmsley, R.; Dwyer, T. Accelerometer measured physical activity and the incidence of cardiovascular disease: Evidence from the UK Biobank cohort study. PLoS Med. 2021, 18, e1003487. [Google Scholar]
- Kokkinos, P. Physical Activity, Health Benefits, and Mortality Risk. Int. Sch. Res. Not. 2012, 2012, 718789. [Google Scholar] [CrossRef]
- Li, H.; Lynn, H.S.; Zipunnikov, V. Sex and Age Differences in Association between Physical Activity and Metabolic Syndrome: Results from NHANES 2003–2006. Healthcare 2023, 11, 1059. [Google Scholar] [CrossRef]
- Laaksonen, D.E.; Lakka, T.A. Physical activity in prevention and treatment of the metabolic syndrome. Appl. Physiol. Nutr. Metab. 2007, 32, 76–88. [Google Scholar] [CrossRef]
- Shin, S.W.; Kim, J. Association of Physical Activity Patterns with the Metabolic Syndrome in Korean Adults: A Nationwide Cross-Sectional Study. Rev. Cardiovasc. Med. 2024, 25, 115. [Google Scholar] [CrossRef]
- Tan, A.; Thomas, R.L.; Campbell, M.D.; Prior, S.L.; Bracken, R.M.; Churm, R. Effects of exercise training on metabolic syndrome risk factors in post-menopausal women—A systematic review and meta-analysis of randomised controlled trials. Clin. Nutr. 2023, 42, 337–351. [Google Scholar] [CrossRef]
- Brady, R.; Brown, W.J.; Hillsdon, M.; Mielke, G.I. Patterns of Accelerometer-Measured Physical Activity and Health Outcomes in Adults: A Systematic Review. Med. Sci. Sport. Exerc. 2022, 54, 1155–1166. [Google Scholar] [CrossRef]
- Andersson, C.; Lyass, A.; Larson, M.G.; Spartano, N.L.; Vita, J.A.; Benjamin, E.J.; Murabito, J.M.; Esliger, D.W.; Blease, S.J.; Hamburg, N.M.; et al. Physical activity measured by accelerometry and its associations with cardiac structure and vascular function in young and middle-age adults. J. Am. Heart Assoc. 2015, 4, e001528. [Google Scholar] [CrossRef]
- Robson, J.; Janssen, I. Intensity of bouted and sporadic physical activity and the metabolic syndrome in adults. PeerJ 2015, 3, e1437. [Google Scholar] [CrossRef] [PubMed]
- Cassidy, S.; Fuller, H.; Chau, J.; Catt, M.; Bauman, A.; Trenell, M.I. Accelerometer-based physical activity in individuals with cardiometabolic disease compared to healthy adults: A UK Biobank study. Acta Diabetol. 2018, 55, 975–979. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, G.L.M.; Victo, E.R.; Kovalskys, I.; Mello, A.V.D.; Previdelli, A.N.; Solé, D.; Fisberg, M. Sedentary behavior, physical activity and body composition in adults. Rev. Assoc. Med. Bras. 2020, 66, 314–320. [Google Scholar] [CrossRef]
- Ballin, M.; Nordstrom, P.; Nordstrom, A. Associations of light, moderate to vigorous, and total physical activity with the prevalence of metabolic syndrome in 4,652 community-dwelling 70-year-olds: A population-based cross-sectional study. J. Aging Phys. Act. 2021, 29, 735–743. [Google Scholar] [CrossRef]
- Mancilla, R.; Brouwers, B.; Schrauwen-Hinderling, V.B.; Hesselink, M.K.; Hoeks, J.; Schrauwen, P. Exercise training elicits superior metabolic effects when performed in the afternoon compared to morning in metabolically compromised humans. Physiol. Rep. 2021, 8, e14669. [Google Scholar] [CrossRef]
- Aqeel, M.; Guo, J.; Lin, L.; Gelfand, S.; Delp, E.; Bhadra, A.; Richards, E.A.; Hennessy, E.; Eicher-Miller, H.A. Temporal physical activity patterns are associated with obesity in US adults. Prev. Med. 2021, 148, 106538. [Google Scholar] [CrossRef]
- Barkley, J.E.; Farnell, G.; Boyko, B.; Turner, B.; Wiet, R. Impact of activity monitoring on physical activity, sedentary behavior, and body weight during the COVID-19 pandemic. Int. J. Environ. Res. Public Health 2021, 18, 7518. [Google Scholar] [CrossRef]
- Lim, J.; Sung, H.; Lee, O.; Kim, Y. Physical activity in South Korea measured by accelerometer: The Korea National Health and Nutrition Examination Survey VI 2014–2015. Korean J. Sport Sci. 2020, 31, 169–179. [Google Scholar] [CrossRef]
- Korea Disease Control and Prevention Agency. Guidelines for the Use of the 7th Korea National Health and Nutrition Examination Survey (KNHANES VII) Raw Data. Korea Disease Control and Prevention Agency: Cheongju, Republic of Korea, 2023. Available online: https://knhanes.kdca.go.kr (accessed on 3 January 2025).
- Troiano, R.P.; Berrigan, D.; Dodd, K.W.; Masse, L.C.; Tilert, T.; McDowell, M. Physical activity in the United States measured by accelerometer. Med. Sci. Sport. Exerc. 2008, 40, 181–188. [Google Scholar] [CrossRef]
- Sung, H.; Lim, J.; Mun, J.; Kim, Y. Objective versus self-reported physical activity and cardiovascular disease risk factors. Korean J. Sport. Med. 2020, 38, 28–36. [Google Scholar] [CrossRef]
- National Cholesterol Education Program (NCEP). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) Final Report. Circulation 2002, 106, 3143–3421. [Google Scholar] [CrossRef]
- Korean Society for the Study of Obesity. Clinical Practice Guidelines for Obesity, 8th ed.; Korean Society for the Study of Obesity: Seoul, Republic of Korea, 2022. [Google Scholar]
- Lim, J.; Kim, J.-S.; Kim, Y. Association between Physical Activity and Metabolic Syndrome in Korean Adults and Older Adults: Using Accelerometer Data from the Korea National Health and Nutrition Examination Survey, 2014–2017. Exerc. Sci. 2022, 31, 357–363. [Google Scholar] [CrossRef]
- Gao, W.; Sanna, M.; Chen, Y.H.; Tsai, M.K.; Wen, C.P. Occupational Sitting Time, Leisure Physical Activity, and All-Cause and Cardiovascular Disease Mortality. JAMA Netw. Open 2024, 7, e2350680. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Yoon, Y.S.; Oh, S.W.; Lee, E.S. The Amount of Physical Activity of Korean Adults Measured from the 2001 Korean National Health and Nutrition Survey. J. Korean Acad. Fam. Med. 2005, 26, 22–30. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024. [Google Scholar]
- Ramsay, J.O.; Hooker, G.; Graves, S. fda: Functional Data Analysis, R package version 5.5.1; CRAN: Vienna, Austria, 2023; Available online: https://CRAN.R-project.org/package=fda (accessed on 3 December 2024).
- Sabag, A.; Ahmadi, M.N.; Francois, M.E.; Postnova, S.; Cistulli, P.A.; Fontana, L.; Stamatakis, E. Timing of moderate to vigorous physical activity, mortality, cardiovascular disease, and microvascular disease in adults with obesity. Diabetes Care 2024, 47, 890–897. [Google Scholar] [CrossRef]
- Moholdt, T.; Parr, E.B.; Devlin, B.L.; Debik, J.; Giskeødegård, G.; Hawley, J.A. The effect of morning vs evening exercise training on glycaemic control and serum metabolites in overweight/obese men: A randomised trial. Diabetologia 2021, 64, 2061–2076. [Google Scholar] [CrossRef]
- Clavero-Jimeno, A.; Dote-Montero, M.; Migueles, J.H.; Camacho-Cardenosa, A.; Oses, M.; Medina, J.E.; Alcantara, J.M.A.; Muñoz-Torres, M. Impact of lifestyle moderate-to-vigorous physical activity timing on glycemic control in sedentary adults with overweight/obesity and metabolic impairments. Obesity 2024, 32, 1465–1473. [Google Scholar] [CrossRef]
- Drobnic, F.; Rueda, J.; Pons, V.; Banquells, M.; Cordobilla, B. Diagnosis and Treatment of Dyslipidaemias in Athletes. Curr. Vasc. Pharmacol. 2017, 15, 238–247. [Google Scholar] [CrossRef]

| Characteristic | Without MetS (n = 1347) | MetS (n = 549) | Total (n = 1896) | p-Value |
|---|---|---|---|---|
| Age, mean (SD) | 41.9 (12.5) | 49.4 (9.4) | 43.5 (12.3) | <0.001 |
| Sex, no. (%) | ||||
| Male | 406 (30.1) | 306 (55.7) | 712 (37.6) | <0.001 |
| Female | 941 (69.9) | 243 (44.3) | 1184 (62.4) | |
| Family income, no. (%) | ||||
| 1st quartile | 186 (13.8) | 109 (19.9) | 295 (15.6) | <0.001 |
| 2nd quartile | 365 (27.1) | 176 (32.1) | 541 (28.5) | |
| 3rd quartile | 420 (31.2) | 129 (23.5) | 549 (29.0) | |
| 4th quartile | 376 (27.9) | 135 (24.6) | 511 (27.0) | |
| Smoking status, no. (%) | ||||
| Non-smoker | 984 (73.1) | 281 (51.2) | 1265 (66.7) | <0.001 |
| Former smoker | 201 (14.9) | 163 (29.7) | 364 (19.2) | |
| Current smoker | 162 (12.0) | 105 (19.1) | 267 (14.1) | |
| Alcohol consumption, no. (%) | ||||
| Non-drinker | 289 (21.5) | 115 (20.9) | 404 (21.3) | <0.001 |
| 1 or less times/week | 492 (36.5) | 149 (27.1) | 641 (33.8) | |
| 2–4 times/month | 342 (25.4) | 133 (24.2) | 475 (25.1) | |
| 2–3 times/week | 176 (13.1) | 100 (18.2) | 276 (14.6) | |
| 4 or more times/week | 48 (3.6) | 52 (9.5) | 100 (5.3) | |
| Occupation type, no. (%) | ||||
| Non-manual worker | 703 (52.2) | 204 (37.2) | 907 (47.8) | <0.001 |
| Manual worker | 554 (41.1) | 284 (51.7) | 838 (44.2) | |
| Economically inactive | 90 (6.7) | 61 (11.1) | 151 (8.0) | |
| Work schedule type, no. (%) | ||||
| Day shift | 822 (61.0) | 402 (73.2) | 1224 (64.6) | <0.001 |
| Evening/night shift | 150 (11.1) | 34 (6.2) | 184 (9.7) | |
| Rotating shift | 42 (3.1) | 19 (3.5) | 61 (3.2) | |
| Other | 5 (0.4) | 4 (0.7) | 9 (0.5) | |
| Not employed | 328 (24.4) | 90 (16.4) | 418 (22.0) | |
| Activity group, no. (%) | ||||
| Inactive (0 min/week) | 318 (23.6) | 129 (23.4) | 447 (23.6) | 0.994 |
| Insufficiently active (1–149 min/week) | 829 (61.5) | 340 (61.9) | 1169 (61.6) | |
| Active (150–299 min/week) | 150 (11.1) | 61 (11.1) | 211 (11.1) | |
| Highly active (≥300 min/week) | 50 (3.7) | 19 (3.4) | 69 (3.6) | |
| Metabolic syndrome factors | ||||
| SBP, mean (SD) | 109.3 (11.5) | 125.9 (14.5) | 114.1 (14.5) | <0.001 |
| DBP, mean (SD) | 72.0 (8.0) | 82.6 (9.9) | 75.1(9.8) | <0.001 |
| GLU, median (IQR) | 91.0 (9.0) | 104.0 (17.0) | 93.0 (13.0) | <0.001 |
| WC, mean (SD) | 76.6 (7.9) | 88.7 (8.6) | 80.1 (9.8) | <0.001 |
| TG, median (IQR) | 81.0 (59.0) | 171.0 (109.0) | 99.0 (89.0) | <0.001 |
| HDL-C, mean (SD) | 55.2 (12.6) | 46.0 (10.4) | 52.5 (12.7) | <0.001 |
| Variable | Model 1 | Model 2 | Model 3 | Model 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | Adjusted OR (95% CI) | p-Value | |
| PC1 | 1.016 (0.921, 1.121) | 0.750 | 1.061 (0.952, 1.182) | 0.285 | 1.048 (0.939, 1.167) | 0.406 | 1.050 (0.941, 1.171) | 0.381 |
| PC2 | 1.004 (0.911, 1.107) | 0.930 | 0.979 (0.881, 1.089) | 0.705 | 0.974 (0.875, 1.084) | 0.629 | 0.976 (0.877, 1.086) | 0.650 |
| PC3 | 1.061 (0.963, 1.169) | 0.232 | 1.105 (0.993, 1.230) | 0.067 | 1.118 (1.004, 1.246) | 0.042 | 1.117 (1.003, 1.244) | 0.044 |
| PC4 | 1.036 (0.939, 1.142) | 0.481 | 1.010 (0.909, 1.123) | 0.848 | 1.009 (0.908, 1.123) | 0.856 | 1.008 (0.906, 1.121) | 0.884 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, S.; Jee, H.-J. Association Between Physical Activity Timing and Metabolic Syndrome in Korea: A Functional Principal Component Approach. Healthcare 2025, 13, 1384. https://doi.org/10.3390/healthcare13121384
Park S, Jee H-J. Association Between Physical Activity Timing and Metabolic Syndrome in Korea: A Functional Principal Component Approach. Healthcare. 2025; 13(12):1384. https://doi.org/10.3390/healthcare13121384
Chicago/Turabian StylePark, Suah, and Hee-Jung Jee. 2025. "Association Between Physical Activity Timing and Metabolic Syndrome in Korea: A Functional Principal Component Approach" Healthcare 13, no. 12: 1384. https://doi.org/10.3390/healthcare13121384
APA StylePark, S., & Jee, H.-J. (2025). Association Between Physical Activity Timing and Metabolic Syndrome in Korea: A Functional Principal Component Approach. Healthcare, 13(12), 1384. https://doi.org/10.3390/healthcare13121384







