Central Venous Access: An Update on Modern Techniques to Avoid Complications
Abstract
1. A Brief History of Central Venous Access
| Choice of Vein | Anatomical Position | |
|---|---|---|
| Internal Jugular Vein | ||
| Anatomy | Originating at jugular foramen, joins subclavian vein behind sternal extremity of clavicle. | |
| Approach | Central Approach: Insert needle 1 cm above apex of Sedillot’s Triangle *. Advance at 60 degrees to skin aiming at the ipsilateral nipple, blood should be obtained within 3 cm. | |
| Lateral/Posterior Approach: Insert 2–3 cm above clavicle along posterior border of sternocleidomastoid (SCM). Direct needle towards jugular notch; blood should be obtained within 5 cm. | ||
| Subclavian Vein | ||
| Anatomy | The subclavian vein lies inferior to midpoint of clavicle and superior to the first rib. Joins internal jugular vein behind the sternal extremity of the clavicle. | |
| Approach | Infraclavicular Approach: Insert needle 1–2 cm inferior and lateral to the clavicular transition point. The needle should be aimed towards the sternal notch in a fashion parallel to the floor to avoid pneumothorax. | |
| Supraclavicular Approach: Insert needle 1 cm superior and lateral to the junction of the lateral border of the clavicular head of SCM and the clavicle. The needle is oriented 5–15 degrees posteriorly off a coronal plane along a line that would bisect the angle of the clavicle and sternocleidomastoid. | ||
| Femoral Vein | ||
| Anatomy | The femoral vein runs medially to the femoral artery in the femoral triangle, becoming the external iliac vein as it passes the inguinal ligament. | |
| Approach | Insert needle 1 cm medial to the point of maximal femoral arterial pulsation in the anterior superior thigh within the femoral triangle. Insert at a 45-degree angle along the same line as the arterial pulsation. | |
| Complications | The common complications for all insertion sites are catheter misplacement/failure, arterial puncture/cannulation, pneumothorax, catheter-associated infection, and deep vein thrombosis. It should be noted that there is a negligible risk of pneumothorax with CVC insertion into a femoral vein. | |
2. The Modern Procedure
3. Complications
| Immediate Complication | Total Catheters Analyzed | Complication % (95%CrI) |
| Placement failure | 17,407 | 2.04% (1.09–3.44) |
| Arterial puncture | 22,296 | 1.62% (1.15–2.20) |
| Arterial cannulation | 6489 | 0.28% (0.01–1.00) |
| Pneumothorax | 32,665 | 0.44% (0.27–0.65) |
| Delayed Complication | Total Catheter Days Analyzed | Complication % per Catheter Day (95%CrI) |
| Deep vein thrombosis | 73,894 | 0.27% (0.1–0.62) |
| Catheter-associated infection | 549,246 | 0.48% (0.34–0.66) |
| Immediate Complications | 2024 (95% CrI) [1] | 2015 [44] | 1997 [45] | 1985 [46] | 1971 [47,48] |
|---|---|---|---|---|---|
| Placement failure | 2.04% (1.09–3.44) | x | (1.9–5.2%) | x | 6.5% |
| Arterial puncture | 1.62% (1.15–2.20) | x | 5.20% | 3.8% | 19% |
| Pneumothorax | 0.44% (0.27–0.65) | x | 0.50% | 2.3% | 6% |
| Major mechanical complication * | x | 0.7–2.1% | x | x | x |
| Delayed Complications | |||||
| Deep vein thrombosis | 0.27% (0.1–0.62) | 0.5–1.4% | x | x | x |
| Infection | 0.48% (0.34–0.66) | 0.5–1.2% | x | 5.7% | 3.5% |
| Complication [1] | Ultrasonography | Landmark Method |
|---|---|---|
| Placement failure | 1.19% (0.51–2.46) | 2.90% (0.75–9.63) |
| Arterial puncture | 1.35% (0.91–1.92) | 6.88% (3.24–13.75) |
| Arterial cannulation | n/a | n/a |
| Pneumothorax | 0.24% (0.12–0.43) | 0.99% (0.33–2.62) |
| Delayed complications [1] | ||
| Deep vein thrombosis | 0.12% (0.02–0.67) | n/a |
| Infections | 0.42% (0.23–0.72) | 2.18% (0.29–15.4) |
| Other complications * [39,40] | ||
| Internal jugular vein | 8 per 1000 (3–17) | 23 per 1000 |
| Subclavian vein | 30 per 1000 (7–127) | 105 per 1000 |
| Femoral vein | 6 per 1000 (1–28) | 13 per 1000 |
4. Inadvertent Arterial Puncture
| Location | Total Catheters Analyzed | Complication % (95%CrI) |
|---|---|---|
| Internal Jugular Vein | 8852 | 1.19% (0.5–2.47) |
| Femoral Vein | 1200 | 2.77% (0.25–22.58) |
| Subclavian Vein | 4253 | 3.59% (1.32–8.61) |
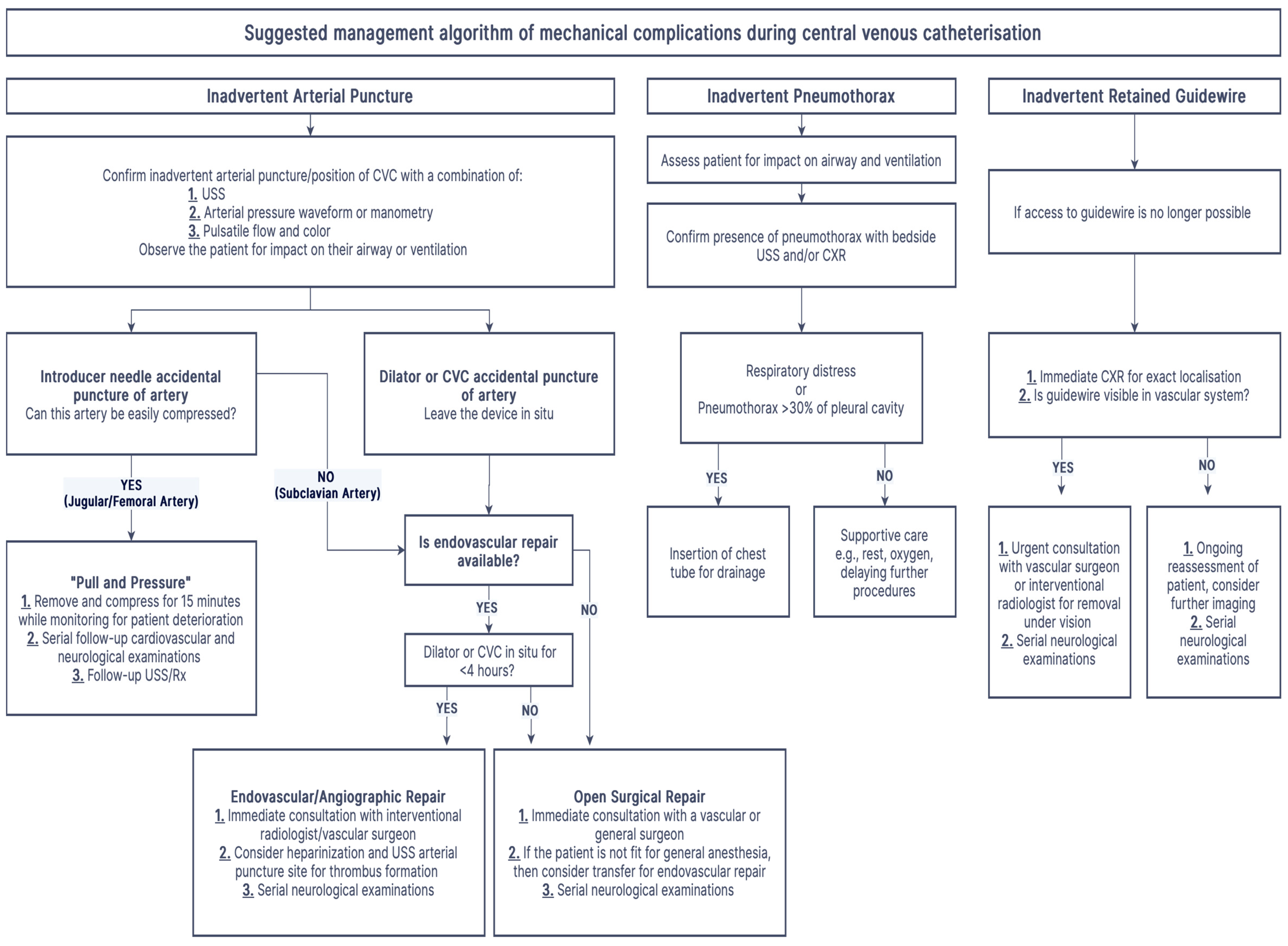
5. Management of Inadvertent Arterial Puncture/Cannulation
| Age | Mechanism of Injury | Outcome |
|---|---|---|
| 67 | Attending emergency physician and resident inserted CVC into an awake septic patient with multilobar pneumonia. The needle went through IJV into carotid artery. | Patient died after failed emergency intubation for worsening respiratory distress from mediastinal hematoma. |
| 75 | Attending physician inserted into awake patient with urosepsis. The catheter went through femoral vein into femoral artery. | Vascular surgery for arteriovenous fistula. |
| 48 | The needle inserted into an intubated patient with pulmonary edema went through a collapsed IJV and entered carotid artery posterior to the IJV. | Surgery for tear and focal dissection of carotid artery. |
| 67 | An awake oncology patient with esophageal cancer had a CVC inserted for pulmonary edema. Guidewire traveled through IJV and its posterior wall and into the carotid artery. | Hematoma with respiratory distress requiring emergent intubation. |
| 69 | An awake patient with respiratory distress had a CVC inserted. The needle penetrated the carotid artery, which was very close to the IJV. | Emergency carotid artery repair; the patient died of complications. |
| 14 | An awake patient with likely urosepsis had a CVC inserted by an emergency physician. The needle penetrated the rear wall of IJV and entered carotid artery. | Central catheter removed; bleeding eventually stopped. |
6. Pneumothorax
7. Other Complications
| Immediate Complications | Diagnosis | Management |
|---|---|---|
| Placement Failure | Historically, CXR has been the gold standard. Ultrasound (USS) and TTE are non-inferior and faster in recent meta-analyses. | Remove misplaced catheter. |
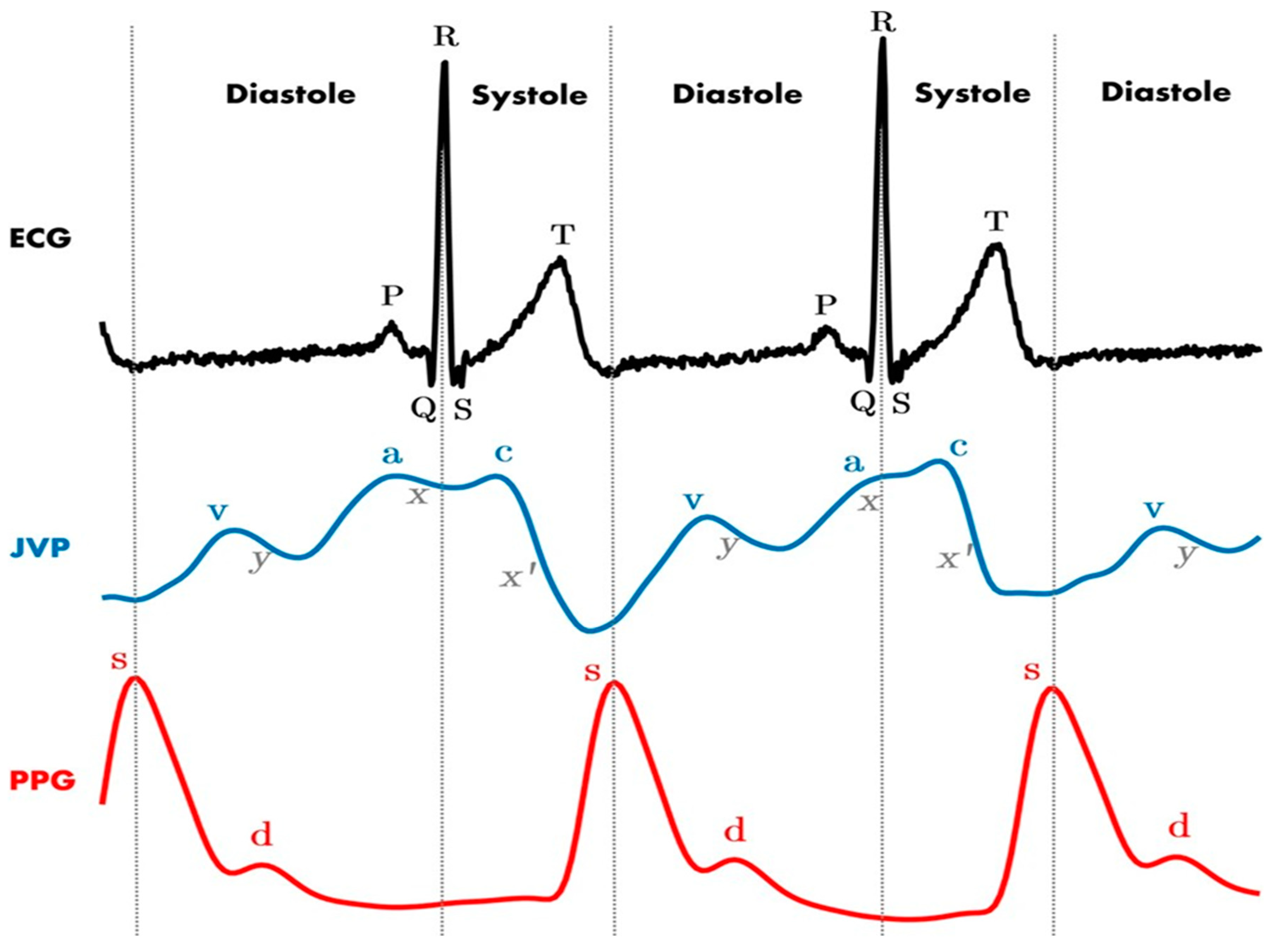
| Arterial Puncture | Combination blood color and pulsatility assessment, pressure manometry, and dynamic USS for needle tip position. | Often, no invasive management is required apart from external compression if it is identified before further cannulation. |
| Arterial Cannulation | Combination of blood color and pulsatility assessment, pressure manometry + waveform analysis, and dynamic USS for catheter position. | Leave the catheter in situ, and follow the pathway documented in Figure 5. |
| Pneumothorax | Bedside ultrasound is faster, more sensitive, and more specific than CXR for identifying pneumothorax after central venous catheter insertion. | Patients with large pneumothoraces >30% or respiratory distress may benefit from the insertion of a chest drain. |
| Delayed Complications | ||
| Deep Vein Thrombosis | Combination of clinical assessment, D-dimer, and USS. | The catheter may be left in situ. Anticoagulation for as long as catheter is in situ or ≥3 months, whichever is longer. |
| Catheter-Associated Infection | Clinical assessment and blood cultures. | Remove the catheter and send for tip culture. Antibiotics per local guidelines. Prophylaxis is not recommended |
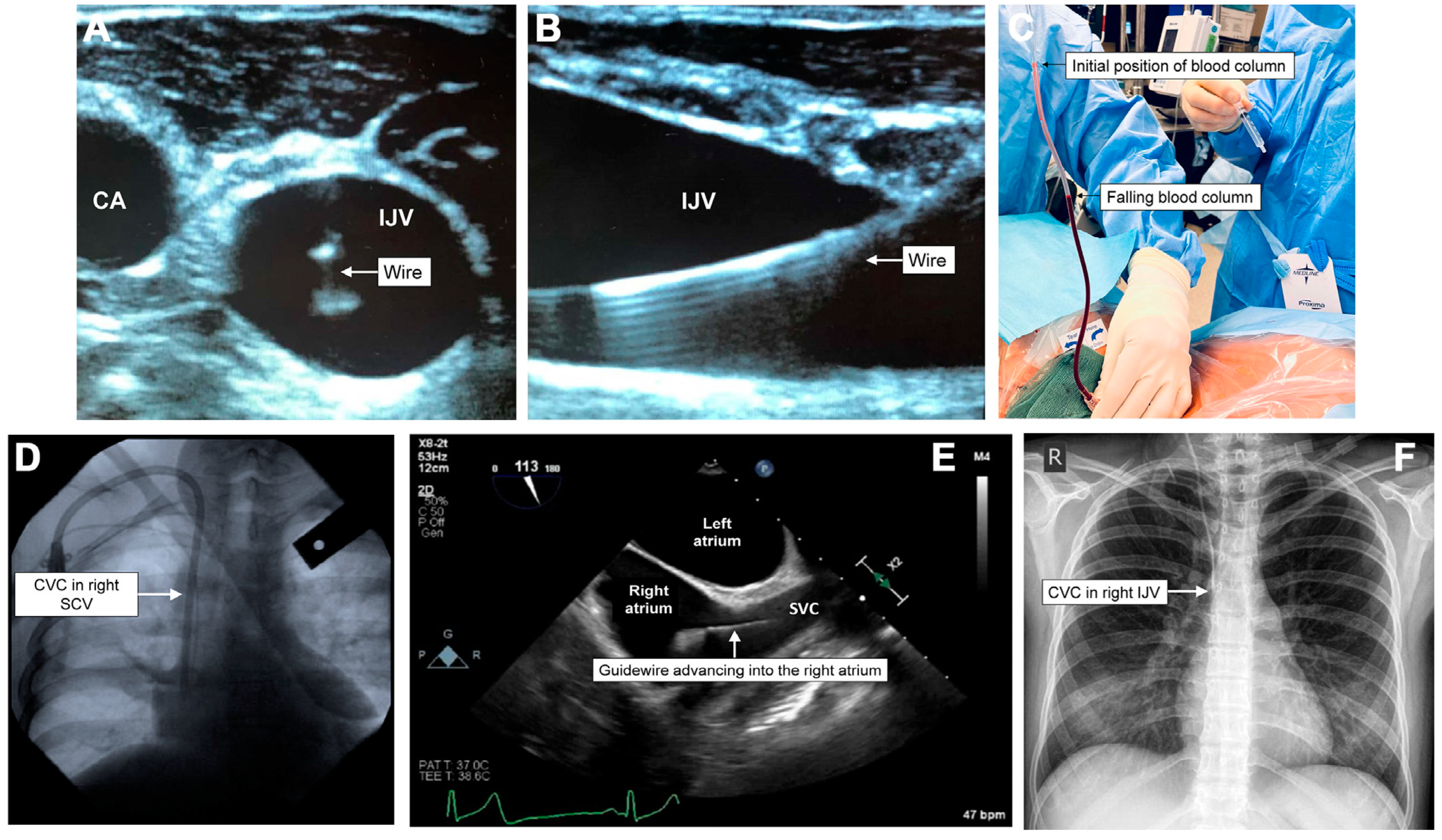
7.1. Retained Guidewire
7.2. Hemothorax/Mediastinal Hematoma/Cardiac Tamponade
7.3. Chylothorax/Infusothorax/Hydrothorax
7.4. Delayed Complications
7.5. Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Teja, B.; Bosch, N.A.; Diep, C.; Pereira, T.V.; Mauricio, P.; Sklar, M.C.; Sankar, A.; Wijeysundera, H.C.; Saskin, R.; Walkey, A.; et al. Complication Rates of Central Venous Catheters: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2024, 184, 474–482. [Google Scholar] [CrossRef] [PubMed]
- Sette, P.; Dorizzi, R.M.; Azzini, A.M. Vascular access: An historical perspective from Sir William Harvey to the 1956 Nobel prize to André F. Cournand, Werner Forssmann, and Dickinson W. Richards. J. Vasc. Access 2012, 13, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.; Du Plessis, M.; Prekupec, M.P.; Gielecki, J.; Zurada, A.; Tubbs, R.S.; Loukas, M. Ultrasound-guided central venous catheterization: A review of the relevant anatomy, technique, complications, and anatomical variations. Clin. Anat. 2017, 30, 237–250. [Google Scholar] [CrossRef] [PubMed]
- English, I.C.W.; Frew, R.M.; Pigott, J.F.G.; Zaki, M. Percutaneous cannulation of the internal jugular vein. Thorax 1969, 24, 496–497. [Google Scholar] [CrossRef]
- Aubaniac, R. Subclavian intravenous injection; advantages and technic. Presse Med. 1952, 60, 1456. [Google Scholar]
- Yoffa, D. Supraclavicular subclavian venepuncture and catheterisation. Lancet 1965, 2, 614–617. [Google Scholar] [CrossRef]
- Moncrief, J.A. Femoral catheters. Ann. Surg. 1958, 147, 166–172. [Google Scholar] [CrossRef]
- Fabian, J.A.; Jesudian, M.C. A simple method for improving the safety of percutaneous cannulation of the internal jugular vein. Anesth. Analg. 1985, 64, 1032–1033. [Google Scholar] [CrossRef]
- Legler, D.; Nugent, M. Doppler localization of the internal jugular vein facilitates central venous cannulation. Anesthesiology 1984, 60, 481–482. [Google Scholar] [CrossRef]
- Bannon, M.P.; Heller, S.F.; Rivera, M. Anatomic considerations for central venous cannulation. Risk Manag. Healthc. Policy 2011, 4, 27–39. [Google Scholar] [CrossRef]
- Practice Guidelines for Central Venous Access 2020: An Updated Report by the American Society of Anesthesiologists Task Force on Central Venous Access. Anesthesiology 2020, 132, 8–43. [CrossRef] [PubMed]
- ANZICS. Central Line Insertion and Maintenance Guideline; Care ACoSaQiH; ANZICS: Prahran, Australia, 2012; Available online: https://www.anzics.org/wp-content/uploads/2018/08/ANZICS_Insertionmaintenance_guideline2012_04.pdf (accessed on 10 March 2025).
- Czyzewska, D.; Ustymowicz, A.; Kosel, J. Internal jugular veins must be measured before catheterization. J. Clin. Anesth. 2015, 27, 129–131. [Google Scholar] [CrossRef]
- Bos, M.J.; van Loon, R.F.H.J.; Heywood, L.; Morse, M.P.; van Zundert, A.A.J. Comparison of the diameter, cross-sectional area, and position of the left and right internal jugular vein and carotid artery in adults using ultrasound. J. Clin. Anesth. 2016, 32, 65–69. [Google Scholar] [CrossRef] [PubMed]
- van Zundert, A.; Bos, M.; Heywood, L. Internal jugular veins must be measured before catheterisation. J. Clin. Anesth. 2015, 27, 435–436. [Google Scholar] [CrossRef]
- Bos, M.J.; van Mook, W.N.K.A.; van Zundert, A.A.J. Asymmetric Jugular Veins: Implication for Central Venous Access in the ICU. Crit. Care Med. 2016, 44, e778–e779. [Google Scholar] [CrossRef]
- Shin, K.W.; Park, S.; Jo, W.-Y.; Choi, S.; Kim, Y.J.; Park, H.-P.; Oh, H. Comparison of Catheter Malposition Between Left and Right Ultrasound-Guided Infraclavicular Subclavian Venous Catheterizations: A Randomized Controlled Trial. Crit. Care Med. 2024, 52, 1557–1566. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.; Kim, B.G.; Lim, Y.J.; Jeon, Y.T.; Hwang, J.W.; Kim, H.C.; Choi, Y.H.; Park, H.P. A prospective randomised trial comparing insertion success rate and incidence of catheterisation-related complications for subclavian venous catheterisation using a thin-walled introducer needle or a catheter-over-needle technique. Anaesthesia 2016, 71, 1030–1036. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, T.K.; Jung, Y.S.; Cho, Y.J.; Yoon, S.; Seo, J.-H.; Jeon, Y.; Bahk, J.H.; Hong, D.M. Comparison of Needle Insertion and Guidewire Placement Techniques During Internal Jugular Vein Catheterization: The Thin-Wall Introducer Needle Technique Versus the Cannula-Over-Needle Technique. Crit. Care Med. 2015, 43, 2112–2116. [Google Scholar] [CrossRef]
- Walsh, E.; Fitzsimons, M.G. Preventing mechanical complications associated with central venous catheter placement. BJA Educ. 2023, 23, 229–237. [Google Scholar] [CrossRef]
- Wright, D.; Williams, D. Central venous catheter tip position on chest radiographs. Anaesthesia 2020, 75, 124–125. [Google Scholar] [CrossRef]
- Aslamy, Z.; Dewald, C.L.; Heffner, J.E. MRI of central venous anatomy: Implications for central venous catheter insertion. Chest 1998, 114, 820–826. [Google Scholar] [CrossRef]
- Schuster, M.; Nave, H.; Piepenbrock, S.; Pabst, R.; Panning, B. The carina as a landmark in central venous catheter placement. Br. J. Anaesth. 2000, 85, 192–194. [Google Scholar] [CrossRef] [PubMed]
- Hade, A.D.; Beckmann, L.A.; Basappa, B.K. A checklist to improve the quality of central venous catheter tip positioning. Anaesthesia 2019, 74, 896–903. [Google Scholar] [CrossRef]
- Ablordeppey, E.A.; Drewry, A.M.M.; Beyer, A.B.M.; Theodoro, D.L.M.; Fowler, S.A.M.; Fuller, B.M.M.; Carpenter, C.R.M. Diagnostic Accuracy of Central Venous Catheter Confirmation by Bedside Ultrasound Versus Chest Radiography in Critically Ill Patients: A Systematic Review and Meta-Analysis. Crit. Care Med. 2017, 45, 715–724. [Google Scholar] [CrossRef] [PubMed]
- Smit, J.M.; Raadsen, R.; Blans, M.J.; Petjak, M.; Van de Ven, P.M.; Tuinman, P.R. Bedside ultrasound to detect central venous catheter misplacement and associated iatrogenic complications: A systematic review and meta-analysis. Crit. Care 2018, 22, 65. [Google Scholar] [CrossRef]
- Swaminathan, L.; Flanders, S.; Horowitz, J.; Zhang, Q.; O’malley, M.; Chopra, V. Safety and Outcomes of Midline Catheters vs Peripherally Inserted Central Catheters for Patients With Short-term Indications: A Multicenter Study. JAMA Intern. Med. 2022, 182, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Bing, S.; Smotherman, C.; Rodriguez, R.G.; Skarupa, D.J.; Ra, J.H.; Crandall, M.L. PICC versus midlines: Comparison of peripherally inserted central catheters and midline catheters with respect to incidence of thromboembolic and infectious complications. Am. J. Surg. 2022, 223, 983–987. [Google Scholar] [CrossRef]
- Duwadi, S.; Zhao, Q.; Budal, B.S. Peripherally inserted central catheters in critically ill patients—complications and its prevention: A review. Int. J. Nurs. Sci. 2019, 6, 99–105. [Google Scholar] [CrossRef]
- Galloway, S.; Bodenham, A. Long-term central venous access. Br. J. Anaesth. 2004, 92, 722–734. [Google Scholar] [CrossRef]
- Kehagias, E.; Galanakis, N.; Tsetis, D. Central venous catheters: Which, when and how. Br. J. Radiol. 2023, 96, 20220894. [Google Scholar] [CrossRef]
- Neville, J.J.; Aye, H.M.; Hall, N.J. Tunnelled external versus implanted port central venous catheters in paediatric oncology: A systematic review and meta-analysis. Arch. Dis. Child. 2023, 108, 975–981. [Google Scholar] [CrossRef] [PubMed]
- Najari, F.; Amirian, M.; Sadjadi, S.; Kayal, I.B. Loss of Guide Wire as an Important Complication of Central Venous Catheterization; a Case Report. Emergency 2018, 6, e17. [Google Scholar] [PubMed]
- Xiao, L.; Chen, X.; Zhang, D. Carotid Artery Aneurysm-Induced Mediastinal Hematoma Leading to Airway Compression-A Rare Complication of Internal Jugular Vein Puncture. Vasc. Endovasc. Surg. 2024, 58, 676–679. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, Y.; Xiang, H. Complete heart block: A rare central venous catheter placement complication. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2019, 44, 1306–1310. [Google Scholar] [CrossRef]
- Aggarwal, R.; Soni, K.D. Delayed massive hydrothorax following subclavian catheter insertion: An unusual complication. Crit. Care Nurs. Q. 2015, 38, 405–408. [Google Scholar] [CrossRef]
- Ali, S.R.; Mukhija, D.; Nagpal, S.J.S. Mediastinal haematoma: A rare complication of central venous catheter insertion. Natl. Med. J. India 2017, 30, 362. [Google Scholar] [CrossRef]
- Schnabel, R.; van Zundert, A. Intracardiac entrapment of a pulmonary artery catheter. Acta Anaesthesiol. Scand. 2007, 51, 781–782. [Google Scholar] [CrossRef]
- Brass, P.; Hellmich, M.; Kolodziej, L.; Schick, G.; Smith, A.F. Ultrasound guidance versus anatomical landmarks for internal jugular vein catheterization. Cochrane Database Syst. Rev. 2015, 1, Cd006962. [Google Scholar] [CrossRef] [PubMed]
- Brass, P.; Hellmich, M.; Kolodziej, L.; Schick, G.; Smith, A.F. Ultrasound guidance versus anatomical landmarks for subclavian or femoral vein catheterization. Cochrane Database Syst. Rev. 2015, 1, Cd011447. [Google Scholar] [CrossRef]
- McGee, D.C.; Gould, M.K. Preventing complications of central venous catheterization. N. Engl. J. Med. 2003, 348, 1123–1133. [Google Scholar] [CrossRef]
- Pikwer, A.; Acosta, S.; Kölbel, T.; Malina, M.; Sonesson, B.; Akeson, J. Management of inadvertent arterial catheterisation associated with central venous access procedures. Eur. J. Vasc. Endovasc. Surg. 2009, 38, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Papastratigakis, G.; Marouli, D.; Proklou, A.; Nasis, N.; Kondili, E.; Kehagias, E. Management of Inadvertent Arterial Catheterization during Central Venous Catheter Placement: A Case Series. J. Pers. Med. 2022, 12, 1537. [Google Scholar] [CrossRef]
- Parienti, J.-J.; Mongardon, N.; Mégarbane, B.; Mira, J.-P.; Kalfon, P.; Gros, A.; Marqué, S.; Thuong, M.; Pottier, V.; Ramakers, M.; et al. Intravascular Complications of Central Venous Catheterization by Insertion Site. N. Engl. J. Med. 2015, 373, 1220–1229. [Google Scholar] [CrossRef] [PubMed]
- Yilmazlar, A.; Bilgin, H.; Korfali, G.; Eren, A.; Ozkan, U. Complications of 1303 central venous cannulations. J. R. Soc. Med. 1997, 90, 319–321. [Google Scholar] [CrossRef]
- Sitzmann, J.V.; Townsend, T.R.; Siler, M.C.; Bartlett, J.G. Septic and technical complications of central venous catheterization. A prospective study of 200 consecutive patients. Ann. Surg. 1985, 202, 766–770. [Google Scholar] [CrossRef]
- Bernard, R.W.; Stahl, W.M. Subclavian vein catheterizations: A prospective study. I. Non-infectious complications. Ann. Surg. 1971, 173, 184–190. [Google Scholar] [CrossRef]
- Bernard, R.W.; Stahl, W.M.; Chase, R.M., Jr. Subclavian vein catheterizations: A prospective study. II. Infectious complications. Ann. Surg. 1971, 173, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Bowdle, A. Vascular Complications of Central Venous Catheter Placement: Evidence-Based Methods for Prevention and Treatment. J. Cardiothorac. Vasc. Anesth. 2014, 28, 358–368. [Google Scholar] [CrossRef]
- Domino, K.B.; Bowdle, T.A.; Posner, K.L.; Spitellie, P.H.; Lee, L.A.; Cheney, F.W. Injuries and Liability Related to Central Vascular Catheters. J. Am. Soc. Anesthesiol. 2004, 100, 1411–1418. [Google Scholar] [CrossRef]
- Ezaru, C.S.; Mangione, M.P.; Oravitz, T.M.; Ibinson, J.W.; Bjerke, R.J. Eliminating arterial injury during central venous catheterization using manometry. Anesth. Analg. 2009, 109, 130–134. [Google Scholar] [CrossRef]
- Jobes, D.R.; Schwartz, A.J.; E Greenhow, D.; Stephenson, L.W.; Ellison, N. Safer jugular vein cannulation: Recognition of arterial puncture and preferential use of the external jugular route. Anesthesiology 1983, 59, 353–355. [Google Scholar] [CrossRef] [PubMed]
- García-López, I.; Rodriguez-Villegas, E. Extracting the Jugular Venous Pulse from Anterior Neck Contact Photoplethysmography. Sci. Rep. 2020, 10, 3466. [Google Scholar] [CrossRef] [PubMed]
- Guilbert, M.-C.; Elkouri, S.; Bracco, D.; Corriveau, M.M.; Beaudoin, N.; Dubois, M.J.; Bruneau, L.; Blair, J.-F. Arterial trauma during central venous catheter insertion: Case series, review and proposed algorithm. J. Vasc. Surg. 2008, 48, 918–925; discussion 925. [Google Scholar] [CrossRef]
- Blaivas, M. Video analysis of accidental arterial cannulation with dynamic ultrasound guidance for central venous access. J. Ultrasound Med. 2009, 28, 1239–1244. [Google Scholar] [CrossRef] [PubMed]
- Al Rayes, A.; Khattak, Y.; Qafani, A.; Anwar, M.; Al Sibaie, A. Acute Management of Iatrogenic Injury to Vertebral Artery With Central Venous Catheter in a Critically Ill Patient. Cureus 2020, 12, e9956. [Google Scholar] [CrossRef]
- Akkan, K.; Cindil, E.; Kilic, K.; Ilgit, E.; Onal, B.; Erbas, G. Misplaced central venous catheter in the vertebral artery: Endovascular treatment of foreseen hemorrhage during catheter withdrawal. J. Vasc. Access 2014, 15, 418–423. [Google Scholar] [CrossRef]
- Gabriele, P.; Emanuele, G.; Ilenia, D.S.; Carlo, G.C.; Luca, F.; Luciano, C. Management of Inadvertent Supra-aortic Arterial Lesions During Central Venous Access Procedures: Report of Six Cases and Proposed Algorithm. Ann. Vasc. Surg. 2021, 75, 308–314. [Google Scholar] [CrossRef]
- Verloh, N.; Scharf, G.; Bäumler, W.; Pfister, K.; Oikonomou, K.; Stroszczynski, C.; Uller, W.; Dollinger, M. Erroneous placement of central venous catheters in subclavian artery: Retrieval and successful hemostasis with a femoral closure device. J. Vasc. Access 2022, 23, 692–697. [Google Scholar] [CrossRef]
- Dixon, O.G.; Smith, G.E.; Carradice, D.; Chetter, I.C. A systematic review of management of inadvertent arterial injury during central venous catheterisation. J. Vasc. Access 2017, 18, 97–102. [Google Scholar] [CrossRef]
- Bechara, C.F.; Barshes, N.R.; Pisimisis, G.; Kougias, P.; Lin, P.H. Management of inadvertent carotid artery sheath insertion during central venous catheter placement. JAMA Surg. 2013, 148, 1063–1066. [Google Scholar] [CrossRef]
- Frykholm, P.; Pikwer, A.; Hammarskjöld, F.; Larsson, A.T.; Lindgren, S.; Lindwall, R.; Taxbro, K.; Öberg, F.; Acosta, S.; Åkeson, J. Clinical guidelines on central venous catheterisation. Swedish Society of Anaesthesiology and Intensive Care Medicine. Acta Anaesthesiol. Scand. 2014, 58, 508–524. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, D.A.; Menu, Y. A bedside ultrasound sign ruling out pneumothorax in the critically ill. Lung sliding. Chest 1995, 108, 1345–1348. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Yousefifard, M.; Kazemi, H.M.; Rasouli, H.R.; Asady, H.; Jafari, A.M.; Hosseini, M. Diagnostic Accuracy of Chest Ultrasonography versus Chest Radiography for Identification of Pneumothorax: A Systematic Review and Meta-Analysis. Tanaffos 2014, 13, 29–40. [Google Scholar] [PubMed]
- Rantanen, N.W. Diseases of the thorax. Vet. Clin. N. Am. Equine Pr. 1986, 2, 49–66. [Google Scholar] [CrossRef]
- Hemsinli, D.; Mutlu, H.; Altun, G.; Pulathan, Z.; Ozdemir, A.C. An unexpected image on a chest radiograph. Scott Med. J. 2017, 62, 156–158. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Díez, D.; Salgado-Fernández, J.; Vázquez-González, N.; Calviño-Santos, R.; Vázquez-Rodríguez, J.M.; Aldama-López, G.; García-Barreiro, J.J.; Castro-Beiras, A. Images in cardiovascular medicine. Percutaneous retrieval of a lost guidewire that caused cardiac tamponade. Circulation 2007, 115, e629–e631. [Google Scholar] [CrossRef]
- Lin, Y.N.; Chou, J.W.; Chen, Y.H.; Liu, C.Y.; Ho, C.M. A 20-year retained guidewire, should it be removed? Qjm Int. J. Med. 2013, 106, 373–374. [Google Scholar] [CrossRef] [PubMed]
- Gunduz, Y.; Vatan, M.B.; Osken, A.; Cakar, M.A. A delayed diagnosis of a retained guidewire during central venous catheterisation: A case report and review of the literature. BMJ Case Rep. 2012, 2012, bcr2012007064. [Google Scholar] [CrossRef]
- Bugnicourt, J.-M.; Belhomme, D.; Bonnaire, B.; Constans, J.-M.; Manaouil, C. Posterior Cerebral Infarction following Loss of Guide Wire. Case Rep. Neurol. Med. 2013, 2013, 164710. [Google Scholar] [CrossRef]
- Sharma, S.; Gupta, P.; Guleria, S. Mediastinal haematoma: A rare complication following insertion of central venous catheter. Indian J. Chest Dis. Allied Sci. 2011, 53, 225–228. [Google Scholar] [CrossRef]
- Mora-Aznar, M. Cardiac tamponade and cardiogenic shock after central venous catheter cannulation. Analysis of a case. Anaesthesiol. Intensiv. Ther. 2023, 55, 71–75. [Google Scholar] [CrossRef]
- Valente-Aguiar, M.S.; de Carvalho, E.R.; Magalhães, T.; Dinis-Oliveira, R.J. Fatal iatrogenic cardiac tamponade due to central venous catheterization. Forensic Sci. Med. Pathol. 2022, 18, 275–279. [Google Scholar] [CrossRef]
- Naguib, M.; Farag, H.; Joshi, R.N. Bilateral hydrothorax and hydromediastinum after a subclavian line insertion. Can. J. Soc. Anaesth. 1985, 32, 412–414. [Google Scholar] [CrossRef] [PubMed]
- Rabia, R.; Liaqat, A.; Mariam, M.; Kanwal, A.; Khan, Z.A.; Mumtaz, H. A case of accidental displacement of a central venous catheter in lung parenchyma leading to hydrothorax: A case report. Int. J. Surg. Case Rep. 2023, 102, 107813. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Chaddha, U.; Kaul, V.; Desai, A.; Gillaspie, E.; Maldonado, F. Multidisciplinary Management of Chylothorax. Chest 2022, 162, 1402–1412. [Google Scholar] [CrossRef]
- Kleinjan, A.; Di Nisio, M.; Beyer-Westendorf, J.; Camporese, G.; Cosmi, B.; Ghirarduzzi, A.; Kamphuisen, P.W.; Otten, H.-M.; Porreca, E.; Aggarwal, A.; et al. Safety and feasibility of a diagnostic algorithm combining clinical probability, d-dimer testing, and ultrasonography for suspected upper extremity deep venous thrombosis: A prospective management study. Ann. Intern. Med. 2014, 160, 451–457. [Google Scholar] [CrossRef]
- Kreuziger, L.B.; Jaffray, J.; Carrier, M. Epidemiology, diagnosis, prevention and treatment of catheter-related thrombosis in children and adults. Thromb. Res. 2017, 157, 64–71. [Google Scholar] [CrossRef]
- Sousa, B.; Furlanetto, J.; Hutka, M.; Gouveia, P.; Wuerstlein, R.; Mariz, J.M.; Pinto, D.; Cardoso, F. Central venous access in oncology: ESMO Clinical Practice Guidelines. Ann. Oncol. 2015, 26 (Suppl. S5), v152–v168. [Google Scholar] [CrossRef] [PubMed]
- O’Grady, N.P.; Alexander, M.; Burns, L.A.; Dellinger, E.P.; Garland, J.; Heard, S.O.; Lipsett, P.A.; Masur, H.; Mermel, L.A.; Pearson, M.L.; et al. Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin. Infect. Dis. 2011, 52, e162–e193. [Google Scholar] [CrossRef]
- Therapeutic Guidelines. Available online: https://app-tg-org-au.ap1.proxy.openathens.net/viewTopic?etgAccess=true&guidelinePage=Antibiotic&topicfile=bartonella-infections&guidelinename=auto§ionId=c_ABG17_Infections-associated-with-intravenous-catheters_topic_11#c_ABG17_Infections-associated-with-intravenous-catheters_topic_11 (accessed on 24 April 2025).
- Collignon RHaP. Controlling Intravascular Catheter Infections. Australian Prescriber. Updated 1 April 2003. Available online: https://australianprescriber.tg.org.au/articles/controlling-intravascular-catheter-infections.html#:~:text=Summary,unit%20will%20also%20be%20necessary (accessed on 24 April 2025).
- Control CfD. Guidelines for the Prevention of Intravascular Catheter-Related Infections. Background Information: Strategies for Prevention of Catheter-Related Infections in Adult and Pediatric Patients. 28 February 2024. Available online: https://www.cdc.gov/infection-control/hcp/intravascular-catheter-related-infection/prevention-strategies.html#cdc_generic_section_10-antimicrobialantiseptic-impregnated-catheters-and-cuffs (accessed on 24 April 2025).
- Rodríguez-Calero, M.A.; Blanco-Mavillard, I.; Morales-Asencio, J.M.; Fernández-Fernández, I.; Castro-Sánchez, E.; de Pedro-Gómez, J.E. Defining risk factors associated with difficult peripheral venous Cannulation: A systematic review and meta-analysis. Heart Lung J. Cardiopulm. Acute Care 2020, 49, 273–286. [Google Scholar] [CrossRef]
- Williamson, K.; Nimegeer, A.; Lean, M. Rising prevalence of BMI ≥ 40 kg/m2: A high-demand epidemic needing better documentation. Obes. Rev. 2020, 21, e12986. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Chang, J.-E.; Won, D.; Lee, J.-M.; Kim, T.K.; Min, S.-W.; Kim, C.; Hwang, J.-Y. Effect of head and shoulder positioning on the cross-sectional area of the subclavian vein in obese subjects. Am. J. Emerg. Med. 2021, 50, 561–565. [Google Scholar] [CrossRef] [PubMed]
- Pei, D.; Wang, S.; Sun, C. Effect of passive leg raising on the cross-sectional area of the right internal jugular vein in patients with obesity: A randomised controlled trial protocol. BMJ Open 2024, 14, e085044. [Google Scholar] [CrossRef] [PubMed]
- Perilli, V.; Aceto, P.; Sacco, T.; Cicco, R.D.; Punzo, G.; Tosi, A.; Ceaichisciuc, I.; Cataldo, A.; Lai, C.; Sollazzi, L. Effect of different manoeuvres on internal jugular vein cross-sectional area in obese and normal-weight patients. Eur. J. Anaesthesiol. 2020, 37, 149–152. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woodfall, K.; van Zundert, A. Central Venous Access: An Update on Modern Techniques to Avoid Complications. Healthcare 2025, 13, 1168. https://doi.org/10.3390/healthcare13101168
Woodfall K, van Zundert A. Central Venous Access: An Update on Modern Techniques to Avoid Complications. Healthcare. 2025; 13(10):1168. https://doi.org/10.3390/healthcare13101168
Chicago/Turabian StyleWoodfall, Kai, and André van Zundert. 2025. "Central Venous Access: An Update on Modern Techniques to Avoid Complications" Healthcare 13, no. 10: 1168. https://doi.org/10.3390/healthcare13101168
APA StyleWoodfall, K., & van Zundert, A. (2025). Central Venous Access: An Update on Modern Techniques to Avoid Complications. Healthcare, 13(10), 1168. https://doi.org/10.3390/healthcare13101168







