Oral Health Status of Healthcare Workers in Ilembula/Tanzania during the COVID-19 Condition
Abstract
1. Introduction
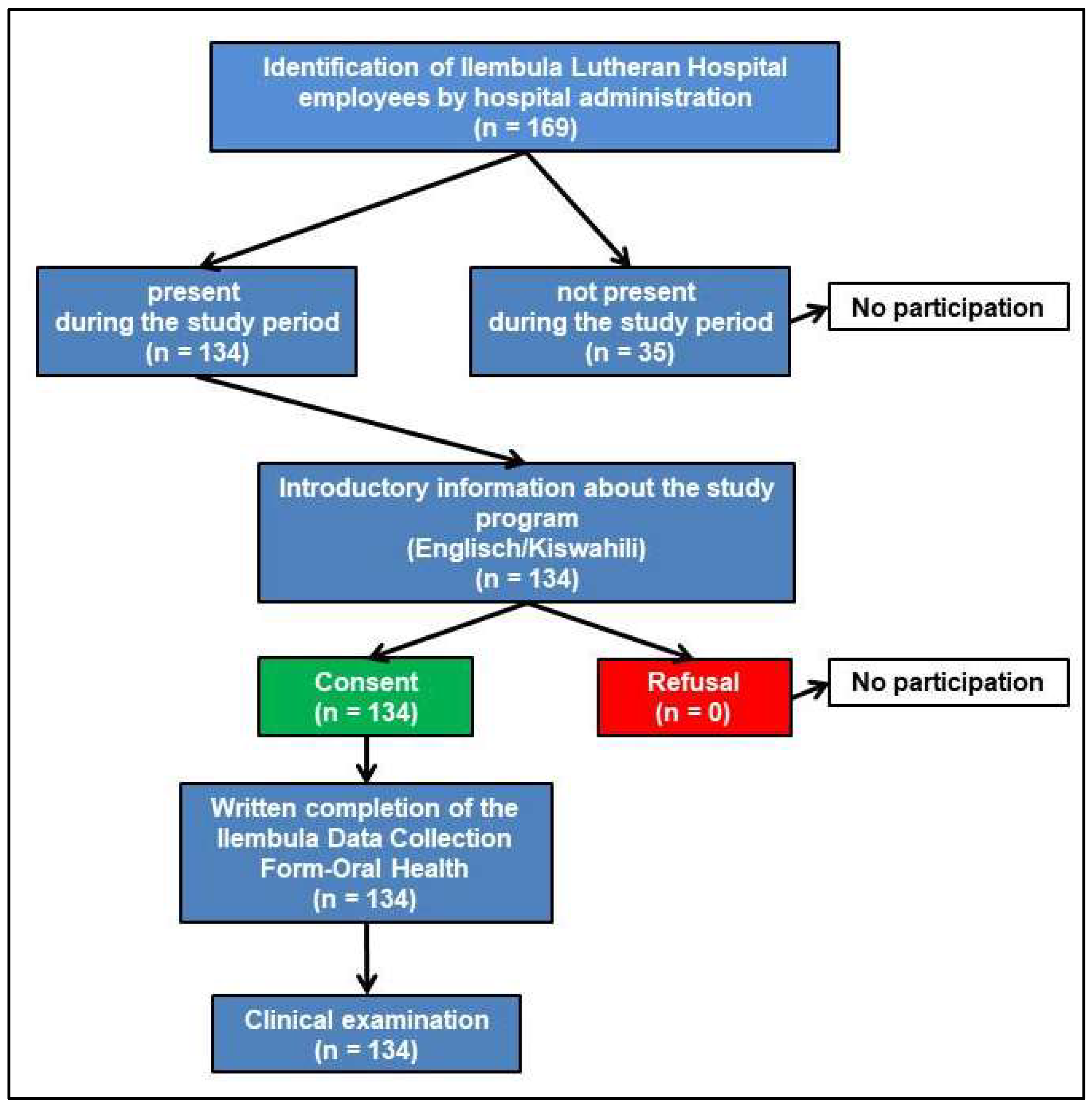
2. Materials and Methods
2.1. Participants
2.2. Study Site
2.3. Data Collection
2.4. Clinical Examination
2.5. Data Analysis
3. Results
3.1. General Medical History and Dental Medical History
3.2. Social and Diet Behaviours
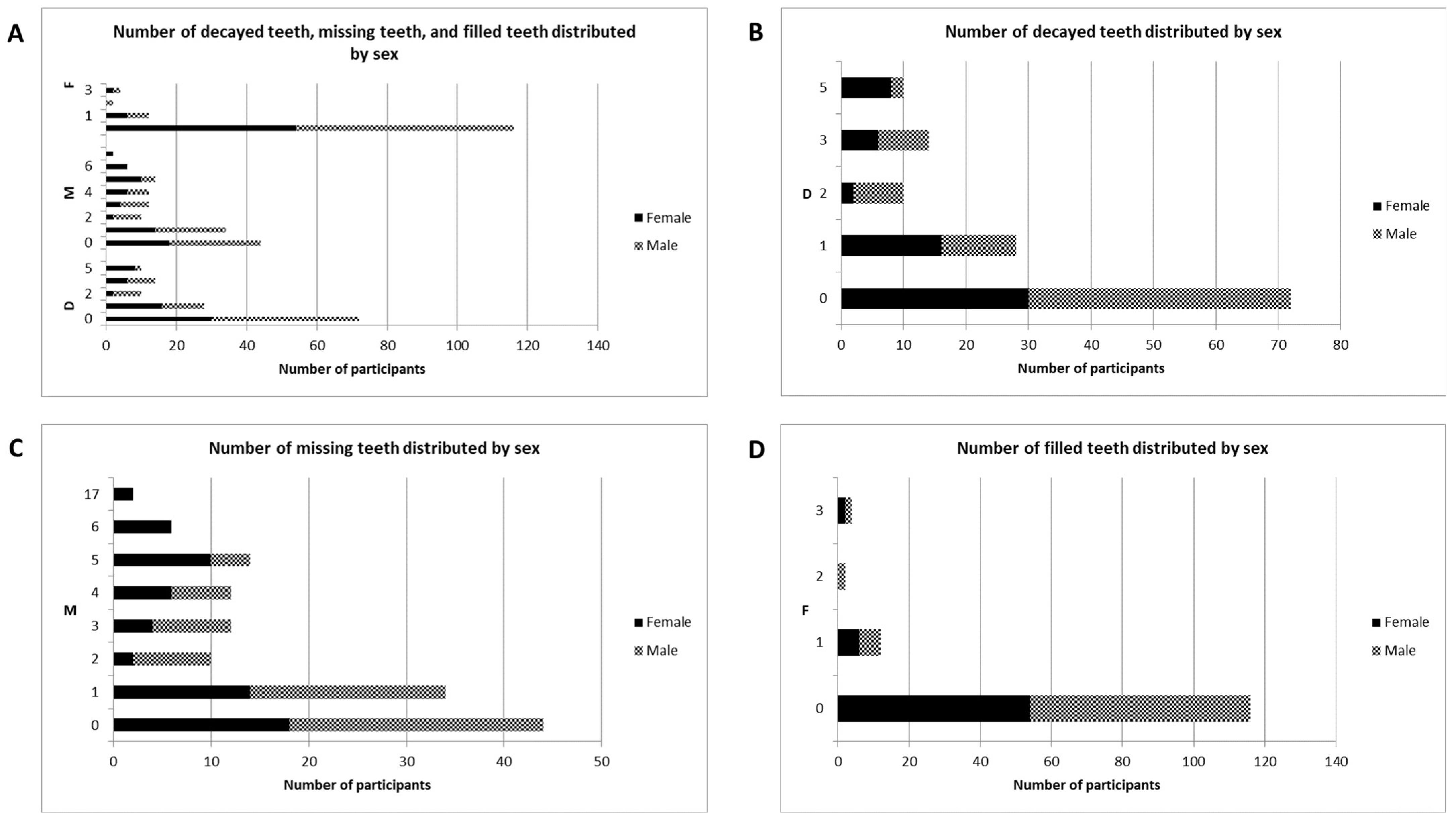
3.3. Oral Health Data
3.4. Tooth Position Anomalies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sarita, P.T.; Witter, D.J.; Kreulen, C.M.; Matee, M.I.; van’t Hof, M.A.; Creugers, N.H. Decayed/missing/filled teeth and shortened dental arches in Tanzanian adults. Int. J. Prosthodont. 2004, 17, 224–230. [Google Scholar] [PubMed]
- Bensel, T.; Erhart, I.; Megiroo, S.; Kronenberg, W.; Bömicke, W.; Hinz, S. Oral Health status of nursing staff in Ilembula, Wanging’ombe District, Njombe region, Tanzania: A cross-sectional study. BMC Oral Health 2022, 22, 169. [Google Scholar] [CrossRef] [PubMed]
- Petersen, P.E.; Bourgeois, D.; Ogawa, H.; Estupinan-Day, S.; Ndiaye, C. The global burden of oral diseases and risks to oral health. Bull. World Health Organ. 2005, 83, 661–669. [Google Scholar] [PubMed]
- Jain, N.; Dutt, U.; Radenkov, I.; Jain, S. WHO’s global oral health status report 2022: Actions, discussion and implementation. Oral Dis. 2023, 30, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; Arora, A.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease, 2017. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [PubMed]
- Rwakatema, D.S.; Ananduni, K.N.; Katiti, V.W.; Msuya, M.; Chugulu, J.; Kapanda, G. Oral Health in nursing students at Kilimanjaro Christian Medical Centre teaching hospital in Moshi, Tanzania. BMC Oral Health 2015, 15, 23. [Google Scholar] [CrossRef] [PubMed]
- Nyamuryekung’e, K.K.; Lahti, S.M.; Tuominen, R.J. The relative patient costs and availability of dental services, materials and equipment in public oral care facilities in Tanzania. BMC Oral Health 2015, 15, 74. [Google Scholar] [CrossRef]
- Çetinkaya, H.; Romaniuk, P. Relationship between consumption of soft and alcoholic drinks and oral health problems. Cent. Eur. J. Public Health 2020, 28, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Masalu, J.R.; Kikwilu, E.N.; Kahabuka, F.K.; Senkoro, A.R.; Kida, I.A. Oral Health related behaviors among adult Tanzanians: A national pathfinder survey. BMC Oral Health 2009, 9, 22. [Google Scholar] [CrossRef]
- Odhiambo, J.; Rwabukwisi, F.C.; Rusangwa, C.; Rusanganwa, V.; Hirschhorn, L.R.; Nahimana, E.; Ngamije, P.; Hedt-Gauthier, B.L. Health worker attrition at a rural district hospital in Rwanda: A need for improved placement and retention strategies. Pan. Afr. Med. J. 2017, 27, 168. [Google Scholar] [CrossRef]
- Zhao, L.; Jin, Y.; Zhou, L.; Yang, P.; Qian, Y.; Huang, X.; Min, M. Evaluation of health system resilience in 60 countries based on their responses to COVID-19. Front. Public Health 2022, 10, 1081068. [Google Scholar] [CrossRef] [PubMed]
- Hugelius, K.; Harada, N.; Marutani, M. Consequences of visiting restrictions during the COVID-19 pandemic: An integrative review. Int. J. Nurs. Stud. 2021, 121, 104000. [Google Scholar] [CrossRef] [PubMed]
- Bellary, S.; Krishnankutty, B.; Latha, M.S. Basics of case report form designing in clinical research. Perspect. Clin. Res. 2014, 5, 159–166. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Oral Health Surveys: Basic Methods; World Health Organization: Geneva, Switzerland, 2013. [Google Scholar]
- Greene, J.C.; Vermillion, J.R. The simplified oral hygiene index. J. Am. Dent. Assoc. 1964, 68, 7–13. [Google Scholar] [CrossRef]
- Miller, E.L. Systems for classifying partially dentulous arches. J. Prosthet. Dent. 1970, 24, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Mfinanga, S.G.; Mnyambwa, N.P.; Minja, D.T.; Ntinginya, N.E.; Ngadaya, E.; Makani, J.; Makubi, A.N. Tanzania’s position on the COVID-19 pandemic. Lancet 2021, 397, 1542–1543. [Google Scholar] [CrossRef]
- Tanzania Commission for AIDS (TACAIDS); Zanzibar AIDS Commission (ZAC). Tanzania HIV Impact Survey (THIS) 2016–2017: Final Report; Tanzania Commission for AIDS (TACAIDS), Zanzibar AIDS Commission (ZAC): Dar es Salaam, Tanzania, 2018. [Google Scholar]
- Carneiro, L.; Kabulwa, M.; Makyao, M.; Mrosso, G.; Choum, R. Oral Health knowledge and practices of secondary school students, Tanga, Tanzania. Int. J. Dent. 2011, 2011, 806258. [Google Scholar] [CrossRef]
- Nyorobi, J.M.; Carneiro, L.C.; Kabulwa, M.N. Knowledge and practices on periodontal health among adults, Misungwi, Tanzania. Int. J. Dent. 2018, 2018, 7189402. [Google Scholar] [CrossRef]
- Furuta, M.; Ekuni, D.; Irie, K.; Azuma, T.; Tomofuji, T.; Ogura, T.; Morita, M. Sex differences in gingivitis relate to interaction of oral health behaviors in young people. J. Periodontol. 2011, 82, 558–565. [Google Scholar] [CrossRef]
- Russell, S.L.; Gordon, S.; Lukacs, J.R.; Kaste, L.M. Sex/gender differences in tooth loss and edentulism—Historical perspectives, biological factors, and sociologic reasons. Dent. Clin. N. Am. 2013, 57, 317–337. [Google Scholar] [CrossRef]
- Etetafia, O.M.; Azodo, C.C. Gender and oral health in Africa. Indian J. Oral Health Res. 2019, 5, 1–5. [Google Scholar] [CrossRef]
- Atkins, D.; Uskul, A.K.; Cooper, N.R. Culture shapes empathic responses to physical and social pain. Emotion 2016, 16, 587–601. [Google Scholar] [CrossRef] [PubMed]
- Simangwa, L.D.; Åstrøm, A.N.; Johansson, A.; Minja, I.K.; Johansson, A.K. Oral diseases and socio-demographic factors in adolescents living in Maasai population areas of Tanzania: A cross-sectional study. BMC Oral Health 2018, 18, 200. [Google Scholar] [CrossRef] [PubMed]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global burden of severe periodontitis in 1990-2010: A systematic review and meta-regression. J. Dent. Res. 2014, 93, 1045–1053. [Google Scholar] [CrossRef]
- Oyapero, A.; Enone, L.L.; Moronkola, R.; Ojikutu, R. Relationship between Periodontal Disease, Dental Caries and the Risk Indicators for Cardiovascular Disease in Lagos, Nigeria. West Afr. J. Med. 2023, 40, 404–413. [Google Scholar] [PubMed]
- Kikwilu, E.N.; Masalu, J.R.; Kahabuka, F.K.; Senkoro, A.R. Prevalence of oral pain and barriers to use of emergency oral care facilities among adult Tanzanians. BMC Oral Health 2008, 8, 28. [Google Scholar] [CrossRef]
- Astrøm, A.N.; Kida, I.A. Perceived dental treatment need among older Tanzanian adults—A cross-sectional study. BMC Oral Health 2007, 7, 9. [Google Scholar] [CrossRef]
- Boniol, M.; Kunjumen, T.; Nair, T.S.; Siyam, A.; Campbell, J.; Diallo, K. The global health workforce stock and distribution in 2020 and 2030: A threat to equity and ‘universal’ health coverage? BMJ Glob. Health 2022, 7, e009316. [Google Scholar] [CrossRef]
- Mosha, H.J.; Scheutz, F. Perceived need and use of oral health services among adolescents and adults in Tanzania. Community Dent. Oral Epidemiol. 1993, 21, 129–132. [Google Scholar] [CrossRef]
- Carneiro, L.C.; Kabulwa, M.N. Dental caries, and supragingival plaque and calculus among students, Tanga, Tanzania. ISRN Dent. 2012, 2012, 245296. [Google Scholar] [CrossRef][Green Version]
- Pengpid, S.; Peltzer, K. Self-rated oral health status and social and health determinants among community dwelling adults in Kenya. Afr. Health Sci. 2019, 19, 3146–3153. [Google Scholar] [CrossRef] [PubMed]
- Mashoto, K.O.; Astrom, A.N.; Skeie, M.S.; Masalu, J.R. Socio-demographic disparity in oral health among the poor: A cross sectional study of early adolescents in Kilwa district, Tanzania. BMC Oral Health 2010, 10, 7. [Google Scholar] [CrossRef] [PubMed]
- Mbulo, L.; Palipudi, K.; Smith, T.; Owusu, D.; Williams, F.; Dean, A.K.; Mamudu, H.M. Secondhand smoke exposure inside the home among adults in eight countries in sub-Saharan Africa: Global adult tobacco survey, 2012–2018. Nicotine Tob. Res. 2023, 25, 828–837. [Google Scholar] [CrossRef] [PubMed]
- Platt, L.; Melendez-Torres, G.J.; O’Donnell, A.; Bradley, J.; Newbury-Birch, D.; Kaner, E.; Ashton, C. How effective are brief interventions in reducing alcohol consumption: Do the setting, practitioner group and content matter? Findings from a systematic review and metaregression analysis. BMJ Open 2016, 6, e011473. [Google Scholar] [CrossRef] [PubMed]
- Mbawalla, H.; Masalu, J.R.; Masatu, M.; Åstrøm, A.N. Changes in adolescents’ oral health status following oral health promotion activities in Tanzania. Acta Odontol. Scand 2013, 71, 333–342. [Google Scholar] [CrossRef] [PubMed]
- Ogunbodede, E.O.; Kida, I.A.; Madjapa, H.S.; Amedari, M.; Ehizele, A.; Mutave, R.; Sodipo, B.; Temilola, S.; Okoye, L. Oral Health inequalities between rural and urban populations of the African and middle east region. Adv. Dent. Res. 2015, 27, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gupta, N.; Pawar, A.P.; Birajdar, S.S.; Natt, A.S.; Singh, H.P. Role of sugar and sugar substitutes in dental caries: A review. ISRN Dent. 2013, 2013, 519421. [Google Scholar] [CrossRef] [PubMed]
- Winkelmann, J.; Gómez Rossi, J.; van Ginneken, E. Oral Health care in Europe: Financing, access and provision. Health Syst. Transit. 2022, 24, 1–176. [Google Scholar] [CrossRef] [PubMed]
- Omotoso, G.; Kadir, E. Midline diastema amongst South-Western Nigerians. Int. J. Dent. Sci. 2009, 8, 1–5. [Google Scholar]
- Mumghamba, E.G.; Honkala, S.; Honkala, E.; Manji, K.P. Gingival recession, oral hygiene and associated factors among Tanzanian women. East Afr. Med. J. 2009, 86, 125–132. [Google Scholar] [CrossRef][Green Version]
- Devishree, R.A.; Sangeetha, S.; Jain, A.R. Prevalence of partial edentulism according to Kennedy’s classification based on age, gender, and arch. Drug Invent. Today 2018, 10, 108–110. [Google Scholar]
- Chen, Q.; Liang, M.; Li, Y.; Guo, J.; Fei, D.; Wang, L.; He, L.; Sheng, C.; Cai, Y.; Li, X.; et al. Mental health care for medical staff in China during the COVID-19 outbreak. Lancet Psychiatry 2020, 7, e15–e16. [Google Scholar] [CrossRef] [PubMed]
- van Palenstein Helderman, W.H.; Nathoo, Z.A. Dental treatment demands among patients in Tanzania. Community Dent. Oral Epidemiol. 1990, 18, 85–87. [Google Scholar] [CrossRef] [PubMed]

| Parameters | n | % | |
|---|---|---|---|
| Sex | |||
| Female | 62 | 46.3 | |
| Male | 72 | 53.7 | |
| Total | 134 | 100.0 | |
| Medical History | Female | Male | |
| None | 50 | 42 | 68.7 |
| Known diseases | 0 | 6 | 4.5 |
| General wound healing disorders | 2 | 0 | 1.5 |
| Medication | 4 | 2 | 4.5 |
| Smoking in the last week | 0 | 2 | 1.5 |
| Alcohol | 2 | 12 | 10.4 |
| Oral habits | 0 | 2 | 1.5 |
| Smoking and alcohol | 0 | 2 | 1.5 |
| Known diseases and medication | 4 | 4 | 6.0 |
| COVID-19 reported in the last 12 months | 0 | 0 | 0 |
| Total | 62 | 72 | 100.0 |
| Dental care products | |||
| None | 2 | 2 | 3.0 |
| Toothbrush | 8 | 10 | 13.4 |
| Toothbrush and toothpaste | 34 | 42 | 56.7 |
| Toothbrush, toothpaste, and toothpick | 12 | 14 | 19.4 |
| Toothbrush and toothpick | 6 | 4 | 7.5 |
| Total | 62 | 72 | 100.0 |
| Brushing teeth per day | |||
| 0 | 4 | 4 | 6.0 |
| 1 | 14 | 14 | 20.9 |
| 2 | 34 | 42 | 56.7 |
| 3 | 10 | 12 | 16.4 |
| Total | 62 | 72 | 100.0 |
| While brushing | |||
| None | 34 | 48 | 61.2 |
| Toothache | 16 | 6 | 16.4 |
| Gum hurt | 2 | 6 | 6.0 |
| Gum bleed | 2 | 6 | 6.0 |
| Toothache and gum hurt | 4 | 0 | 3.0 |
| Toothache and gum bleed | 2 | 0 | 1.5 |
| Gum hurt and gum bleed | 0 | 2 | 1.5 |
| Toothache, gum hurt, and gum bleed | 2 | 4 | 4.5 |
| Total | 62 | 72 | 100.0 |
| Dental treatment | |||
| None | 32 | 44 | 56.7 |
| Check-up (2 year) | 12 | 16 | 20.9 |
| Extraction of a tooth (2 year) | 10 | 0 | 7.5 |
| Filling (2 year) | 0 | 2 | 1.5 |
| Other reason (2 year) | 0 | 2 | 1.5 |
| Check-up and tooth extraction | 4 | 6 | 7.5 |
| Check-up, tooth extraction, and filling | 2 | 0 | 1.5 |
| Check-up, tooth extraction, and other | 2 | 0 | 1.5 |
| Check-up and filling | 0 | 2 | 1.5 |
| Total | 62 | 72 | 100.0 |
| Crossbite | |||
| No | 54 | 62 | 86.6 |
| Yes | 8 | 10 | 13.4 |
| Total | 62 | 72 | 100.0 |
| Dental trauma | |||
| No | 62 | 70 | 98.5 |
| Yes | 0 | 2 | 1.5 |
| Total | 62 | 72 | 100.0 |
| Recession | |||
| No | 50 | 54 | 77.6 |
| Yes | 12 | 18 | 22.4 |
| Total | 62 | 72 | 100.0 |
| Gingival hyperplasia | |||
| No | 62 | 68 | 97.0 |
| Yes | 0 | 4 | 3.0 |
| Total | 62 | 72 | 100.0 |
| Candidiasis | |||
| No | 62 | 72 | 100.0 |
| Yes | 0 | 0 | 0.0 |
| Total | 62 | 72 | 100.0 |
| Mucosal diseases | |||
| No | 62 | 68 | 97.0 |
| Yes | 0 | 4 | 3.0 |
| Total | 62 | 72 | 100.0 |
| Parameters | n | % | |
|---|---|---|---|
| Smoking last week | Female | Male | |
| No | 36 | 34 | 52.2 |
| Never | 26 | 32 | 43.3 |
| Occasionally | 0 | 4 | 3.0 |
| Every day | 0 | 2 | 1.5 |
| Total | 62 | 72 | 100.0 |
| Alcohol | |||
| No | 60 | 58 | 88.1 |
| Yes | 2 | 14 | 11.9 |
| Total | 62 | 72 | 100.0 |
| Meals per day | |||
| 1 | 2 | 2 | 3.0 |
| 2 | 14 | 28 | 31.3 |
| 3 | 46 | 42 | 65.7 |
| Total | 62 | 72 | 100.0 |
| Sweets per day | |||
| 0 | 14 | 30 | 32.8 |
| 1 | 40 | 24 | 47.8 |
| 2 | 4 | 12 | 11.9 |
| 3 | 4 | 4 | 6.0 |
| 5 | 0 | 2 | 1.5 |
| Total | 62 | 72 | 100.0 |
| Sugar-sweetened tea per day | |||
| 0 | 4 | 2 | 4.5 |
| 1 | 48 | 62 | 82.1 |
| 2 | 6 | 6 | 9.0 |
| 3 | 2 | 2 | 3.0 |
| 4 | 2 | 0 | 1.5 |
| Total | 62 | 72 | 100.0 |
| Soft drinks per day | |||
| 0 | 20 | 22 | 31.3 |
| 1 | 36 | 34 | 52.2 |
| 2 | 6 | 16 | 16.4 |
| Total | 62 | 72 | 100.0 |
| Parameters | DMF/T | DIS | CIS | OHIS | |
|---|---|---|---|---|---|
| Mean | 3.33 | 0.4403 | 0.3333 | 0.7736 | |
| Standard error of the mean | 0.294 | 0.06281 | 0.05332 | 0.09833 | |
| Median | 3.00 | 0.0000 | 0.0000 | 0.3333 | |
| Standard deviation | 3.409 | 0.72703 | 0.61721 | 1.13820 | |
| Minimum | 0 | 0.00 | 0.00 | 0.00 | |
| Maximum | 17 | 3.00 | 3.00 | 6.00 | |
| Percentile | 25 | 1.00 | 0.0000 | 0.0000 | 0.0000 |
| 50 | 3.00 | 0.0000 | 0.0000 | 0.3333 | |
| 75 | 5.00 | 0.5000 | 0.5000 | 1.0000 | |
| DMF/T | ||||||
|---|---|---|---|---|---|---|
| R2 = 0.382 | Regression Coefficient B | SD | Beta | T | Sig. | 95% Confidence Interval (CI) for B (Min–Max) |
| Constant | 8.829 | 1.966 | 4.491 | <0.001 | 4.939–12.720 | |
| Sex | −1.747 | 0.494 | −0.256 | −3.533 | <0.001 | −2.725–−0.768 |
| Age | 0.127 | 0.025 | 0.356 | 4.979 | <0.001 | 0.076–0.177 |
| Brushing teeth per day | −0.612 | 0.344 | −0.138 | −1.780 | 0.077 | −1.292–0.068 |
| Meals per day | −1.814 | 0.476 | −0.289 | −3.814 | <0.001 | −2.756–−0.873 |
| Sweets per day | 0.263 | 0.268 | 0.074 | 0.983 | 0.328 | −0.267–0.792 |
| Sugar–sweetened tea per day | −0.348 | 0.439 | −0.062 | −0.792 | 0.430 | −1.218–0.522 |
| Soft drinks per day | −0.794 | 0.393 | −0.158 | −2.020 | 0.045 | −1.571–0.016 |
| OHIS | ||||||
|---|---|---|---|---|---|---|
| R2 = 0.171 | Regression Coefficient B | SD | Beta | T | Sig. | 95% Confidence Interval (CI) for B (Min–Max) |
| Constant | −0.140 | 0.760 | −0.184 | 0.854 | −1.644–1.364 | |
| Sex | 0.390 | 0.191 | 0.171 | 2.040 | 0.043 | 0.012–0.768 |
| Age | 0.030 | 0.010 | 0.248 | 3.003 | 0.003 | 0.010–0.049 |
| Brushing teeth per day | −0.148 | 0.133 | −0.100 | −1.112 | 0.268 | −0.411–0.115 |
| Meals per day | −0.085 | 0.184 | −0.040 | −0.460 | 0.646 | −0.448–0.279 |
| Sweets per day | −0.091 | 0.103 | −0.077 | −0.879 | 0.381 | −0.296–0.114 |
| Sugar–sweetened tea per day | 0.286 | 0.170 | 0.153 | 1.686 | 0.094 | −0.050–0.623 |
| Soft drinks per day | −0.137 | 0.152 | −0.081 | −0.901 | 0.369 | −0.437–0.164 |
| Parameters | n | % | |
|---|---|---|---|
| Dental Status without Wisdom Teeth | Female | Male | |
| Full dentition | 18 | 26 | 32.8 |
| Partially edentulous | 44 | 46 | 67.2 |
| Total | 62 | 72 | 100.0 |
| Eichner classification | |||
| None | 14 | 14 | 20.9 |
| A1 | 4 | 14 | 13.4 |
| A2 | 14 | 20 | 25.4 |
| A3 | 10 | 12 | 16.4 |
| B1 | 14 | 12 | 19.4 |
| B2 | 4 | 0 | 3.0 |
| B4 | 2 | 0 | 1.5 |
| Total | 62 | 72 | 100.0 |
| Maxilla—Kennedy classification (wisdom teeth included) | |||
| None | 28 | 44 | 53.7 |
| Class I/1 | 2 | 0 | 1.5 |
| Class II | 10 | 4 | 10.4 |
| Class II/1 | 2 | 0 | 1.5 |
| Class III | 4 | 18 | 16.4 |
| Class III/1 | 10 | 6 | 11.9 |
| Class III/2 | 6 | 0 | 4.5 |
| Total | 62 | 72 | 100.0 |
| Mandible—Kennedy classification (wisdom teeth included) | |||
| None | 24 | 36 | 44.8 |
| Class I | 6 | 4 | 7.5 |
| Class I/2 | 2 | 0 | 1.5 |
| Class II | 8 | 2 | 7.5 |
| Class II/1 | 4 | 4 | 6.0 |
| Class II/2 | 0 | 2 | 1.5 |
| Class III | 6 | 18 | 17.9 |
| Class III/1 | 12 | 6 | 13.4 |
| Total | 62 | 72 | 100.0 |
| Teeth (FDI) | Existing | D | M | F | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |
| 18 | 88 | 65.7 | 8 | 6.0 | 38 | 28.4 | 0 | 0.0 |
| 17 | 108 | 80.6 | 12 | 9.0 | 12 | 9.0 | 2 | 1.5 |
| 16 | 108 | 80.6 | 10 | 7.5 | 12 | 9.0 | 4 | 3.0 |
| 15 | 114 | 85.1 | 4 | 3.0 | 16 | 11.9 | 0 | 0.0 |
| 14 | 116 | 86.6 | 4 | 3.0 | 14 | 10.4 | 0 | 0.0 |
| 13 | 130 | 97.0 | 0 | 0.0 | 4 | 3.0 | 0 | 0.0 |
| 12 | 132 | 98.5 | 2 | 1.5 | 0 | 0.0 | 0 | 0.0 |
| 11 | 126 | 94.0 | 6 | 4.5 | 2 | 1.5 | 0 | 0.0 |
| 21 | 124 | 92.5 | 6 | 4.5 | 4 | 3.0 | 0 | 0.0 |
| 22 | 128 | 95.5 | 4 | 3.0 | 2 | 1.5 | 0 | 0.0 |
| 23 | 128 | 95.5 | 2 | 1.5 | 4 | 3.0 | 0 | 0.0 |
| 24 | 122 | 91.0 | 4 | 3.0 | 8 | 6.0 | 0 | 0.0 |
| 25 | 116 | 86.6 | 8 | 6.0 | 10 | 7.5 | 0 | 0.0 |
| 26 | 104 | 77.6 | 8 | 6.0 | 18 | 13.4 | 4 | 3.0 |
| 27 | 96 | 71.6 | 20 | 14.9 | 18 | 13.4 | 0 | 0.0 |
| 28 | 84 | 62.7 | 12 | 9.0 | 38 | 28.4 | 0 | 0.0 |
| 38 | 82 | 61.2 | 14 | 10.4 | 36 | 26.9 | 2 | 1.5 |
| 37 | 78 | 58.2 | 16 | 11.9 | 30 | 22.4 | 10 | 7.5 |
| 36 | 92 | 68.7 | 10 | 7.5 | 32 | 23.9 | 0 | 0.0 |
| 35 | 122 | 91.0 | 2 | 1.5 | 6 | 4.5 | 4 | 3.0 |
| 34 | 134 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 33 | 132 | 98.5 | 2 | 1.5 | 0 | 0.0 | 0 | 0.0 |
| 32 | 134 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 31 | 130 | 97.0 | 2 | 1.5 | 2 | 1.5 | 0 | 0.0 |
| 41 | 130 | 97.0 | 2 | 1.5 | 2 | 1.5 | 0 | 0.0 |
| 42 | 132 | 98.5 | 0 | 0.0 | 2 | 1.5 | 0 | 0.0 |
| 43 | 134 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| 44 | 130 | 97.0 | 0 | 0.0 | 4 | 3.0 | 0 | 0.0 |
| 45 | 124 | 92.5 | 2 | 1.5 | 8 | 6.0 | 0 | 0.0 |
| 46 | 90 | 67.2 | 6 | 4.5 | 36 | 26.9 | 2 | 1.5 |
| 47 | 86 | 64.2 | 14 | 10.4 | 32 | 23.9 | 2 | 1.5 |
| 48 | 92 | 68.7 | 4 | 3.0 | 38 | 28.4 | 0 | 0.0 |
| Tooth Position | n | % | |
|---|---|---|---|
| Female | Male | ||
| Normal | 42 | 44 | 64.2 |
| Midline diastema | 12 | 16 | 20.9 |
| Edge-to-edge bite | 0 | 4 | 3.0 |
| Anterior open bite | 4 | 0 | 3.0 |
| Anterior crowding | 0 | 2 | 1.5 |
| Nonocclusion | 2 | 2 | 3.0 |
| Anterior tooth trauma (maxilla) | 0 | 2 | 1.5 |
| Midline diastema and anterior open bite | 2 | 0 | 1.5 |
| Edge-to-edge bite and anterior crowding | 0 | 2 | 1.5 |
| Total | 62 | 72 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bensel, T.; Megiroo, S.; Kronenberg, W.; Bömicke, W.; Ulrichs, T.; Hinz, S. Oral Health Status of Healthcare Workers in Ilembula/Tanzania during the COVID-19 Condition. Healthcare 2024, 12, 920. https://doi.org/10.3390/healthcare12090920
Bensel T, Megiroo S, Kronenberg W, Bömicke W, Ulrichs T, Hinz S. Oral Health Status of Healthcare Workers in Ilembula/Tanzania during the COVID-19 Condition. Healthcare. 2024; 12(9):920. https://doi.org/10.3390/healthcare12090920
Chicago/Turabian StyleBensel, Tobias, Simon Megiroo, Werner Kronenberg, Wolfgang Bömicke, Timo Ulrichs, and Sebastian Hinz. 2024. "Oral Health Status of Healthcare Workers in Ilembula/Tanzania during the COVID-19 Condition" Healthcare 12, no. 9: 920. https://doi.org/10.3390/healthcare12090920
APA StyleBensel, T., Megiroo, S., Kronenberg, W., Bömicke, W., Ulrichs, T., & Hinz, S. (2024). Oral Health Status of Healthcare Workers in Ilembula/Tanzania during the COVID-19 Condition. Healthcare, 12(9), 920. https://doi.org/10.3390/healthcare12090920






