The Association Between Chronic Heart Failure and Metabolic Syndrome Increases the Cost of Hospitalization
Abstract
1. Introduction
2. Materials and Methods
2.1. Data
2.2. Hospitalization Costs
2.3. Statistics
3. Results
4. Discussion
4.1. Sex Difference in CHF Prevalence
4.2. High Frequency of CHF Among Admission Causes
4.3. The Cost of CHF and MetS
4.4. Policy Implications of Observed CHF Costs
4.5. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIDS | Acquired Immunodeficiency Syndrome |
| CCI | Charleston Comorbidity Index |
| CHF | chronic heart failure |
| CRP | C-reactive protein |
| DRGs | Diagnosis-Related Groups |
| HDLs | high-density lipoproteins |
| ICD | International Classification of Diseases |
| IDF | International Diabetes Foundation |
| IQR | interquartile range |
| MetS | metabolic syndrome |
| SD | standard deviation |
References
- Brake, R.; Jones, I.D. Chronic heart failure part 1: Pathophysiology, signs and symptoms. Nurs. Stand. 2017, 31, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Elendu, C.; Amaechi, D.C.; Elendu, T.C.; Fiemotonghan, B.E.; Okoye, O.K.; Agu-Ben, C.M.; Onyekweli, S.O.; Amapu, D.A.; Ikpegbu, R.; Asekhauno, M.; et al. A comprehensive review of heart failure: Unraveling the etiology, decoding pathophysiological mechanisms, navigating diagnostic modalities, exploring pharmacological interventions, advocating lifestyle modifications, and charting the horizon of emerging therapies in the complex landscape of chronic cardiac dysfunction. Medicine 2024, 103, e36895. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2023, 44, 3627–3639. [Google Scholar] [CrossRef]
- Tomasoni, D.; Fonarow, G.C.; Adamo, M.; Anker, S.D.; Butler, J.; Coats, A.J.S.; Filippatos, G.; Greene, S.J.; McDonagh, T.A.; Ponikowski, P.; et al. Sodium-glucose co-transporter 2 inhibitors as an early, first-line therapy in patients with heart failure and reduced ejection fraction. Eur. J. Heart Fail. 2022, 24, 431–441. [Google Scholar] [CrossRef]
- Cunningham, J.W.; Vaduganathan, M.; Claggett, B.L.; Kulac, I.J.; Desai, A.S.; Jhund, P.S.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; et al. Dapagliflozin in Patients Recently Hospitalized with Heart Failure and Mildly Reduced or Preserved Ejection Fraction. J. Am. Coll. Cardiol. 2022, 80, 1302–1310. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Szarek, M.; Steg, P.G.; Cannon, C.P.; Leiter, L.A.; McGuire, D.K.; Lewis, J.B.; Riddle, M.C.; Voors, A.A.; Metra, M.; et al. Sotagliflozin in Patients with Diabetes and Recent Worsening Heart Failure. N. Engl. J. Med. 2021, 384, 117–128. [Google Scholar] [CrossRef]
- Savarese, G.; Becher, P.M.; Lund, L.H.; Seferovic, P.; Rosano, G.M.C.; Coats, A.J.S. Global burden of heart failure: A comprehensive and updated review of epidemiology. Cardiovasc. Res. 2023, 118, 3272–3287. [Google Scholar] [CrossRef] [PubMed]
- Pocock, S.J.; Ariti, C.A.; McMurray, J.J.; Maggioni, A.; Kober, L.; Squire, I.B.; Swedberg, K.; Dobson, J.; Poppe, K.K.; Whalley, G.A.; et al. Predicting survival in heart failure: A risk score based on 39 372 patients from 30 studies. Eur. Heart J. 2013, 34, 1404–1413. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Fonarow, G.C.; Opsha, Y.; Sandhu, A.T.; Sweitzer, N.K.; Warraich, H.J.; HFSA Scientific Statement Committee Members Chair. Economic Issues in Heart Failure in the United States. J. Card. Fail. 2022, 28, 453–466. [Google Scholar] [CrossRef]
- Tran, D.T.; Ohinmaa, A.; Thanh, N.X.; Howlett, J.G.; Ezekowitz, J.A.; McAlister, F.A.; Kaul, P. The current and future financial burden of hospital admissions for heart failure in Canada: A cost analysis. CMAJ Open 2016, 4, E365–E370. [Google Scholar] [CrossRef]
- Khan, S.U.; Khan, M.Z.; Alkhouli, M. Trends of Clinical Outcomes and Health Care Resource Use in Heart Failure in the United States. J. Am. Heart Assoc. 2020, 9, e016782. [Google Scholar] [CrossRef] [PubMed]
- Rosano, G.M.C.; Seferovic, P.; Savarese, G.; Spoletini, I.; Lopatin, Y.; Gustafsson, F.; Bayes-Genis, A.; Jaarsma, T.; Abdelhamid, M.; Miqueo, A.G.; et al. Impact analysis of heart failure across European countries: An ESC-HFA position paper. ESC Heart Fail. 2022, 9, 2767–2778. [Google Scholar] [CrossRef] [PubMed]
- Norhammar, A.; Bodegard, J.; Vanderheyden, M.; Tangri, N.; Karasik, A.; Maggioni, A.P.; Sveen, K.A.; Taveira-Gomes, T.; Botana, M.; Hunziker, L.; et al. Prevalence, outcomes and costs of a contemporary, multinational population with heart failure. Heart 2023, 109, 548–556. [Google Scholar] [CrossRef]
- Baras Shreibati, J.; Goldhaber-Fiebert, J.D.; Banerjee, D.; Owens, D.K.; Hlatky, M.A. Cost-Effectiveness of Left Ventricular Assist Devices in Ambulatory Patients With Advanced Heart Failure. JACC Heart Fail. 2017, 5, 110–119. [Google Scholar] [CrossRef]
- Urbich, M.; Globe, G.; Pantiri, K.; Heisen, M.; Bennison, C.; Wirtz, H.S.; Di Tanna, G.L. A Systematic Review of Medical Costs Associated with Heart Failure in the USA (2014–2020). Pharmacoeconomics 2020, 38, 1219–1236. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Murdoch, D.R.; McMurray, J.J. Economics of chronic heart failure. Eur. J. Heart Fail. 2001, 3, 283–291. [Google Scholar] [CrossRef]
- Khan, M.S.; Shahid, I.; Bennis, A.; Rakisheva, A.; Metra, M.; Butler, J. Global epidemiology of heart failure. Nat. Rev. Cardiol. 2024, 21, 717–734. [Google Scholar] [CrossRef]
- Koutlas, A.; Jenkins, P. Reducing Hospital Admissions for Patients with Heart Failure by Implementing the Chronic Care Management Framework: A Cost, Quality and Satisfaction Improvement Project. J. Dr. Nurs. Pract. 2022, 15, 96–104. [Google Scholar] [CrossRef]
- Leon-Justel, A.; Morgado Garcia-Polavieja, J.I.; Alvarez-Rios, A.I.; Caro Fernandez, F.J.; Merino, P.A.P.; Galvez Rios, E.; Vazquez-Rico, I.; Fernandez, J.F.D. Biomarkers-based personalized follow-up in chronic heart failure improves patient’s outcomes and reduces care associate cost. Health Qual. Life Outcomes 2021, 19, 142. [Google Scholar] [CrossRef]
- Maru, S.; Byrnes, J.; Carrington, M.J.; Chan, Y.K.; Thompson, D.R.; Stewart, S.; Scuffham, P.A. Cost-effectiveness of home versus clinic-based management of chronic heart failure: Extended follow-up of a pragmatic, multicentre randomized trial cohort—The WHICH? study (Which Heart Failure Intervention is Most Cost-Effective & Consumer Friendly in Reducing Hospital Care). Int. J. Cardiol. 2015, 201, 368–375. [Google Scholar] [CrossRef]
- Miura, Y.; Fukumoto, Y.; Shiba, N.; Miura, T.; Shimada, K.; Iwama, Y.; Takagi, A.; Matsusaka, H.; Tsutsumi, T.; Yamada, A.; et al. Prevalence and clinical implication of metabolic syndrome in chronic heart failure. Circ. J. 2010, 74, 2612–2621. [Google Scholar] [CrossRef] [PubMed]
- Alberti, K.G.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef]
- Purwowiyoto, S.L.; Prawara, A.S. Metabolic syndrome and heart failure: Mechanism and management. Med. Pharm. Rep. 2021, 94, 15–21. [Google Scholar] [CrossRef]
- van der Hoef, C.C.S.; Boorsma, E.M.; Emmens, J.E.; van Essen, B.J.; Metra, M.; Ng, L.L.; Anker, S.D.; Dickstein, K.; Mordi, I.R.; Dihoum, A.; et al. Biomarker signature and pathophysiological pathways in patients with chronic heart failure and metabolic syndrome. Eur. J. Heart Fail. 2023, 25, 163–173. [Google Scholar] [CrossRef]
- Roytman, A.P.; Sedova, N.A.; Godkov, M.A. Laboratory indicators of pathological changes in patients with chronic heart failure with metabolic syndrome. Klin. Lab. Diagn. 2021, 66, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Bossone, E.; Arcopinto, M.; Iacoviello, M.; Triggiani, V.; Cacciatore, F.; Maiello, C.; Limongelli, G.; Masarone, D.; Perticone, F.; Sciacqua, A.; et al. Multiple hormonal and metabolic deficiency syndrome in chronic heart failure: Rationale, design, and demographic characteristics of the T.O.S.CA. Registry. Intern. Emerg. Med. 2018, 13, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Kearney, M.T. Chronic heart failure: A missing component of the metabolic syndrome? Diabetes Vasc. Dis. Res. 2009, 6, 145. [Google Scholar] [CrossRef]
- Nichols, G.A.; Amitay, E.L.; Chatterjee, S.; Steubl, D. The Bidirectional Association of Chronic Kidney Disease, Type 2 Diabetes, Atherosclerotic Cardiovascular Disease, and Heart Failure: The Cardio-Renal-Metabolic Syndrome. Metab. Syndr. Relat. Disord. 2023, 21, 261–266. [Google Scholar] [CrossRef]
- Leon-Roman, J.; Azancot, M.A.; Marouco, C.; Patricio-Liebana, M.; Zamora, J.I.; Ramos Terrades, N.; Toapanta, N.; Núñez-Delgado, S.; Fernandez, A.B.M.; Soler, M.J. A New Era in the Management of Cardiorenal Syndrome: The Importance of Cardiorenal Units. Cardiorenal Med. 2025, 15, 174–183. [Google Scholar] [CrossRef]
- Yaqoob, N.; Khalid, F.; Khan, M.F.; Anwar, W.; Khan, M.F.; Iqbal, M.H. Prevalence of Cardiorenal Syndrome in Patients Admitted for Acute Decompensated Heart Failure and Its Correlation With In-Hospital Outcomes. J. Ayub Med. Coll. Abbottabad 2024, 36, 773–777. [Google Scholar] [CrossRef]
- Perrone-Filardi, P.; Savarese, G.; Scarano, M.; Cavazzina, R.; Trimarco, B.; Minneci, S.; Maggioni, A.P.; Tavazzi, L.; Tognoni, G.; Marchioli, R. Prognostic impact of metabolic syndrome in patients with chronic heart failure: Data from GISSI-HF trial. Int. J. Cardiol. 2015, 178, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Tadaki, S.; Sakata, Y.; Miura, Y.; Miyata, S.; Asakura, M.; Shimada, K.; Yamamoto, T.; Fukumoto, Y.; Kadokami, T.; Yasuda, S.; et al. Prognostic Impacts of Metabolic Syndrome in Patients With Chronic Heart Failure—A Multicenter Prospective Cohort Study. Circ. J. 2016, 80, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Ricardo, S.J.; Araujo, M.Y.C.; Santos, L.L.D.; Romanzini, M.; Fernandes, R.A.; Turi-Lynch, B.C.; Codogno, J.S. Burden of metabolic syndrome on primary healthcare costs among older adults: A cross-sectional study. Sao Paulo Med. J. 2024, 142, e2023215. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- Hivert, M.F.; Grant, R.W.; Shrader, P.; Meigs, J.B. Identifying primary care patients at risk for future diabetes and cardiovascular disease using electronic health records. BMC Health Serv. Res. 2009, 9, 170. [Google Scholar] [CrossRef]
- Hivert, M.F.; Dusseault-Bélanger, F.; Cohen, A.; Courteau, J.; Vanasse, A. Modified metabolic syndrome criteria for identification of patients at risk of developing diabetes and coronary heart diseases: Longitudinal assessment via electronic health records. Can. J. Cardiol. 2012, 28, 744–749. [Google Scholar] [CrossRef] [PubMed]
- Radu, C.P.; Chiriac, D.N.; Vladescu, C. Changing patient classification system for hospital reimbursement in Romania. Croat. Med. J. 2010, 51, 250–258. [Google Scholar] [CrossRef]
- Bonapace, S.; Mantovani, A. Do Sex and Gender-Related Differences Account to Different Risk of Developing Heart Failure in Middle-Aged People with Metabolic Syndrome? Metabolites 2024, 14, 528. [Google Scholar] [CrossRef]
- Chandra, A.; Skali, H.; Claggett, B.; Solomon, S.D.; Rossi, J.S.; Russell, S.D.; Matsushita, K.; Kitzman, D.W.; Konety, S.H.; Mosley, T.H.; et al. Race- and Gender-Based Differences in Cardiac Structure and Function and Risk of Heart Failure. J. Am. Coll. Cardiol. 2022, 79, 355–368. [Google Scholar] [CrossRef]
- Ciutac, A.M.; Pana, T.; Dawson, D.; Myint, P.K. Sex-related differences in heart failure patients: Physiological mechanisms of cardiovascular ageing and evidence-based sex-specific medical therapies. Ther. Adv. Cardiovasc. Dis. 2025, 19, 17539447241309673. [Google Scholar] [CrossRef]
- Kim, T.E.; Kim, D.Y.; Kim, H.; Kim, S.H. Sex and Age Differences in the Impact of Metabolic Syndrome on Heart Failure Development. Metabolites 2024, 14, 653. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wen, Y.; Peng, H.; Zhu, H.; Wang, W.E.; Zhou, J. Gender Differences in Anxiety, Depression, Insomnia, and Quality of Life in Heart Failure with Preserved Ejection Fraction: A Multicenter, Cross-sectional Study. J. Cardiovasc. Nurs. 2023, 38, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Tapia, J.; Basalo, M.; Enjuanes, C.; Calero, E.; Jose, N.; Ruiz, M.; Calvo, E.; Garcimartín, P.; Moliner, P.; Hidalgo, E.; et al. Psychosocial factors partially explain gender differences in health-related quality of life in heart failure patients. ESC Heart Fail. 2023, 10, 1090–1102. [Google Scholar] [CrossRef] [PubMed]
- Lim, A.; Benjasirisan, C.; Tebay, J.; Liu, X.; Badawi, S.; Himmelfarb, C.D.; Davidson, P.M.; Koirala, B. Gender Differences in Disease Burden, Symptom Burden, and Quality of Life Among People Living with Heart Failure and Multimorbidity: Cross-Sectional Study. J. Adv. Nurs. 2025. [Google Scholar] [CrossRef] [PubMed]
- Shi, K.; Zhang, G.; Fu, H.; Li, X.M.; Jiang, L.; Gao, Y.; Qian, W.-L.; Shen, L.-T.; Xu, H.-Y.; Li, Y.; et al. Sex differences in clinical profile, left ventricular remodeling and cardiovascular outcomes among diabetic patients with heart failure and reduced ejection fraction: A cardiac-MRI-based study. Cardiovasc. Diabetol. 2024, 23, 266. [Google Scholar] [CrossRef]
- Tang, W.H.W. Targeting Inflammation in Heart Failure Prevention: Are There Sex Differences? JACC Heart Fail. 2025, 13, 450–452. [Google Scholar] [CrossRef]
- Fluschnik, N.; Strangl, F.; Kondziella, C.; Gossling, A.; Becher, P.M.; Schrage, B.; Schnabel, R.B.; Bernadyn, J.; Bremer, W.; Grahn, H.; et al. Gender differences in characteristics and outcomes in heart failure patients referred for end-stage treatment. ESC Heart Fail. 2021, 8, 5031–5039. [Google Scholar] [CrossRef]
- Saldarriaga, C.; Garcia-Arango, M.; Valentina Lopez, L.; Contreras, J. Sex Differences in Worsening Heart Failure: Learning From Real-world Evidence. J. Card. Fail. 2024, 30, 991–993. [Google Scholar] [CrossRef]
- 47 Kocabas, U.; Kivrak, T.; Yilmaz Oztekin, G.M.; Tanik, V.O.; Ozdemir, I.; Kaya, E.; Yüce, E.I.; Demir, F.A.; Doğduş, M.; Altınsoy, M.; et al. Gender-related clinical and management differences in patients with chronic heart failure with reduced ejection fraction. Int. J. Clin. Pract. 2021, 75, e13765. [Google Scholar] [CrossRef]
- Cediel, G.; Codina, P.; Spitaleri, G.; Domingo, M.; Santiago-Vacas, E.; Lupon, J.; Bayes-Genis, A. Gender-Related Differences in Heart Failure Biomarkers. Front. Cardiovasc. Med. 2020, 7, 617705. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.M. Sex differences in diagnosis and treatment of heart failure: Toward precision medicine. Korean J. Intern. Med. 2025, 40, 196–207. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Cai, A.; Wu, S.; Zhu, Y.; Zheng, H.; Feng, Y. Sex- and age-specific differences in the associations between comorbidity and incident heart failure. QJM Int. J. Med. 2025. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Wang, W.; Wu, S.; Zhu, Y.; Zheng, H.; Feng, Y. Sex differences in long-term heart failure prognosis: A comprehensive meta-analysis. Eur. J. Prev. Cardiol. 2024, 31, 2013–2023. [Google Scholar] [CrossRef]
- Chen, C.C.; Chiu, C.C.; Hao, W.R.; Hsu, M.H.; Liu, J.C.; Lin, J.L. Sex differences in clinical characteristics and long-term clinical outcomes in Asian hospitalized heart failure patients. ESC Heart Fail. 2024, 11, 3095–3104. [Google Scholar] [CrossRef]
- Salem, K.; ElKhateeb, O. Gender-adjusted and age-adjusted economic inpatient burden of congestive heart failure: Cost and disability-adjusted life-year analysis. ESC Heart Fail. 2017, 4, 259–265. [Google Scholar] [CrossRef]
- Cremers, H.P.; Theunissen, L.J.H.J.; Essers, P.P.M.; van de Ven, A.R.T.; Spee, R.; Verbunt, R.; Otterspoor, L.; Post, J.C.; Tio, R.; van Asperdt, F.G.M.H.; et al. Gender differences in Heart Failure; Data on Outcomes and Costs. Eur. Soc. Cardiol. Virtual J. 2020. Available online: https://www.escardio.org/The-ESC/What-we-do/Initiatives/Virtual-Journal/gender-differences-in-heart-failure-data-on-outcomes-and-costs (accessed on 6 March 2025).
- Janwanishstaporn, S.; Karaketklang, K.; Krittayaphong, R. National trend in heart failure hospitalization and outcome under public health insurance system in Thailand 2008–2013. BMC Cardiovasc. Disord. 2022, 22, 203. [Google Scholar] [CrossRef]
- Aizpuru, F.; Millan, E.; Garmendia, I.; Mateos, M.; Librero, J. Hospitalizations for heart failure: Epidemiology and health system burden based on data gathered in routine practice. Med. Clínica Práctica 2020, 3, 100140. [Google Scholar] [CrossRef]
- Alosaimi, F.D.; Abalhassan, M.; Alhaddad, B.; Alzain, N.; Fallata, E.; Alhabbad, A.; Alassiry, M.Z. Prevalence of metabolic syndrome and its components among patients with various psychiatric diagnoses and treatments: A cross-sectional study. Gen. Hosp. Psychiatry 2017, 45, 62–69. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Nguyen, T.N.; Nguyen, K.M.; Tran, H.P.N.; Huynh, K.L.A.; Hoang, S.V. Prevalence and impact of metabolic syndrome on in-hospital outcomes in patients with acute myocardial infarction: A perspective from a developing country. Medicine 2023, 102, e35924. [Google Scholar] [CrossRef]
- Park, H.J.; Jung, J.H.; Han, K.; Shin, J.; Lee, Y.; Chang, Y.; Park, K.; Cho, Y.J.; Choi, Y.S.; Kim, S.M.; et al. Association between metabolic syndrome and mortality in patients with COVID-19: A nationwide cohort study. Obes. Res. Clin. Pract. 2022, 16, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Checa, C.; Canelo-Aybar, C.; Suclupe, S.; Ginesta-Lopez, D.; Berenguera, A.; Castells, X.; Brotons, C.; Posso, M. Effectiveness and Cost-Effectiveness of Case Management in Advanced Heart Failure Patients Attended in Primary Care: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2022, 19, 13823. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ba, Y.; Ni, J.; Huang, R.; Du, X. Role of Telemedicine Intervention in the Treatment of Patients with Chronic Heart Failure: A Systematic Review and Meta-analysis. Anatol. J. Cardiol. 2024, 28, 177–186. [Google Scholar] [CrossRef] [PubMed]
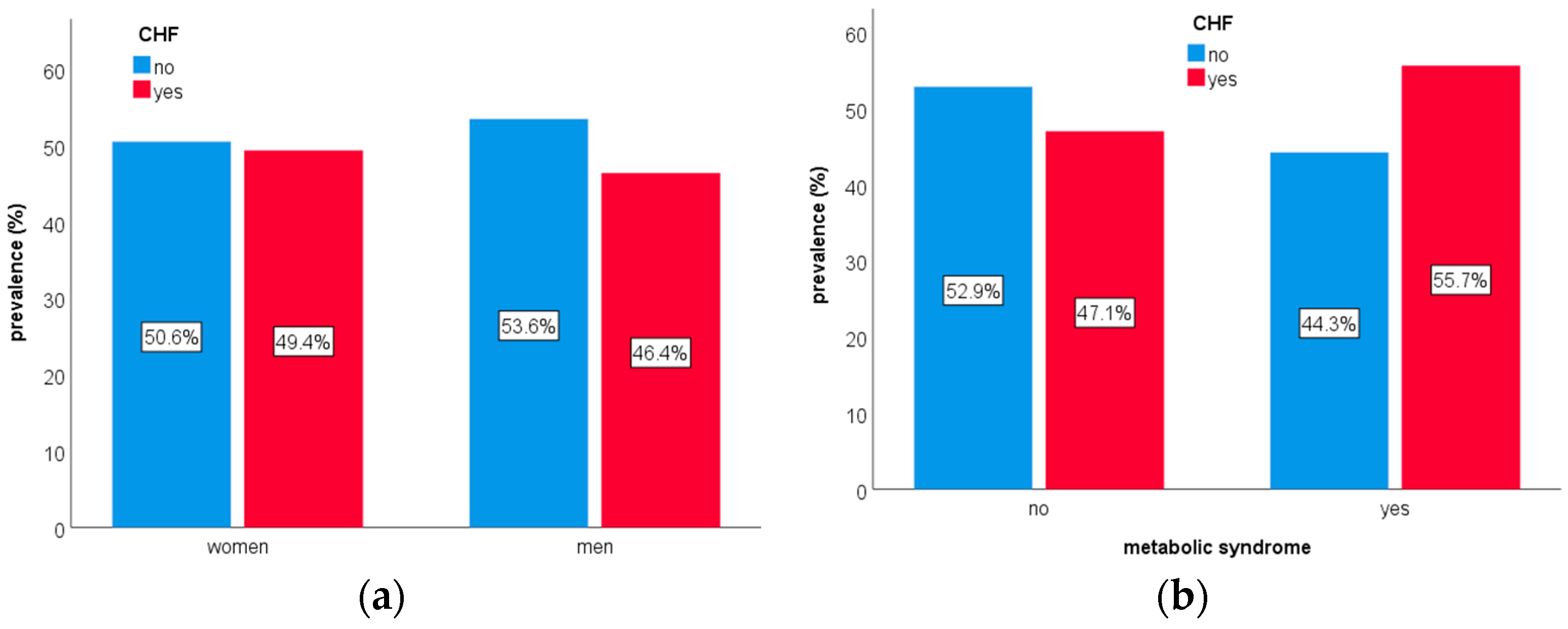

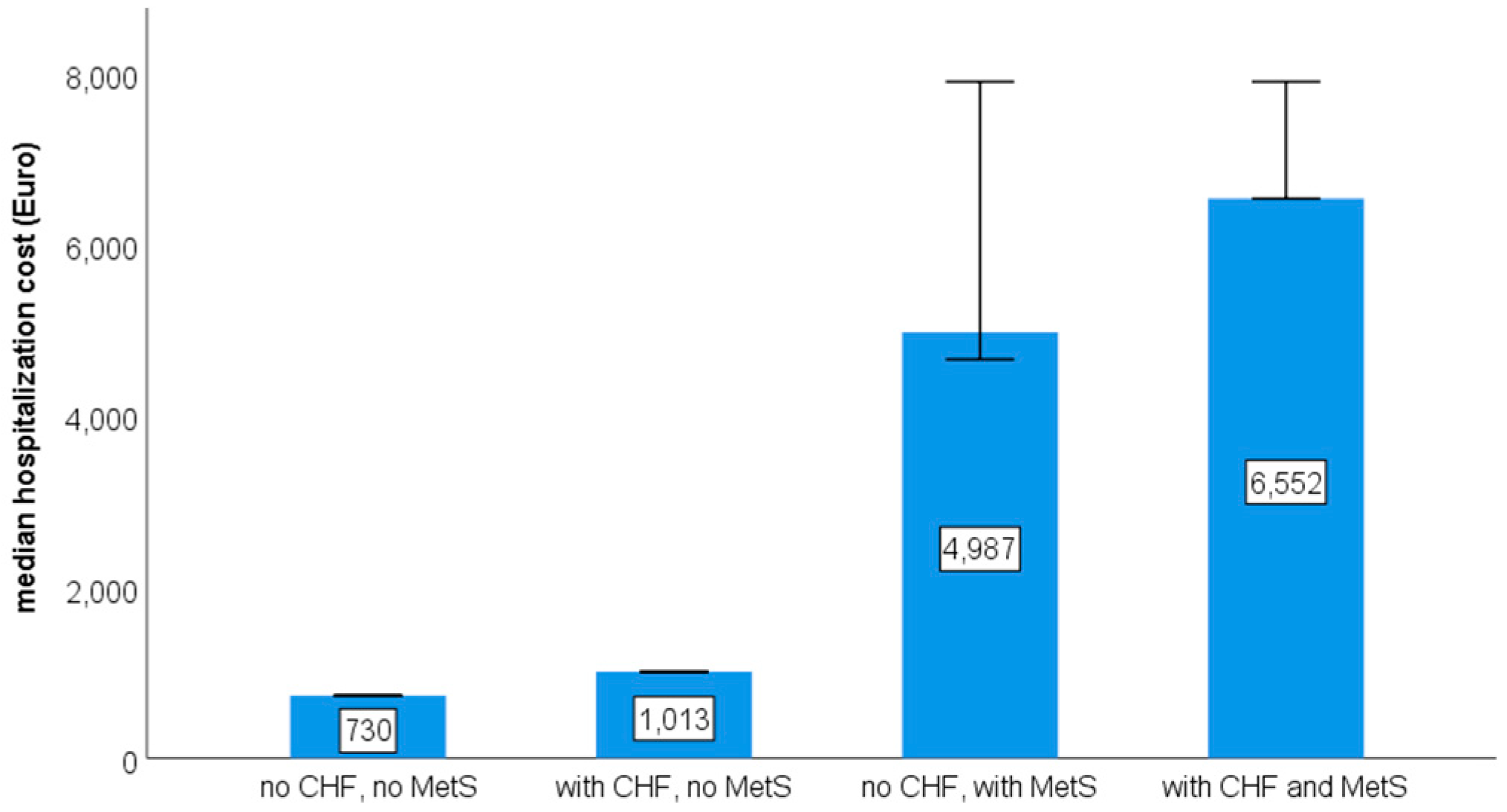
| Diagnosis | ICD10 Code |
|---|---|
| chronic heart failure | I50 |
| myocardial infarction | I21, I25 |
| peripheral vascular disease | I73 |
| cerebrovascular disease | I60, I61, I63, I64, I67, G45.8, G45.9 |
| dementia | F00, F01, F03 |
| chronic pulmonary disease | J44 |
| rheumatic disease | M05, M06, M45, L40.5, M07, M32, M33, M34, M35 |
| peptic ulcer disease | K27 |
| cirrhosis | K70, K71, K74, K76 |
| variceal bleeding | I85.0, I98.3 |
| diabetes mellitus | E10, E11, E12, E13, E14 |
| hemiplegia | G81 |
| chronic kidney disease | N18 |
| solid tumor | C |
| metastatic cancer | C78, C79, C80 |
| leukemia | C90, C91, C92, C93, C94, C95 |
| lymphoma | C81, C82, C83, C84, C85, C86, C88 |
| AIDS | B20, B21, B22, B23, B24 |
| obesity | E66 |
| arterial hypertension | I10 |
| dyslipidemia | E78 |
| Variable | Observed |
|---|---|
| women | 53.9% |
| age (years, average ± SD) | 68.7 ± 13.4 |
| hospitalization duration (days, median (IQR)) | 4.0 (5.9) |
| total cost of hospitalization (EUR, median (IQR)) | 1002.1 (7338.3) |
| total cost of hospitalization (RON, median (IQR)) | 5010.2 (36,690.1) |
| cost per day of hospitalization (EUR, median (IQR)) | 322.2 (1476.8) |
| cost per day of hospitalization (RON, median (IQR)) | 1611.2 (7347.9) |
| Charlson Comorbidity Index (average ± SD) | 4.8 ± 2.5 |
| Diagnosis | Prevalence | Diagnosis | Prevalence |
|---|---|---|---|
| chronic heart failure | 48.0% | hemiplegia | 0.4% |
| myocardial infarction | 18.8% | chronic kidney disease | 19.8% |
| peripheral vascular disease | 0.9% | solid tumor | 8.7% |
| cerebrovascular disease | 6.5% | metastatic cancer | 2.8% |
| dementia | 4.8% | leukemia | 0.5% |
| chronic pulmonary disease | 9.6% | lymphoma | 0.3% |
| rheumatic disease | 2.9% | AIDS | 0.1% |
| peptic ulcer disease | 0.0% | obesity | 19.8% |
| cirrhosis | 17.3% | arterial hypertension | 50.0% |
| variceal bleeding | 0.0% | dyslipidemia | 38.5% |
| diabetes mellitus | 30.5% | metabolic syndrome | 11.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mincă, A.; Popescu, C.C.; Mincă, D.I.; Călinoiu, A.L.; Ciobanu, A.; Gheorghiță, V.; Mincă, D.G. The Association Between Chronic Heart Failure and Metabolic Syndrome Increases the Cost of Hospitalization. Healthcare 2025, 13, 1239. https://doi.org/10.3390/healthcare13111239
Mincă A, Popescu CC, Mincă DI, Călinoiu AL, Ciobanu A, Gheorghiță V, Mincă DG. The Association Between Chronic Heart Failure and Metabolic Syndrome Increases the Cost of Hospitalization. Healthcare. 2025; 13(11):1239. https://doi.org/10.3390/healthcare13111239
Chicago/Turabian StyleMincă, Alexandra, Claudiu C. Popescu, Dragoș I. Mincă, Amalia L. Călinoiu, Ana Ciobanu, Valeriu Gheorghiță, and Dana G. Mincă. 2025. "The Association Between Chronic Heart Failure and Metabolic Syndrome Increases the Cost of Hospitalization" Healthcare 13, no. 11: 1239. https://doi.org/10.3390/healthcare13111239
APA StyleMincă, A., Popescu, C. C., Mincă, D. I., Călinoiu, A. L., Ciobanu, A., Gheorghiță, V., & Mincă, D. G. (2025). The Association Between Chronic Heart Failure and Metabolic Syndrome Increases the Cost of Hospitalization. Healthcare, 13(11), 1239. https://doi.org/10.3390/healthcare13111239







