Surface Electromyography Combined with Artificial Intelligence in Predicting Neuromuscular Falls in the Elderly: A Narrative Review of Present Applications and Future Perspectives
Abstract
1. Introduction
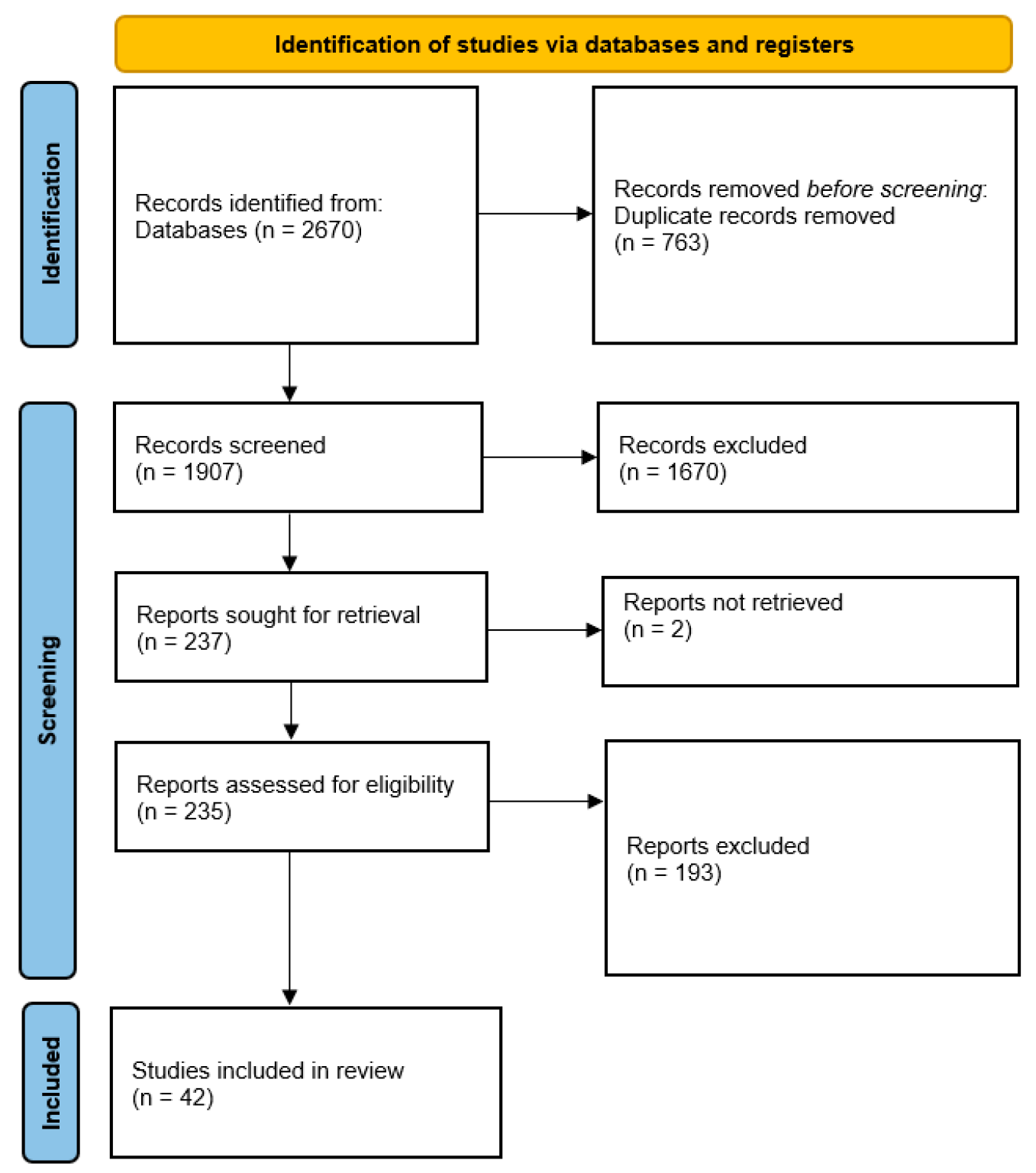
2. Methods
3. Research Status of Global Elderly Falls
4. The Application of Artificial Intelligence and sEMG in the Medical Field
4.1. The Application of Artificial Intelligence in the Medical Field
4.2. Research on sEMG
5. The Insufficiency of Existing Research on Neuromuscular Falls in the Elderly
5.1. Research Methods for Early Warning of Neuromuscular Falls in Elderly
5.2. The Application of sEMG in the Prediction of Neuromuscular Falls in the Elderly
6. Improvement and Prospect of Artificial Intelligence Combined with sEMG in Prediction of Neuromuscular Falls in Elderly
6.1. Lack of Realistic Data
6.2. Lack of Consistency in Prediction Methods
6.3. Limitations of Fall Warning Systems
6.4. Emphasis on Mitigating Consequences Rather than Preventing Falls
6.5. Improvements
6.5.1. Data Collection and Research Approach
6.5.2. Portable sEMG Acquisition and Analysis System
6.5.3. Integration with Alarm Devices and Exoskeletons
6.5.4. Novelty and Potential Impact
6.5.5. Social and Economic Implications
6.5.6. Future Clinical Work in Elderly Populations
7. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bloom, D.E.; Canning, D.; Fink, G. Implications of population ageing for economic growth. Oxf. Rev. Econ. Policy 2010, 26, 583–612. [Google Scholar] [CrossRef]
- Ambrose, A.F.; Cruz, L.; Paul, G. Falls and fractures: A systematic approach to screening and prevention. Maturitas 2015, 82, 85–93. [Google Scholar] [CrossRef]
- WHO; Ageing and Life Course Unit. WHO Global Report on Falls Prevention in Older Age; World Health Organization: Geneva, Switzerland, 2008. [Google Scholar]
- Appeadu, M.K.; Bordoni, B. Falls and Fall Prevention in Older Adults. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Wu, H.; Ouyang, P. Fall prevalence, time trend and its related risk factors among elderly people in China. Arch. Gerontol. Geriatr. 2017, 73, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Larsson, L.; Degens, H.; Li, M.; Salviati, L.; Lee, Y.I.; Thompson, W.; Kirkland, J.L.; Sandri, M. Sarcopenia: Aging-Related Loss of Muscle Mass and Function. Physiol. Rev. 2019, 99, 427–511. [Google Scholar] [CrossRef]
- Sarıhan, K.; Uzkeser, H.; Erdal, A. Evaluation of balance, fall risk, and related factors in patients with fibromyalgia syndrome. Turk. J. Phys. Med. Rehabil. 2021, 67, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Svensson, H. Finding Ways Forward with Pain as a Fellow Traveler. Older Women’s Experience of Living with Osteoporotic Vertebral Compression Fractures and Back Pain. 2018. Available online: https://gupea.ub.gu.se/handle/2077/55627 (accessed on 1 October 2024).
- Kennedy, H.L. Aging and Health for the US Elderly: A Health Primer for Ages 60 to 90 Years; University of Missouri Press: Columbia, MO, USA, 2021. [Google Scholar]
- American Thoracic Society. ATS/ERS statement on respiratory muscle testing. Am. J. Respir. Crit. Care Med. 2002, 166, 518–624. [Google Scholar] [CrossRef]
- Barbero, M.; Merletti, R.; Rainoldi, A. Atlas of Muscle Innervation Zones: Understanding Surface Electromyography and Its Applications; Springer: Milano, Italy, 2012. [Google Scholar]
- Reaz, M.B.I.; Hussain, M.S.; Mohd-Yasin, F. Techniques of EMG signal analysis: Detection, processing, classification and applications. Biol. Proced. Online 2006, 8, 11–35. [Google Scholar] [CrossRef]
- Ai, Q.; Zhang, Y.; Qi, W.; Liu, Q.; Chen, K. Research on lower limb motion recognition based on fusion of sEMG and accelerometer signals. Symmetry 2017, 9, 147. [Google Scholar] [CrossRef]
- Suarez-Patiño, L.V.; Roldan-Vasco, S.; Suarez-Escudero, J.C.; Orozco-Duque, A.; Perez-Giraldo, E. sEMG as complementary tool for VFSS: A synchronized study in patients with neurogenic oropharyngeal dysphagia. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2024, 78, 102913. [Google Scholar] [CrossRef]
- Kulesa-Mrowiecka, M.; Barański, R.; Kłaczyński, M. sEMG and Vibration System Monitoring for Differential Diagnosis in Temporomandibular Joint Disorders. Sensors 2022, 22, 3811. [Google Scholar] [CrossRef]
- Coratella, G.; Tornatore, G.; Caccavale, F.; Longo, S.; Esposito, F.; Cè, E. The Activation of Gluteal, Thigh, and Lower Back Muscles in Different Squat Variations Performed by Competitive Bodybuilders: Implications for Resistance Training. Int. J. Environ. Res. Public Health 2021, 18, 772. [Google Scholar] [CrossRef]
- Huang, Q.; Gao, M.; Guo, M.; Wei, Y.; Zhang, J.; Jin, X. Vibration comfort assessment of tractor drivers based on sEMG and vibration signals. Comput. Methods Biomech. Biomed. Eng. 2024, 27, 1875–1892. [Google Scholar] [CrossRef] [PubMed]
- Moore, A.; Li, J.; Contag, C.H.; Currano, L.J.; Pyles, C.O.; Hinkle, D.A.; Patil, V.S. Wearable Surface Electromyography System to Predict Freeze of Gait in Parkinson’s Disease Patients. Sensors 2024, 24, 7853. [Google Scholar] [CrossRef] [PubMed]
- Shefa, F.R.; Sifat, F.H.; Uddin, J.; Ahmad, Z.; Kim, J.M.; Kibria, M.G. Deep Learning and IoT-Based Ankle-Foot Orthosis for Enhanced Gait Optimization. Healthcare 2024, 12, 2273. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Orban, M.; Lu, J.; Al-Quraishi, M.S.; Yang, H.; Elsamanty, M. Empowering Hand Rehabilitation with AI-Powered Gesture Recognition: A Study of an sEMG-Based System. Bioengineering 2023, 10, 557. [Google Scholar] [CrossRef]
- Vinik, A.I.; Camacho, P.; Reddy, S.; Valencia, W.M.; Trence, D.; Matsumoto, A.M.; Morley, J.E. Aging, diabetes, and falls. Endocr. Pract. 2017, 23, 1120–1142. [Google Scholar] [CrossRef]
- von Haehling, S.; Morley, J.E.; Anker, S.D. An overview of sarcopenia: Facts and numbers on prevalence and clinical impact. J. Cachexia Sarcopenia Muscle 2010, 1, 129–133. [Google Scholar] [CrossRef]
- Labbé, A.P. Social Network Development of the Stay on Your Feet (SOYF) Implementation in the Greater Sudbury Region: A Case Study. Master’s Thesis, Laurentian University of Sudbury, Sudbury, ON, Canada, 2017. [Google Scholar]
- Bischoff-Ferrari, H.A.; Orav, J.; Kanis, J.A.; Rizzoli, R.; Schlögl, M.; Staehelin, H.; Willett, W.C.; Dawson-Hughes, B. Comparative performance of current definitions of sarcopenia against the prospective incidence of falls among community-dwelling seniors age 65 and older. Osteoporos. Int. 2015, 26, 2793–2802. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Reijnierse, E.M.; Pham, V.K.; Trappenburg, M.C.; Lim, W.K.; Meskers, C.G.M.; Maier, A.B. Sarcopenia and its association with falls and fractures in older adults: A systematic review and meta-analysis. J. Cachexia Sarcopenia Muscle 2019, 10, 485–500. [Google Scholar] [CrossRef]
- Stroup, T.S.; Gray, N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018, 17, 341–356. [Google Scholar] [CrossRef]
- Phelan, E.A.; Ritchey, K. Fall prevention in community-dwelling older adults. Ann. Intern. Med. 2018, 169, ITC81–ITC96. [Google Scholar] [CrossRef]
- Rubenstein, L.Z. Falls in older people: Epidemiology, risk factors and strategies for prevention. Age Ageing 2006, 35 (Suppl. 2), ii37–ii41. [Google Scholar] [CrossRef]
- Moreland, J.; Richardson, J.; Chan, D.H.; O’Neill, J.; Bellissimo, A.; Grum, R.M.; Shanks, L. Evidence-based guidelines for the secondary prevention of falls in older adults. Gerontology 2003, 49, 93–116. [Google Scholar] [CrossRef]
- Ye, P.; Er, Y.; Wang, H.; Fang, L.; Li, B.; Ivers, R.; Keay, L.; Duan, L.; Tian, M. Burden of falls among people aged 60 years and older in mainland China, 1990–2019: Findings from the Global Burden of Disease Study 2019. Lancet Public Health 2021, 6, e907–e918. [Google Scholar] [CrossRef]
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and fall injuries among adults aged ≥65 years—United States, 2014. Morb. Mortal. Wkly. Rep. 2016, 65, 993–998. [Google Scholar] [CrossRef]
- US Preventive Services Task Force; Grossman, D.C.; Curry, S.J.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.W.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; et al. Interventions to Prevent Falls in Community-Dwelling Older Adults: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 319, 1696–1704. [Google Scholar] [CrossRef]
- Moreland, B.L.; Burns, E.R.; Haddad, Y.K. Differences in fall-related emergency departments visits with and without an Injury, 2018. J. Saf. Res. 2022, 82, 367–370. [Google Scholar] [CrossRef]
- Reiber, G.D. Fatal falls in childhood: How far must children fall to sustain fatal head injury? Report of cases and review of the literature. Am. J. Forensic Med. Pathol. 1993, 14, 201–207. [Google Scholar] [CrossRef]
- Brewer, L.M.; Kelly, R.; Donegan, C.; Moore, A.R.; Williams, D. Poor return of functional mobility after hip fracture in older patients—it’s time to improve on hip fracture prevention. J. Am. Geriatr. Soc. 2011, 59, 1562–1563. [Google Scholar] [CrossRef]
- Kanis, J.; Brazier, J.; Stevenson, M.; Calvert, N.; Lloyd Jones, M. Treatment of established osteoporosis: A systematic review and cost-utility analysis. Health Technol. Assess. 2002, 6, 1–146. [Google Scholar] [CrossRef]
- Martinez Perez, J.A.; Palacios, S.; García, F.C.; Pérez, M. Assessing osteoporosis risk factors in Spanish menopausal women. Gynecol. Endocrinol. 2011, 27, 807–813. [Google Scholar] [CrossRef]
- Aboel-Fetoh, N.M.; Abukanna, A.; Hamad, A.; Ibn Idris, H.; Adlan, N.; Olama, S.M. Risk factors for osteoporosis in women forty years and above in Arar City, Kingdom of Saudi Arabia (KSA). Med. Sci. Clin. Res. 2015, 3, 7461–7468. [Google Scholar] [CrossRef]
- Jacobs, J.M.; Maaravi, Y.; Cohen, A.; Bursztyn, M.; Ein-Mor, E.; Stessman, J. Changing profile of health and function from age 70 to 85 years. Gerontology 2012, 58, 313–321. [Google Scholar] [CrossRef]
- Vellas, B.; Cayla, F.; Bocquet, H.; De Pemille, F.; Albarede, J. Prospective study of restriction of acitivty in old people after falls. Age Ageing 1987, 16, 189–193. [Google Scholar] [CrossRef]
- Elliott, D.B. The Glenn A. Fry award lecture 2013: Blurred vision, spectacle correction, and falls in older adults. Optom. Vis. Sci. 2014, 91, 593–601. [Google Scholar] [CrossRef]
- LeLaurin, J.H.; Shorr, R.I. Preventing falls in hospitalized patients: State of the science. Clin. Geriatr. Med. 2019, 35, 273–283. [Google Scholar] [CrossRef]
- Berry, S.D.; Miller, R.R. Falls: Epidemiology, pathophysiology, and relationship to fracture. Curr. Osteoporos. Rep. 2008, 6, 149–154. [Google Scholar] [CrossRef]
- Palanca, M.; Perilli, E.; Martelli, S. Body anthropometry and bone strength conjointly determine the risk of hip fracture in a sideways fall. Ann. Biomed. Eng. 2021, 49, 1380–1390. [Google Scholar] [CrossRef]
- Li, W.; Lu, W.; Sha, X.; Xing, H.; Lou, J.; Sun, H.; Zhao, Y. Wearable gait recognition systems based on MEMS pressure and inertial sensors: A review. IEEE Sens. J. 2021, 22, 1092–1104. [Google Scholar] [CrossRef]
- Townsend, A.M. Smart Cities: Big Data, Civic Hackers, and the Quest for a New Utopia; WW Norton & Company: New York, NY, USA, 2013. [Google Scholar]
- Teizer, J.; Allread, B.S.; Fullerton, C.E.; Hinze, J. Autonomous pro-active real-time construction worker and equipment operator proximity safety alert system. Autom. Constr. 2010, 19, 630–640. [Google Scholar] [CrossRef]
- Kosse, N.M.; Brands, K.; Bauer, J.M.; Hortobágyi, T.; Lamoth, C.J. Sensor technologies aiming at fall prevention in institutionalized old adults: A synthesis of current knowledge. Int. J. Med. Inform. 2013, 82, 743–752. [Google Scholar] [CrossRef]
- Stead, W.W. Clinical Implications and Challenges of Artificial Intelligence and Deep Learning. JAMA 2018, 320, 1107–1108. [Google Scholar] [CrossRef]
- Nguyen, Q.; Woof, W.; Kabiri, N.; Sen, S.; Eye2Gene Patient Advisory Group. Can artificial intelligence accelerate the diagnosis of inherited retinal diseases? Protocol for a data-only retrospective cohort study (Eye2Gene). BMJ Open 2023, 13, e071043. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Wang, F.; Zhang, C.; Wang, Y.; Yuan, M.; Yang, G. Towards reliable and explainable AI model for pulmonary nodule diagnosis. Biomed Signal Process Control. 2024, 88, 105646. [Google Scholar] [CrossRef]
- Blasiak, A.; Khong, J.; Kee, T. CURATE.AI: Optimizing Personalized Medicine with Artificial Intelligence. Slas Technol. 2020, 25, 95–105. [Google Scholar] [CrossRef]
- Liebman, M. The role of artificial intelligence in drug discovery and development. Chem. Int. 2022, 44, 16–19. [Google Scholar] [CrossRef]
- Kim, J.I.; Kim, G. Evaluation of health factors on artificial intelligence and the internet of things-based older adults healthcare programmes. Digit. Health. 2024, 10, 20552076241258663. [Google Scholar] [CrossRef]
- Kong, L. A study on the AI-based online triage model for hospitals in sustainable smart city. Future Gener. Comput. Syst. 2021, 125, 59–70. [Google Scholar] [CrossRef]
- Qin, P.; Shi, X. Evaluation of Feature Extraction and Classification for Lower Limb Motion Based on sEMG Signal. Entropy 2020, 22, 852. [Google Scholar] [CrossRef]
- Jamal, M.Z. Signal acquisition using surface EMG and circuit design considerations for robotic prosthesis. In Computational Intelligence in Electromyography Analysis—A Perspective on Current Applications and Future Challenges; InTech: London, UK, 2012; Volume 18, pp. 427–448. [Google Scholar]
- Papagiannis, G.I.; Triantafyllou, A.I.; Roumpelakis, I.M.; Zampeli, F.; Garyfallia Eleni, P.; Koulouvaris, P.; Papadopoulos, E.C.; Papagelopoulos, P.J.; Babis, G.C. Methodology of surface electromyography in gait analysis: Review of the literature. J. Med. Eng. Technol. 2019, 43, 59–65. [Google Scholar] [CrossRef]
- Wei, P.; Zhang, J.; Wei, P.; Wang, B.; Hong, J. In Different sEMG and EEG features analysis for gait phase recognition. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; IEEE: Piscataway, NJ, USA, 2020; pp. 1002–1006. [Google Scholar]
- Naik, G.R.; Selvan, S.E.; Arjunan, S.P.; Acharyya, A.; Kumar, D.K.; Ramanujam, A.; Nguyen, H.T. An ICA-EBM-based sEMG classifier for recognizing lower limb movements in individuals with and without knee pathology. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 675–686. [Google Scholar] [CrossRef]
- De Luca, C.J.; Gilmore, L.D.; Kuznetsov, M.; Roy, S.H. Filtering the surface EMG signal: Movement artifact and baseline noise contamination. Ornal Biomech. 2010, 43, 1573–1579. [Google Scholar] [CrossRef]
- Young, A.; Kuiken, T.; Hargrove, L. Analysis of using EMG and mechanical sensors to enhance intent recognition in powered lower limb prostheses. J. Neural Eng. 2014, 11, 056021. [Google Scholar] [CrossRef]
- Agostini, V.; Ghislieri, M.; Rosati, S.; Balestra, G.; Knaflitz, M. Surface electromyography applied to gait analysis: How to improve its impact in clinics? Front. Neurol. 2020, 11, 994. [Google Scholar] [CrossRef]
- Chen, P.J.; Chang, C.H.; Kuo, Y.L.; Lin, Y.C. Designing and evaluating a wearable sEMG device for the elderly. In Proceedings of the 14th International Conference on ICT, Society, and Human Beings, ICT 2021, 18th International Conference on Web Based Communities and Social Media, WBC 2021 and 13th International Conference on e-Health, EH 2021-Held at the 15th Multi-Conference on Computer Science and Information Systems, MCCSIS 2021, Virtual, 20–22 July 2021; pp. 136–143. [Google Scholar]
- Muñoz Esquivel, K.; Gillespie, J.; Kelly, D.; Condell, J.; Davies, R.; McHugh, C.; Duffy, W.; Nevala, E.; Alamäki, A.; Jalovaara, J. Factors Influencing Continued Wearable Device Use in Older Adult Populations: Quantitative Study. JMIR Aging 2023, 6, e36807. [Google Scholar] [CrossRef]
- Nouredanesh, M.; Kukreja, S.L.; Tung, J. Detection of compensatory balance responses using wearable electromyography sensors for fall-risk assessment. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, IEEE Engineering in Medicine and Biology Society, Annual International Conference, Orlando, FL, USA, 16–20 August 2016; pp. 1680–1683. [Google Scholar] [CrossRef]
- Clemente, J.; Li, F.; Valero, M.; Song, W. Smart Seismic Sensing for Indoor Fall Detection, Location, and Notification. IEEE J. Biomed. Health Inform. 2020, 24, 524–532. [Google Scholar] [CrossRef]
- Xi, X.; Tang, M.; Miran, S.M.; Luo, Z. Evaluation of Feature Extraction and Recognition for Activity Monitoring and Fall Detection Based on Wearable sEMG Sensors. Sensors 2017, 17, 1229. [Google Scholar] [CrossRef]
- Leone, A.; Rescio, G.; Caroppo, A.; Siciliano, P. An EMG-based system for pre-impact fall detection. In Proceedings of the 2015 IEEE Sensors, Busan, Republic of Korea, 1–4 November 2015; IEEE: Piscataway, NJ, USA, 2015; pp. 1–4. [Google Scholar]
- Lord, S.R.; Close, J.C. New horizons in falls prevention. Age Ageing 2018, 47, 492–498. [Google Scholar] [CrossRef]
- Choi, A.; Kim, T.H.; Yuhai, O.; Jeong, S.; Kim, K.; Kim, H.; Mun, J.H. Deep Learning-Based Near-Fall Detection Algorithm for Fall Risk Monitoring System Using a Single Inertial Measurement Unit. IEEE Trans. Neural Syst. Rehabil. Eng. 2022, 30, 2385–2394. [Google Scholar] [CrossRef]
- Sakai, M.; Shiba, Y.; Sato, H.; Takahira, N. Motor adaptation during slip-perturbed gait in older adults. J. Phys. Ther. Sci. 2008, 20, 109–115. [Google Scholar] [CrossRef]
- Howcroft, J.; Kofman, J.; Lemaire, E.D. Review of fall risk assessment in geriatric populations using inertial sensors. J. Neuroeng. Rehabil. 2013, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mohawish, A.Y.; Fayadh, R.A.; Humadi, A.F. Wireless VSMS and accelerometer sensors with abdomen position for fall detection and health monitoring. AIP Conf. Proc. 2023, 2804, 040004. [Google Scholar] [CrossRef]
- Hemmatpour, M.; Karimshoushtari, M.; Ferrero, R.; Montrucchio, B.; Rebaudengo, M.; Novara, C. Polynomial classification model for real-time fall prediction system. In Proceedings of the 2017 IEEE 41st Annual Computer Software and Applications Conference (COMPSAC), Turin, Italy, 4–8 July 2017; Volume 1, pp. 973–978. [Google Scholar] [CrossRef]
- Paramasivam, A.; Bargavi, S.; Priyadharshini, R.; Subhiksha, M.; Vijayalakshmi, S.; NM, M.B. Internet of things based fall prediction and alerting device. In Proceedings of the International Conference on Communication, Computing and Internet of Things (IC3IoT), Chennai, India, 10–11 March 2022; pp. 1–5. [Google Scholar] [CrossRef]
- Zheng, L.; Zhao, J.; Dong, F.; Huang, Z.; Zhong, D. Fall detection algorithm based on inertial sensor and hierarchical decision. Sensors 2022, 23, 107. [Google Scholar] [CrossRef]
- Li, T.; Yan, Y.; Yin, M.; An, J.; Chen, G.; Wang, Y.; Liu, C.; Xue, N. Elderly fall detection based on GCN-LSTM multi-task learning using nursing aids integrated with multi-array flexible tactile sensors. Biosensors 2023, 13, 862. [Google Scholar] [CrossRef]
- Hassan, M.M.; Gumaei, A.; Aloi, G.; Fortino, G.; Zhou, M. A smartphone-enabled fall detection framework for elderly people in connected home healthcare. IEEE Netw. 2019, 33, 58–63. [Google Scholar] [CrossRef]
- Fanca, A.; Puscasiu, A.; Gota, D.I.; Valean, H. Methods to minimize false detection in accidental fall warning systems. In Proceedings of the 2019 23rd International Conference on System Theory, Control and Computing (ICSTCC), Sinaia, Romania, 9–11 October 2019; pp. 851–855. [Google Scholar] [CrossRef]
- Lee, N.T.; Resnick, P.; Barton, G. Algorithmic Bias Detection and Mitigation: Best Practices and Policies to Reduce Consumer Harms; Brookings Institute: Washington, DC, USA, 2019; p. 2. [Google Scholar]
- O’Connor, S.; Gasteiger, N.; Stanmore, E.; Wong, D.C.; Lee, J.J. Artificial intelligence for falls management in older adult care: A scoping review of nurses’ role. J. Nurs. Manag. 2022, 30, 3787–3801. [Google Scholar] [CrossRef]
- Xiao, J.; Ren, W.; Huang, X.; Wang, H. A surface electromyography-based pre-impact fall detection method. In Proceedings of the 2018 Chinese Automation Congress (CAC), Xi’an, China, 30 November–2 December 2018; pp. 681–685. [Google Scholar] [CrossRef]
- Leone, A.; Rescio, G.; Giampetruzzi, L.; Siciliano, P. Smart EMG-based socks for leg muscles contraction assessment. In Proceedings of the 2019 IEEE International Symposium on Measurements & Networking (M&N), Catania, Italy, 8–10 July 2019; pp. 1–6. [Google Scholar] [CrossRef]
- Moore, K.; O’Shea, E.; Kenny, L.; Barton, J.; Tedesco, S.; Sica, M.; Crowe, C.; Alamäki, A.; Condell, J.; Nordström, A.; et al. Older adults’ experiences with using wearable devices: Qualitative systematic review and meta-synthesis. JMIR Mhealth Uhealth 2021, 9, e23832. [Google Scholar] [CrossRef]
- Rescio, G.; Leone, A.; Giampetruzzi, L.; Siciliano, P. Fall Risk Assessment Using New sEMG-Based Smart Socks. In Advances in Data Science: Methodologies and Applications; Springer: Berlin/Heidelberg, Germany, 2021; pp. 147–166. [Google Scholar] [CrossRef]
- Xi, X.; Jiang, W.; Lü, Z.; Miran, S.M.; Luo, Z.Z. Daily activity monitoring and fall detection based on surface electromyography and plantar pressure. Complexity 2020, 2020, 9532067. [Google Scholar] [CrossRef]
- Razak, A.H.A.; Zayegh, A.; Begg, R.K.; Wahab, Y. Foot plantar pressure measurement system: A review. Sensors 2012, 12, 9884–9912. [Google Scholar] [CrossRef]
- Picard, R.W.; Vyzas, E.; Healey, J. Toward machine emotional intelligence: Analysis of affective physiological state. IEEE Trans. Pattern Anal. Mach. Intell. 2001, 23, 1175–1191. [Google Scholar] [CrossRef]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: What, why and how it influences muscle function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liao, Y.; Tan, G.; Zhang, H. Surface Electromyography Combined with Artificial Intelligence in Predicting Neuromuscular Falls in the Elderly: A Narrative Review of Present Applications and Future Perspectives. Healthcare 2025, 13, 1204. https://doi.org/10.3390/healthcare13101204
Liao Y, Tan G, Zhang H. Surface Electromyography Combined with Artificial Intelligence in Predicting Neuromuscular Falls in the Elderly: A Narrative Review of Present Applications and Future Perspectives. Healthcare. 2025; 13(10):1204. https://doi.org/10.3390/healthcare13101204
Chicago/Turabian StyleLiao, Yuandan, Gang Tan, and Hui Zhang. 2025. "Surface Electromyography Combined with Artificial Intelligence in Predicting Neuromuscular Falls in the Elderly: A Narrative Review of Present Applications and Future Perspectives" Healthcare 13, no. 10: 1204. https://doi.org/10.3390/healthcare13101204
APA StyleLiao, Y., Tan, G., & Zhang, H. (2025). Surface Electromyography Combined with Artificial Intelligence in Predicting Neuromuscular Falls in the Elderly: A Narrative Review of Present Applications and Future Perspectives. Healthcare, 13(10), 1204. https://doi.org/10.3390/healthcare13101204






