Effects of Neuromuscular Electrical Stimulation with Gastrocnemius Strengthening on Foot Morphology in Stroke Patients: A Randomized Controlled Trial
Abstract
1. Introduction
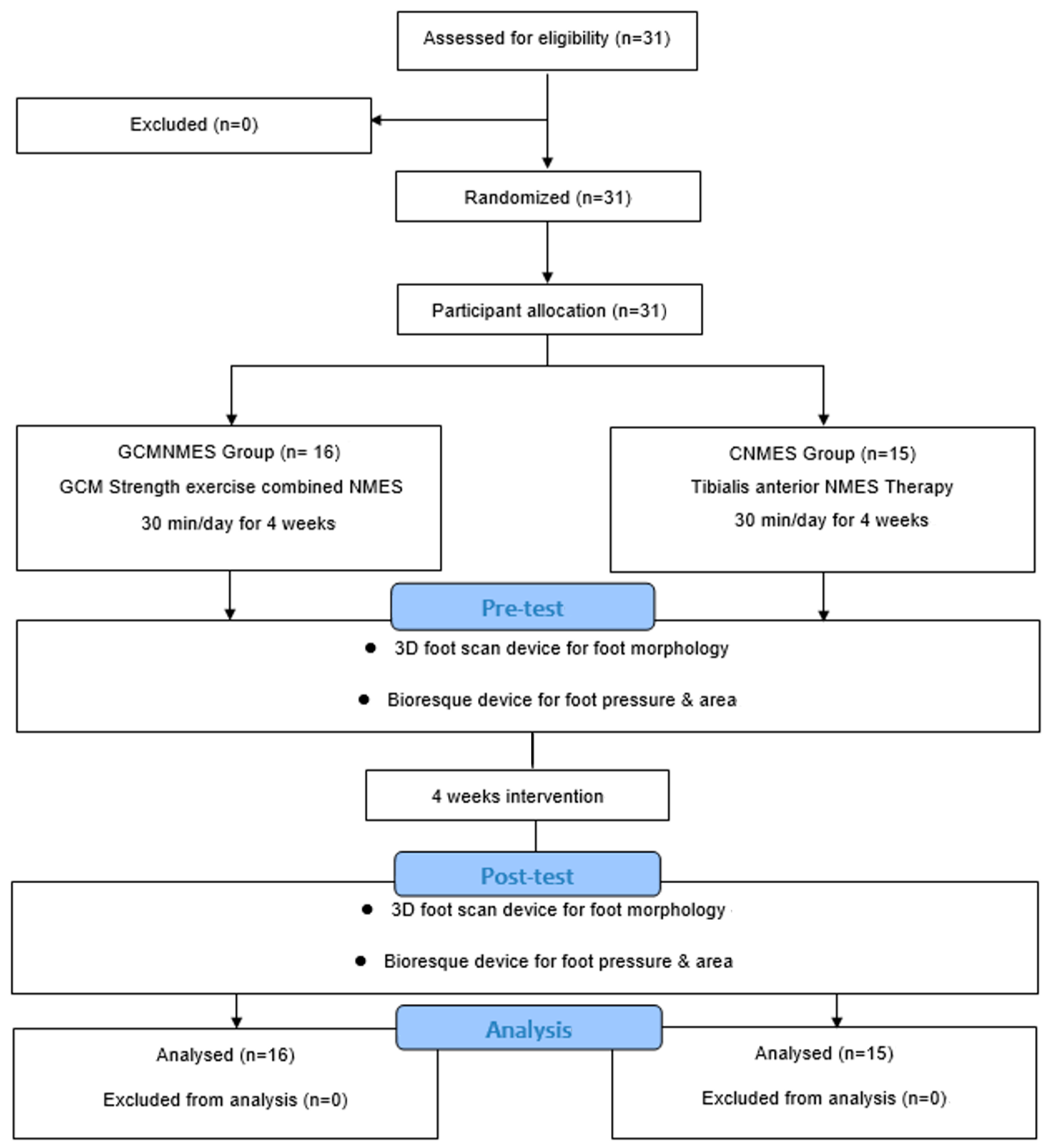
2. Materials and Methods
2.1. Study Design
2.2. Ethical Considerations
2.3. Participants
2.4. Intervention
2.4.1. GCMNMES
2.4.2. CNMES
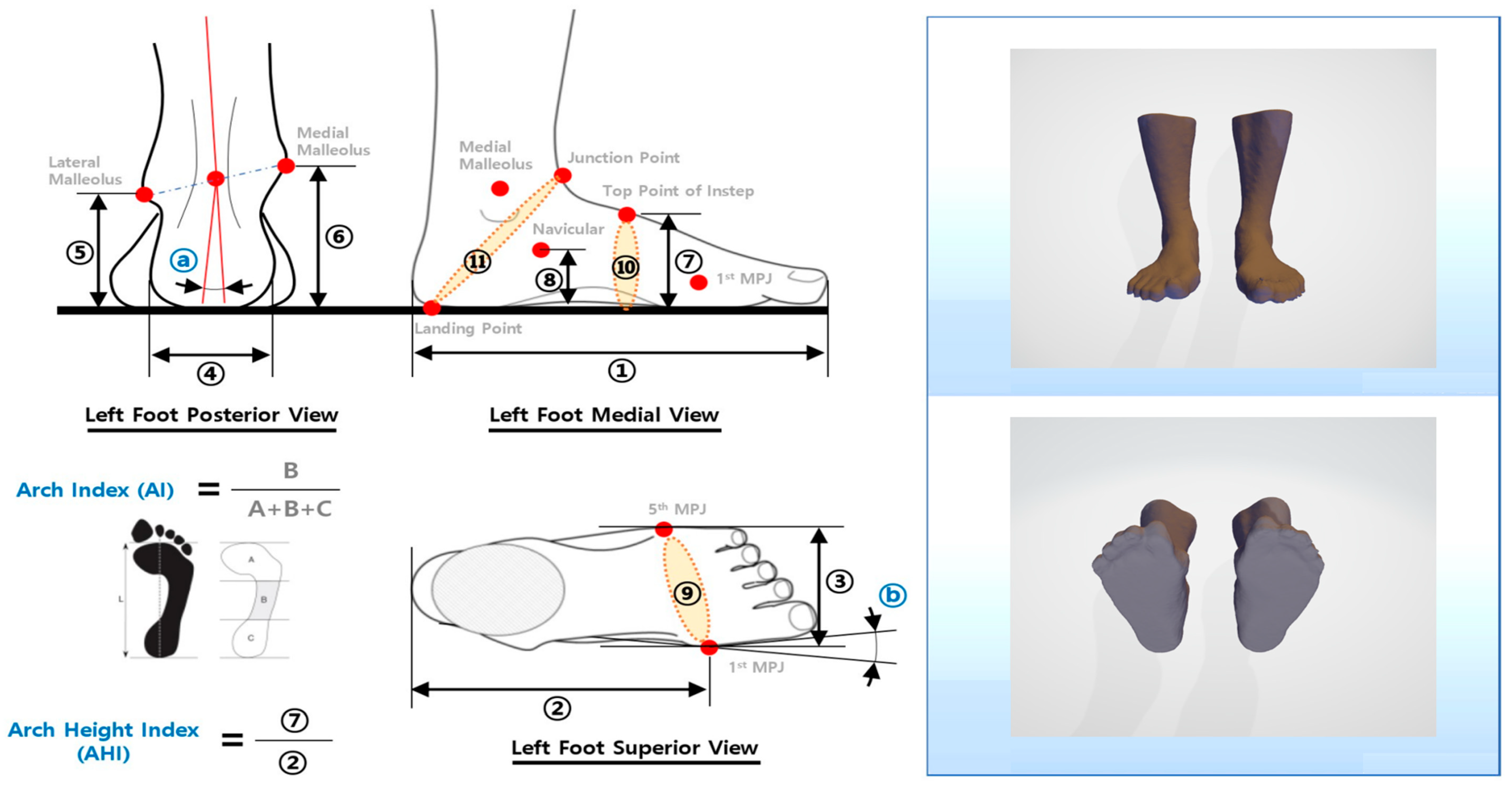
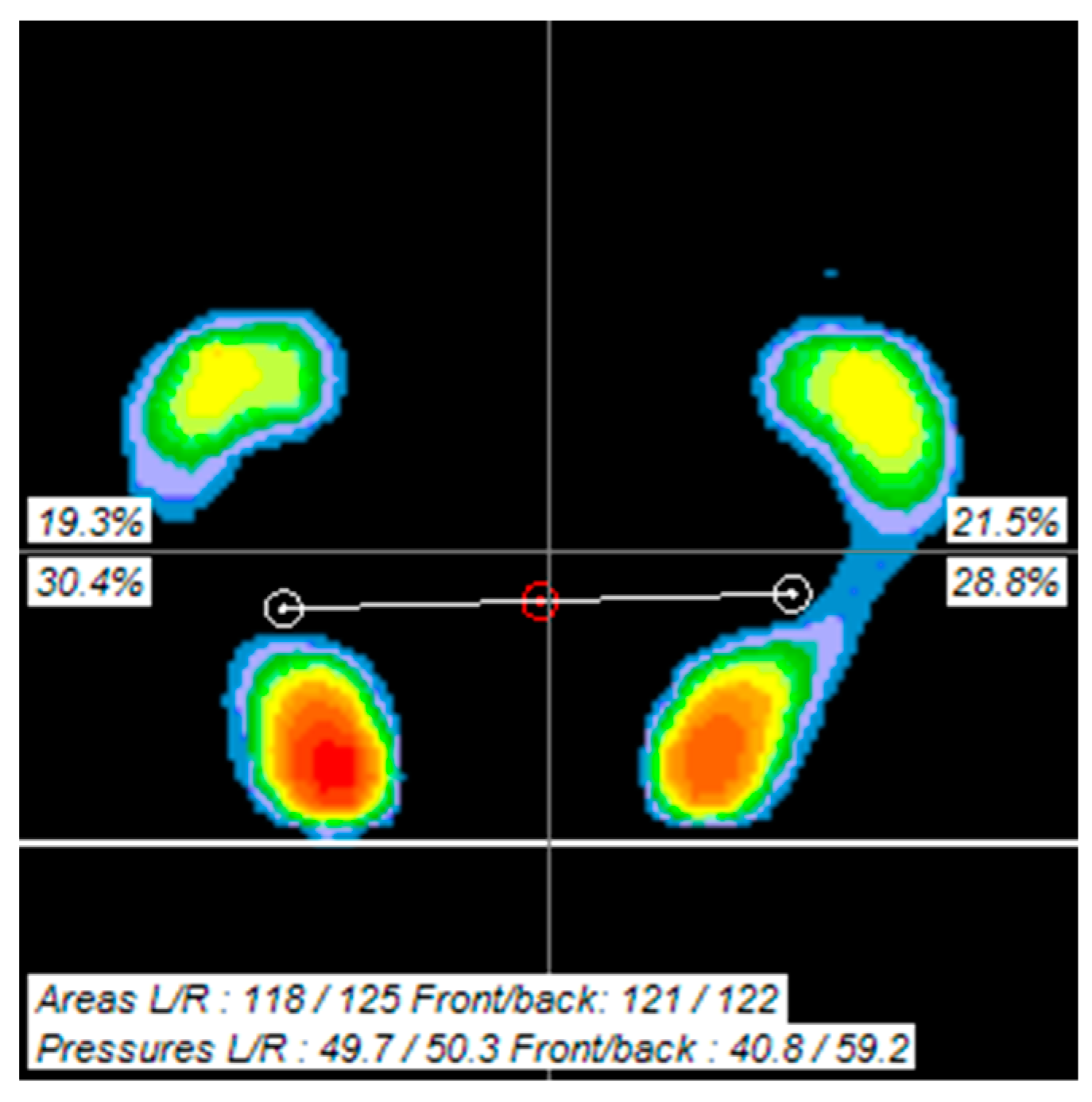
2.5. Outcome Measures
2.5.1. Foot Morphology
2.5.2. Foot Pressure
2.6. Statistical Analysis
3. Results
3.1. General Characteristics of Study Subjects
3.2. Comparison of Foot Shape before and after Intervention
3.3. Comparison of Foot Pressure before and after Intervention
4. Discussion
4.1. Foot Morphology
4.2. Foot Pressure
4.3. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kolb, B.; Mychasiuk, R.; Williams, P.; Gibb, R. Brain plasticity and recovery from early cortical injury. Dev. Med. Child Neurol. 2011, 53, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.M.; Eng, J.J. The relationship of lower-extremity muscle torque to locomotor performance in people with stroke. Phys. Ther. 2003, 83, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; Gustafson, Y. Patient falls in stroke rehabilitation. A challenge to rehabilitation strategies. Stroke 1995, 26, 838–842. [Google Scholar] [CrossRef]
- Knutsson, E.; Richards, C. Different types of disturbed motor control in gait of hemiparetic patients. Brain 1979, 102, 405–430. [Google Scholar] [CrossRef] [PubMed]
- Holowka, N.B.; O’Neill, M.C.; Thompson, N.E.; Demes, B. Chimpanzee and human midfoot motion during bipedal walking and the evolution of the longitudinal arch of the foot. J. Hum. Evol. 2017, 104, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.S.; McClay, I.S. Measurements used to characterize the foot and the medial longitudinal arch: Reliability and validity. Phys. Ther. 2000, 80, 864–871. [Google Scholar] [CrossRef]
- Shibuya, N.; Jupiter, D.C.; Ciliberti, L.J.; VanBuren, V.; La Fontaine, J. Characteristics of adult flatfoot in the United States. J. Foot Ankle Surg. 2010, 49, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Gould, N.; Moreland, M.; Alvarez, R.; Trevino, S.; Fenwick, J. Development of the child’s arch. Foot Ankle 1989, 9, 241–245. [Google Scholar] [CrossRef]
- Scott, G.; Menz, H.B.; Newcombe, L. Age-related differences in foot structure and function. Gait Posture 2007, 26, 68–75. [Google Scholar] [CrossRef]
- Jacquelin Perry, M. Gait Analysis: Normal and Pathological Function; SLACK: Princeton, NJ, USA, 2010. [Google Scholar]
- Ha, N.; Kim, M.; Han, S. The correlation between changes of ankle joint position sense and sway area through unstable surface training. J. Korea Inst. Electron. Commun. Sci. 2013, 8, 1383–1389. [Google Scholar] [CrossRef][Green Version]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Acceleration patterns of the head and pelvis when walking on level and irregular surfaces. Gait Posture 2003, 18, 35–46. [Google Scholar] [CrossRef] [PubMed]
- Shumway-Cook, A.; Woollacott, M.H. Motor Control: Theory and Practical Applications, 2nd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1995. [Google Scholar]
- Muscolino, J.E. Know the Body: Muscle, Bone, and Palpation Essentials, 1st ed.; Mosby: Maryland Heights, MO, USA, 2011. [Google Scholar]
- Sahrmann, S. Movement System Syndrome of the Foot and Ankle. In Movement System Impairment Syndromes of the Extremities, Cervical and Thoracic Spines, 1st ed.; Mosby: Maryland Heights, MO, USA, 2011. [Google Scholar]
- Thompson, A.J.; Jarrett, L.; Lockley, L.; Marsden, J.; Stevenson, V.L. Clinical management of spasticity. J. Neurol. Neurosurg. Psychiatry 2005, 76, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Alon, G.; Levitt, A.F.; McCarthy, P.A. Functional electrical stimulation enhancement of upper extremity functional recovery during stroke rehabilitation: A pilot study. Neurorehabil. Neural Repair 2007, 21, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.R.; Wang, R.Y.; Lin, K.H.; Chu, M.Y.; Chan, R.C. Task-oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clin. Rehabil. 2006, 20, 860–870. [Google Scholar] [CrossRef] [PubMed]
- Combs, S.; Miller, E.W.; Forsyth, E. Motor and functional outcomes of a patient post-stroke following combined activity and impairment level training. Physiother. Theory Pract. 2007, 23, 219–229. [Google Scholar] [CrossRef]
- Sabut, S.K.; Sikdar, C.; Kumar, R.; Mahadevappa, M. Functional electrical stimulation of dorsiflexor muscle: Effects on dorsiflexor strength, plantarflexor spasticity, and motor recovery in stroke patients. NeuroRehabilitation 2011, 29, 393–400. [Google Scholar] [CrossRef] [PubMed]
- Hur, Y.G. The Effects on Spasticity, Range of Motion, Balance on Combining Active-Stretching Exercise of Plantarflexor on Ankle with FES in the Patients with Stroke. Master’s Thesis, Yongin University, Yongin-si, Republic of Korea, 2013. [Google Scholar]
- George, M.S.; Lisanby, S.H.; Sackeim, H.A. Transcranial magnetic stimulation: Applications in neuropsychiatry. Arch. Gen. Psychiatry 1999, 56, 300–311. [Google Scholar] [CrossRef] [PubMed]
- George, M.S.; Taylor, J.J.; Short, E.B. The expanding evidence base for rTMS treatment of depression. Curr. Opin. Psychiatry 2013, 26, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, J.; Lee, B.H. Effect of an EMG–FES interface on ankle joint training combined with real-time feedback on balance and gait in patients with stroke hemiparesis. Healthcare 2020, 8, 292. [Google Scholar] [CrossRef]
- Kobravi, H.R.; Farzaneh, Y.; Faryar Majd, M.; Sheikh, M.; Akbarzadeh, A. A human interactive hybrid FES-robotic system applicable to improvement of foot drop after stroke: Case report of a patient with chronic stroke. Arch. Bone Jt. Surg. 2020, 8, 744–747. [Google Scholar] [CrossRef]
- Sharif, F.; Ghulam, S.; Malik, A.N.; Saeed, Q. Effectiveness of functional electrical stimulation (FES) versus conventional electrical stimulation in gait rehabilitation of patients with stroke. J. Coll. Physicians Surg. Pak. 2017, 27, 703–706. [Google Scholar]
- Mahadevappa, M.; Shendkar, C.; Lenka, P.; Biswas, A.; Kumar, R. Modelling a BCI system to estimate FES stimulation intensity for individual stroke survivors in foot drop cases. Biomed. Tech. 2013, 58, 000010151520134031. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 Statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Robbins, S.M.; Houghton, P.E.; Woodbury, M.G.; Brown, J.L. The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: A meta-analysis. Arch. Phys. Med. Rehabil. 2006, 87, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.W.; Kim, J.H.; Kim, J.H. Effects of eccentric activation training of plantar flexors for the patients with stroke. J. Korean Soc. Neurother. 2020, 24, 33–39. [Google Scholar]
- Choi, Y.S.; Chang, W.N. Effects of Ankle Control Training using NMES on Ankle Joint Mobility and Balance in a Healthy Adult: A Single Case Study. J. Korean. Soc. Neurother. 2022, 26, 19–29. [Google Scholar]
- Lee, W. Effects of gastrocnemius muscle length on the dynamic balance and antero-posterior pressure distribution of foot. J. Korea Acad. Ind. Coop. Soc. 2019, 20, 150–157. [Google Scholar]
- Matsunaga, T.; Shimada, Y.; Sato, K. Muscle fatigue from intermittent stimulation with low and high frequency electrical pulses. Arch. Phys. Med. Rehabil. 1999, 80, 48–53. [Google Scholar] [CrossRef]
- Cheng, J.S.; Yang, Y.R.; Cheng, S.J.; Lin, P.Y.; Wang, R.Y. Effects of combining electric stimulation with active ankle dorsiflexion while standing on a rocker board: A pilot study for subjects with spastic foot after stroke. Arch. Phys. Med. Rehabil. 2010, 91, 505–512. [Google Scholar] [CrossRef]
- Kim, B.H.; An, K.B.; Chang, W.N. Effects of the backward walking training on balance and gait in patient with chronic stroke: Single subject research design. J. Korean Soc. Neurother. 2022, 26, 7–15. [Google Scholar]
- Chang, W.N. The Effects of Facilitation of Trunk Stability Based on Bobath Concept on Trunk Alignment, Postural Control and Gait in Patients with Stroke. Ph.D. Thesis, Yongin University, Yongin-si, Republic of Korea, 2017. [Google Scholar]
- An, K.B.; Lee, K.B.; Hwang, B.Y. Effect of foot perceptual training on balance and gait of patient with chronic stroke: Single subject research design. J. Korean Soc. Neurother. 2019, 23, 19–27. [Google Scholar]
- Zhang, Y.J.; Du, J.Y.; Chen, B.; Jin, R.L.; Hu, J.G.; Lin, X.J. Correlation between three-dimensional medial longitudinal arch joint complex mobility and medial arch angle in stage II posterior tibial tendon dysfunction. Foot Ankle Surg. 2019, 25, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.H.; Lee, K.H.; Ryu, H.J.; Joo, I.W.; Seo, A.; Kim, D.H.; Kim, S.J. Ankle-foot orthosis made by 3D printing technique and automated design software. Appl. Bionics Biomech. 2017, 2017, 9610468. [Google Scholar] [CrossRef] [PubMed]
- Olivia, A.P.; Jeff, R.P.; Jason, M.W. Reliability and validity of 3D limb scanning for ankle foot orthosis fitting. Prosthet. Orthot. Int. 2022, 46, 84–90. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, N.J. The effects of balance training and ankle training on the gait of elderly people who have fallen. J. Phys. Ther. Sci. 2015, 27, 139–142. [Google Scholar] [CrossRef]
- Herzig, D.; Maffiuletti, N.A.; Eser, P. The application of neuromuscular electrical stimulation training in various non-neurologic patient populations: A narrative review. PMR 2015, 7, 1167–1178. [Google Scholar] [CrossRef]
- Bogataj, U.; Gros, N.; Kljajić, M.; Aćimović, R.; Maležič, M. The rehabilitation of gait in patients with hemiplegia: A comparison between conventional therapy and multichannel functional electrical stimulation therapy. Phys. Ther. 1995, 75, 490–502. [Google Scholar] [CrossRef]
- McPoil, T.G.; Cornwall, M.W. Use of the longitudinal arch angle to predict dynamic foot posture in walking. J. Am. Podiatr. Med. Assoc. 2005, 95, 114–120. [Google Scholar] [CrossRef]
- Jang, G.U.; Kweon, M.G.; Park, S.; Kim, J.Y.; Park, J.W. A study of structural foot deformity in stroke patients. J. Phys. Ther. Sci 2015, 27, 191–194. [Google Scholar] [CrossRef]
- Lentell, G.L.; Katzman, L.L.; Walters, M.R. The relationship between muscle function and ankle stability. J. Orthop. Sports Phys. Ther. 1990, 11, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Billis, E.; Katsakiori, E.; Kapodistrias, C.; Kapreli, E. Assessment of foot posture: Correlation between different clinical techniques. Foot 2007, 17, 65–72. [Google Scholar] [CrossRef]
- Chen, G.; Patten, C.; Kothari, D.H.; Zajac, F.E. Gait differences between individuals with post-stroke hemiparesis and non-disabled controls at matched speeds. Gait Posture 2005, 22, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Welte, L.; Kelly, L.A.; Lichtwark, G.A.; Rainbow, M.J. Influence of the windlass mechanism on arch-spring mechanics during dynamic foot arch deformation. J. R. Soc. Interface 2018, 15, 20180270. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.Y.; Koh, E.K.; Kwon, O.Y.; Yi, C.H.; Oh, J.S.; Weon, J.H. Effect of medial arch support on displacement of the myotendinous junction of the gastrocnemius during standing wall stretching. J. Orthop. Sports Phys. Ther. 2009, 39, 867–874. [Google Scholar] [CrossRef]
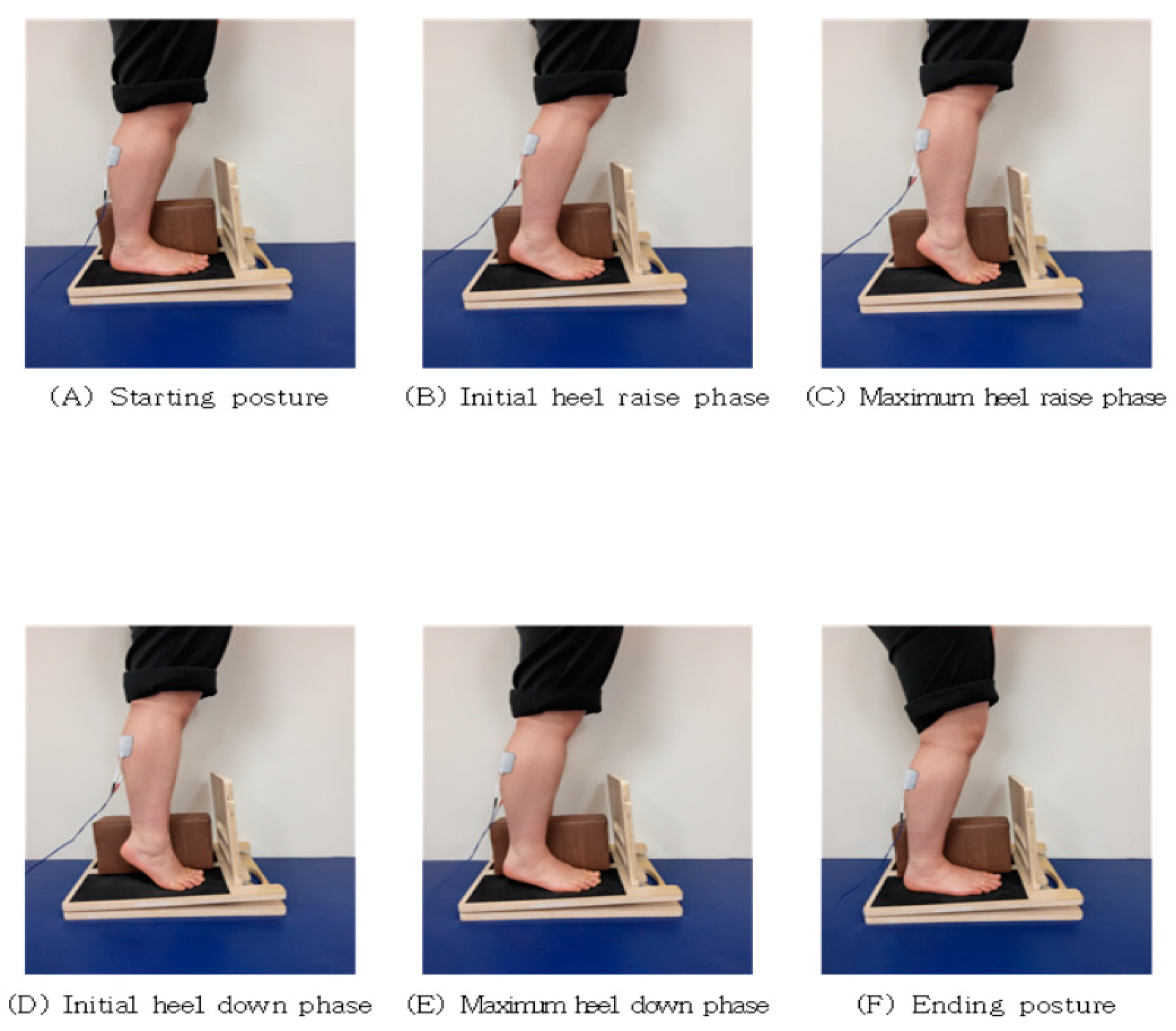

| Stage | GCMNMES |
|---|---|
| A | Start with your knees bent at 5°. |
| B | Allow your heels to slowly lift off the floor for 5 s while the current is running. |
| C | Raise your heels as far as you can in a plantar flexion. |
| D | For 5 s without current, let your heels slowly touch the floor. |
| E | Slowly move your heels until they are fully on the floor. |
| F | Slowly return to a 5° knee bend. |
| Variables | GCMNMES (n = 16) | CNMES (n = 15) | x/t2 | p |
|---|---|---|---|---|
| Sex (Male/Female) | 10/6 (63/37) | 8/7 (53/47) | 0.271 b | 0.721 |
| Stroke type (ICH/CI, %) | 10/6 (63/37) | 5/10 (33/67) | 2.644 | 0.165 |
| Paretic side (R/L, %) | 9/7 (56/44) | 8/7 (53/47) | 0.032 | 1.000 |
| Age (year) | 46.19 ± 13.36 a | 63.93 ± 11.95 | 0.976 c | 0.336 |
| Height (cm) | 168.56 ± 7.84 | 165.27 ± 6.79 | 1.241 | 0.221 |
| Duration (months) | 68.10 ± 12.84 | 61.97 ± 8.36 | 1.562 | 0.133 |
| MMSE-K (score) | 44.25 ± 23.14 | 38.46 ± 34.07 | 0.568 | 0.585 |
| K-MBI (score) | 29.31 ± 1.31 | 26.93 ± 2.40 | 3.452 | 0.097 |
| Variables | GCMNMES Group (n = 16) | CNMES Group (n = 15) | p | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Mean Difference (95% CI) | Pre-Test | Post-Test | Mean Difference (95% CI) | |||
| LAA [◦] | Paretic | 142.77 ± 6.69 | 151.36 ± 7.99 *,a | 8.58 (6.9, 10.3) | 147.23 ± 10.14 | 148.89 ± 8.40 | 1.67 (−1.9, 5.2) | 0.148 b |
| nonparetic | 144.79 ± 6.14 | 148.3 ± 11.79 | 3.55 (−1.0, 8.1) | 148.91 ± 10.46 | 149.91 ± 10.46 | 0.15 (−3.5, 3.8) | 0.390 | |
| MLAA [◦] | Paretic | 137.23 ± 4.79 | 131.38 ± 5.84 ** | −5.85 (−7.4, −4.3) | 133.16 ± 7.02 | 132.03 ± 6.41 | −1.13 (−3.2, 0.9) | 0.049 * |
| nonparetic | 135.51 ± 5.41 | 134.0 ± 7.56 | −1.47 (−4.1, 1.2) | 132.11 ± 7.80 | 131.64 ± 5.77 | −0.47 (−2.1, 1.2) | 0.214 | |
| TAA [◦] | Paretic | 116.08 ± 8.57 | 107.14 ± 9.23 ** | −8.93 (−12.4, −5.4) | 111.23 ± 6.02 | 105.03 ± 8.19 * | −6.02 (−9.0, −3.0) | 0.394 |
| nonparetic | 118.88 ± 7.16 | 112.19 ± 8.79 * | −6.69 (−10.3, −3.1) | 114.63 ± 8.34 | 107.10 ± 7.62 * | −7.50 (−10.1, −4.9) | 0.782 | |
| RA [◦] | Paretic | 7.45 ± 8.78 | 4.25 ± 7.22 * | −3.03 (−5.3, −0.8) | 5.00 ± 8.04 | 5.20 ± 10.31 | 0.20 (−1.9, 2.3) | 0.013 * |
| nonparetic | 9.54 ± 7.04 | 7.45 ± 8.13 | −2.09 (−4.2, −0.1) | 8.00 ± 7.10 | 6.80 ± 8.90 | −1.20 (−3.4, 1.0) | 0.184 | |
| FL [mm] | Paretic | 249.54 ± 13.02 | 247.02 ± 12.72 * | −2.52 (−3.9, −1.2) | 243.38 ± 11.93 | 244.67 ± 10.80 | 1.29 (−0.7, 3.2) | 0.381 |
| nonparetic | 247.73 ± 12.72 | 247.48 ± 14.08 | −0.24 (−1.6, 1.2) | 246.45 ± 9.48 | 245.83 ± 9.38 | −0.61 (−1.6, 0.4) | 0.135 | |
| FW [mm] | Paretic | 100.51 ± 7.08 | 98.89 ± 6.65 * | −1.61 (−2.7, −0.6) | 97.03 ± 5.32 | 95.60 ± 4.98 * | −1.43 (−2.3, −0.6) | 0.849 |
| nonparetic | 101.26 ± 6.29 | 99.78 ± 6.12 | −2.52 (−3.9, −1.2) | 96.07 ± 5.12 | 94.49 ± 4.12 | −1.59 (−2.7, −0.5) | 0.925 | |
| AHI [index] | Paretic | 0.39 ± 0.03 | 0.41 ± 0.04 ** | 0.02 (0.0, 0.0) | 0.40 ± 0.04 | 0.41 ± 0.04 | 0.01 (0.0, 0.0) | 0.318 |
| nonparetic | 950.41 ± 0.04 | 0.43 ± 0.03 * | 0.02 (0.0, 0.0) | 0.41 ± 0.03 | 0.44 ± 0.04 * | 0.03 (0.0, 0.0) | 0.890 | |
| Variables | GCMNMES Group (n = 16) | CNMES Group (n = 15) | p | |||||
|---|---|---|---|---|---|---|---|---|
| Pre-Test | Post-Test | Mean Difference (95% CI) | Pre-Test | Post-Test | Mean Difference (95% CI) | |||
| FPA [mm2] | paretic | 95.51 ± 23.04 | 107.42 ± 22.23 *,a | 11.91 (6.4, 17.4) | 107.44 ± 24.00 | 106.65 ± 24.65 | −1.24 (−5.9, 3.5) | 0.236 b |
| nonparetic | 111.69 ± 12.06 | 112.93 ± 17.66 | 0.79 (−4.9, 6.5) | 113.15 ± 25.56 | 118.27 ± 21.03 | 5.13 (−0.4, 10.6) | 0.706 | |
| symmetry | 0.16 ± 0.16 | 0.22 ± 0.30 * | 0.05 (−0.0, 0.1) | 0.17 ± 0.17 | 0.19 ± 0.15 * | 0.02 (−0.0, 0.1) | 0.481 | |
| FPP [%] | paretic | 41.80 ± 7.72 | 46.37 ± 10.80 ** | 2.59 (−1.7, 6.9) | 49.46 ± 8.29 | 52.06 ± 9.35 | 4.58 (1.07, 8.1) | 0.627 |
| nonparetic | 58.22 ± 6.72 | 53.83 ± 10.80 * | −3.22 (−6.9, 0.5) | 50.54 ± 8.15 | 47.32 ± 8.23 * | −4.60 (−8.1, −1.1) | 0.712 | |
| symmetry | 0.26 ± 0.22 | 0.33 ± 0.37 | 0.07 (−0.1, 0.2) | 0.29 ± 0.18 | 0.31 ± 0.19 | 0.02 (−0.0, 0.1) | 0.611 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.; Lee, S.; Kim, M.; Chang, W. Effects of Neuromuscular Electrical Stimulation with Gastrocnemius Strengthening on Foot Morphology in Stroke Patients: A Randomized Controlled Trial. Healthcare 2024, 12, 777. https://doi.org/10.3390/healthcare12070777
Choi Y, Lee S, Kim M, Chang W. Effects of Neuromuscular Electrical Stimulation with Gastrocnemius Strengthening on Foot Morphology in Stroke Patients: A Randomized Controlled Trial. Healthcare. 2024; 12(7):777. https://doi.org/10.3390/healthcare12070777
Chicago/Turabian StyleChoi, Yusik, Sooyong Lee, Minhyuk Kim, and Woonam Chang. 2024. "Effects of Neuromuscular Electrical Stimulation with Gastrocnemius Strengthening on Foot Morphology in Stroke Patients: A Randomized Controlled Trial" Healthcare 12, no. 7: 777. https://doi.org/10.3390/healthcare12070777
APA StyleChoi, Y., Lee, S., Kim, M., & Chang, W. (2024). Effects of Neuromuscular Electrical Stimulation with Gastrocnemius Strengthening on Foot Morphology in Stroke Patients: A Randomized Controlled Trial. Healthcare, 12(7), 777. https://doi.org/10.3390/healthcare12070777








