Hemispheric Lateralization in Older Adults Who Habitually Play Darts: A Cross-Sectional Study Using Functional Near-Infrared Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
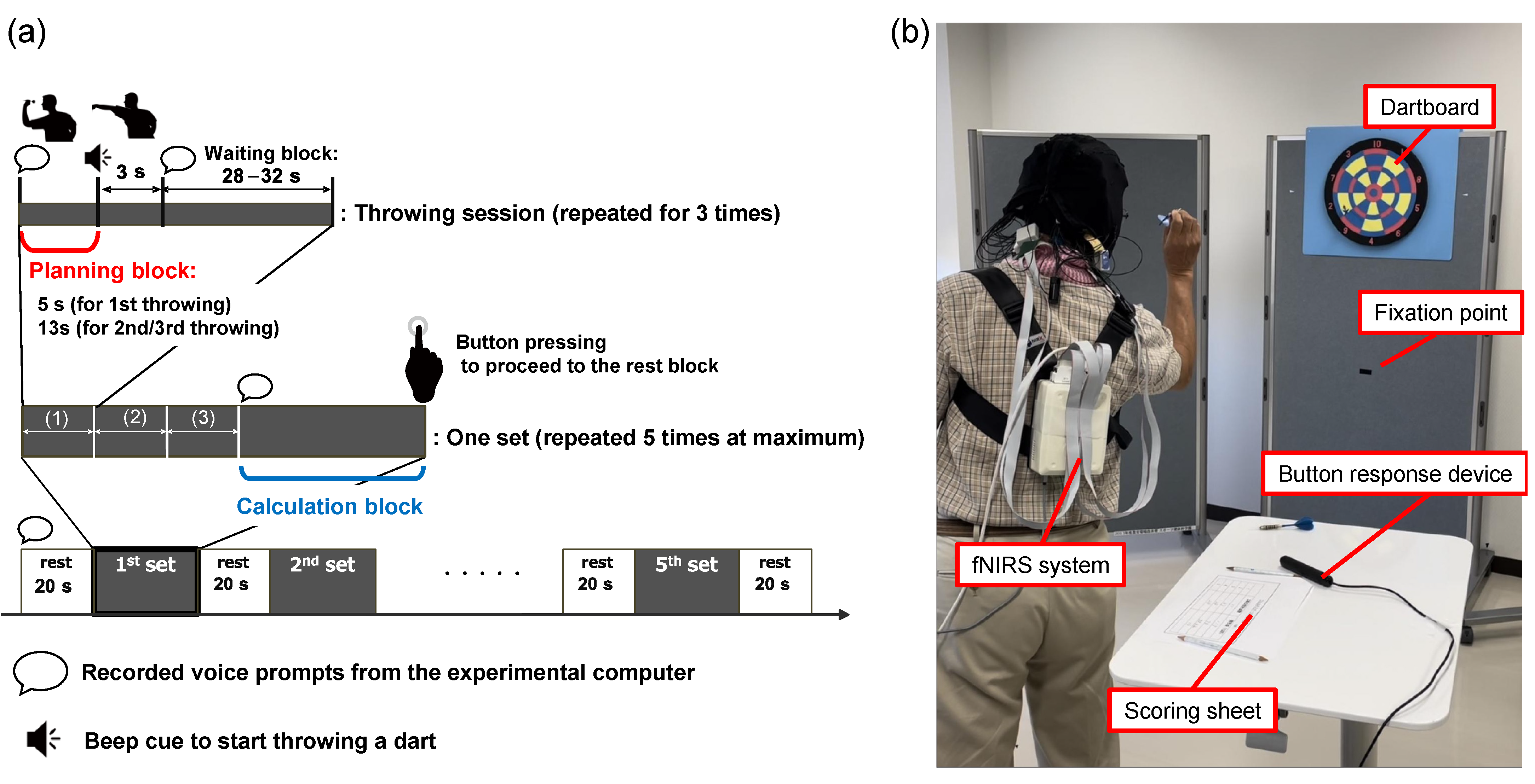
2.2. Wellness Darts Task
2.3. Procedure
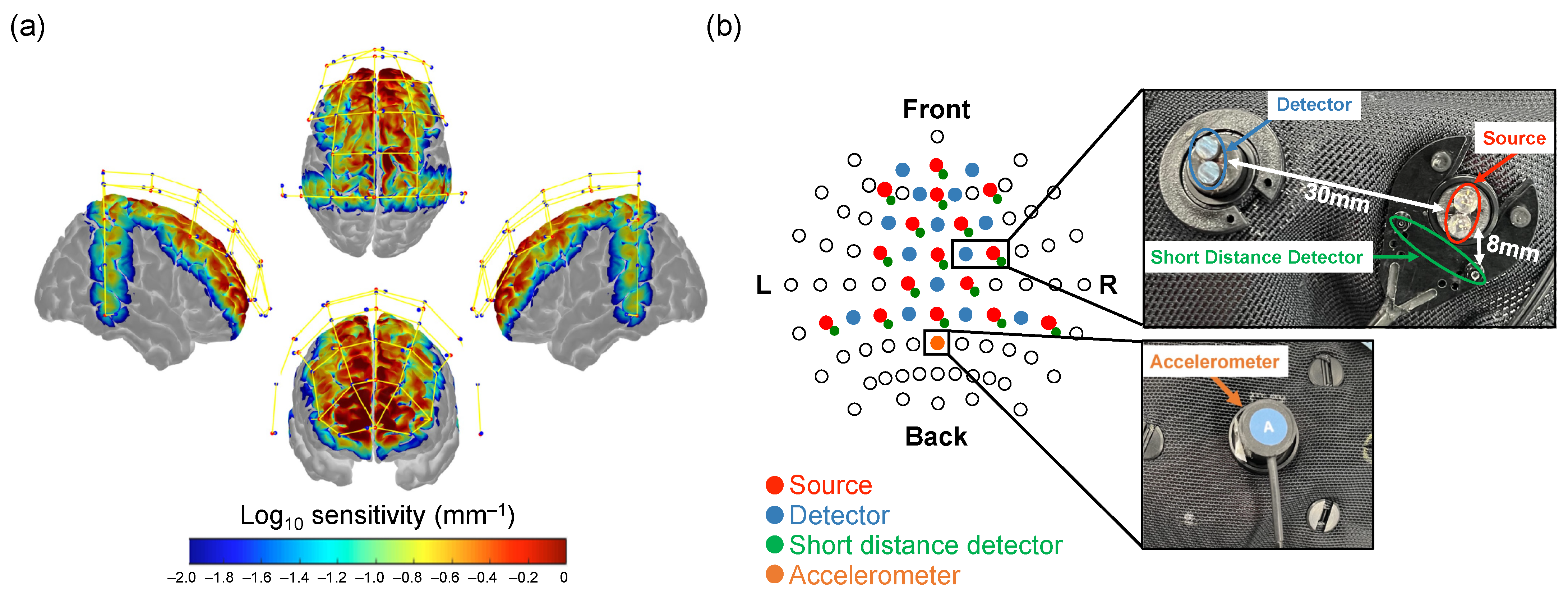
2.4. fNIRS Data Acquisition
2.5. Cognitive Tests
2.5.1. Simple Cognitive Test
2.5.2. Computational Ability Test
2.5.3. STM
2.5.4. CogEvo
2.5.5. TMPT
2.6. Dart-Throwing Performance Test
2.7. Questionnaires
2.8. fNIRS Data Processing
2.8.1. Preprocessing
2.8.2. Activation Analysis
2.8.3. Hemispheric Lateralization Analysis
2.9. Statistical Analysis
2.9.1. Activation Analysis
2.9.2. Hemispheric Lateralization Analysis
3. Results
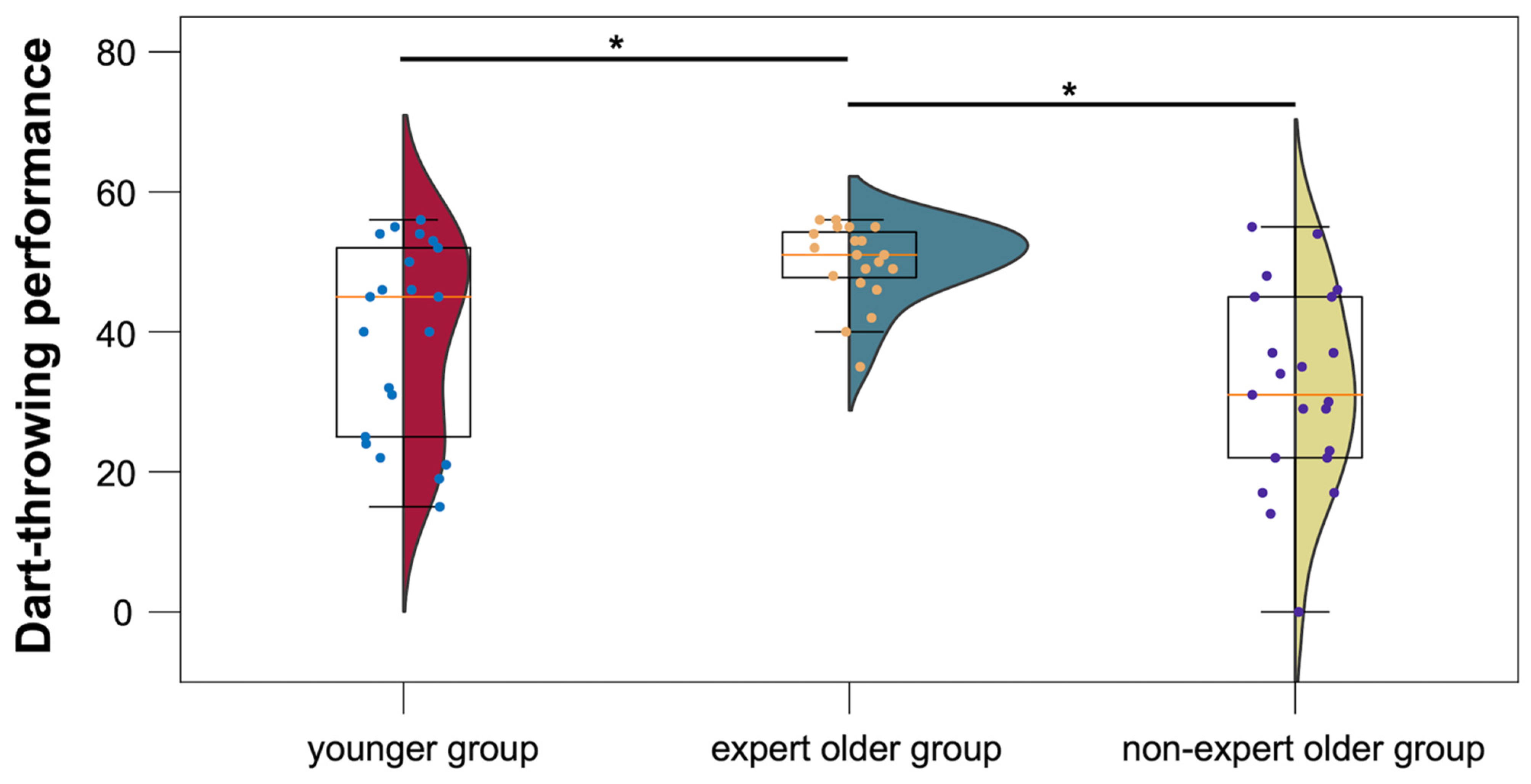
3.1. Descriptive Statistics on Three Groups
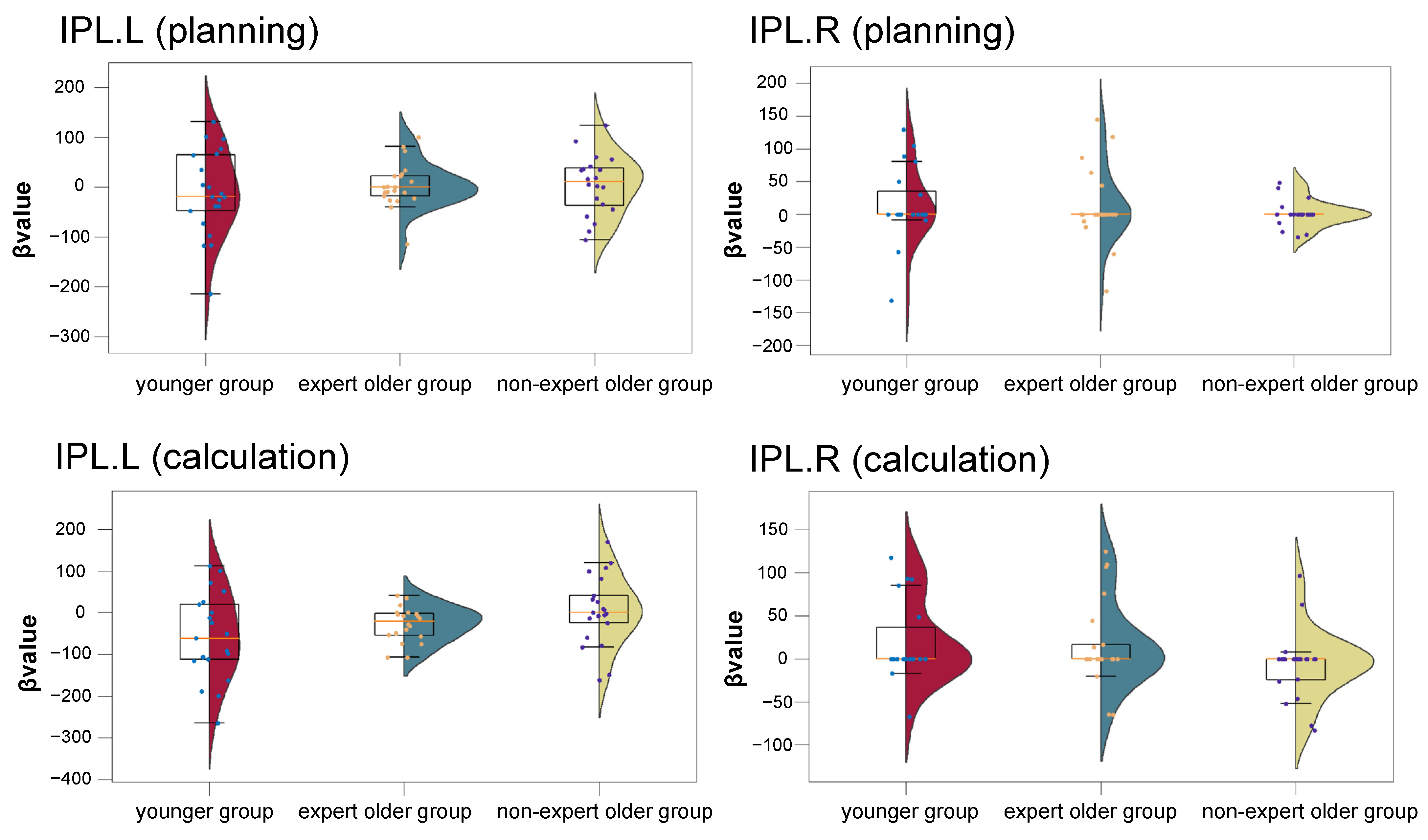
3.2. fNIRS Data
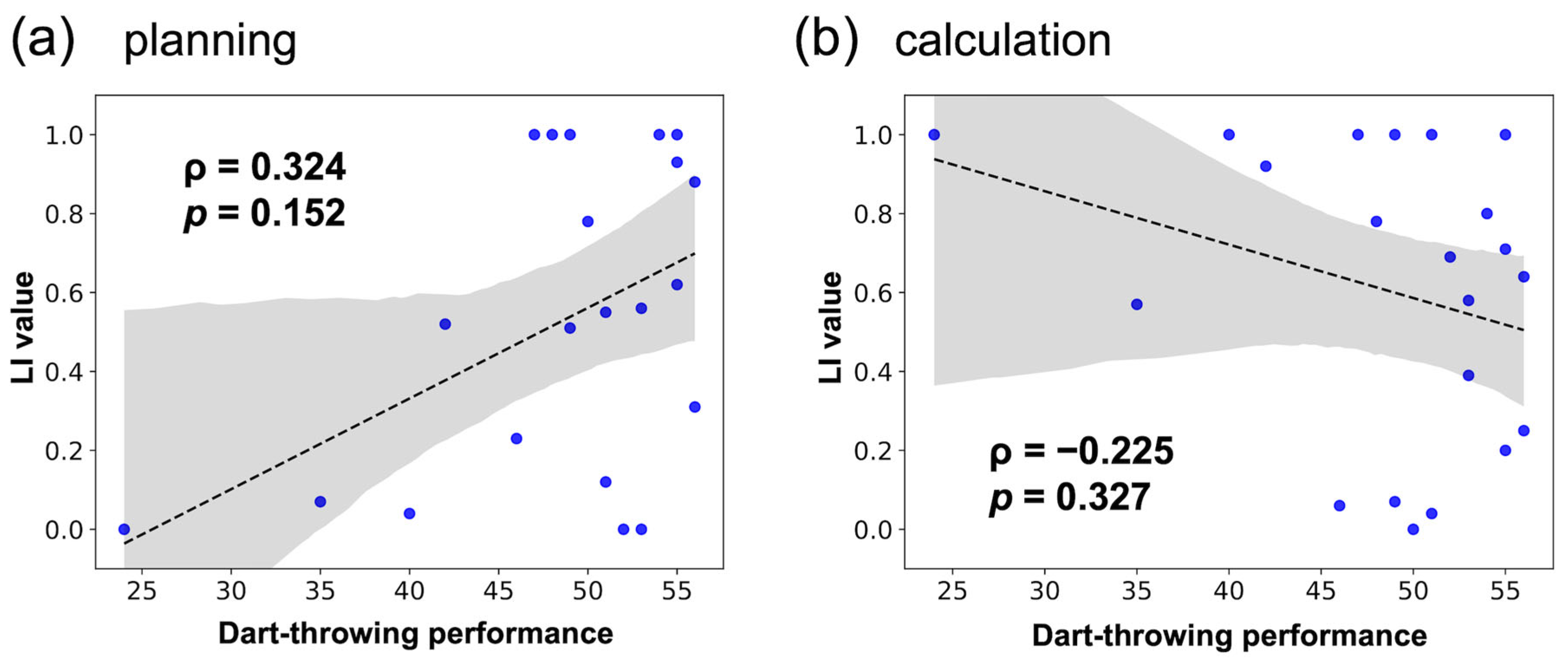
3.2.1. Hemispheric Lateralization Analysis
3.2.2. Activation Analysis
3.3. Cognitive Tests
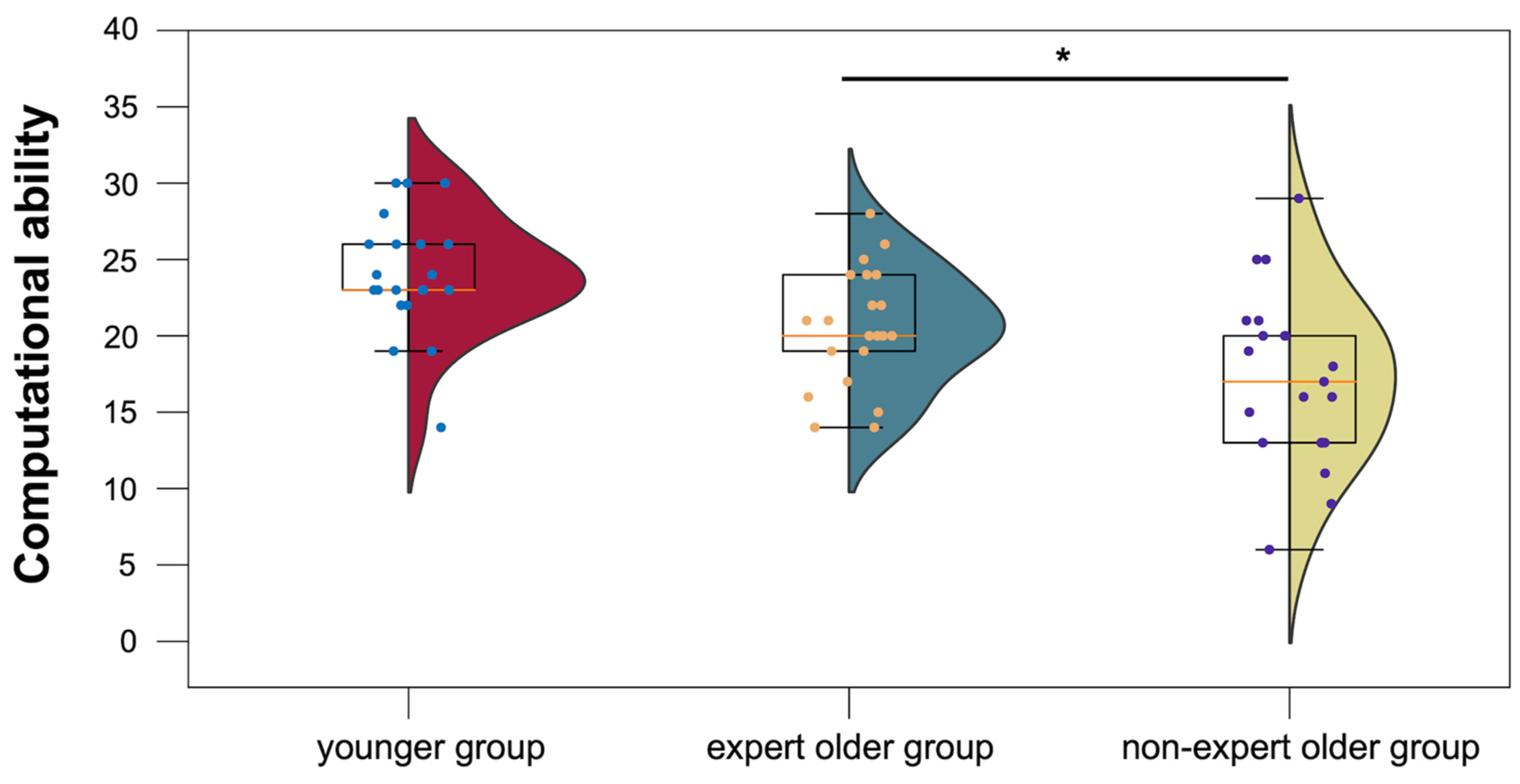
3.3.1. Computational Ability Test
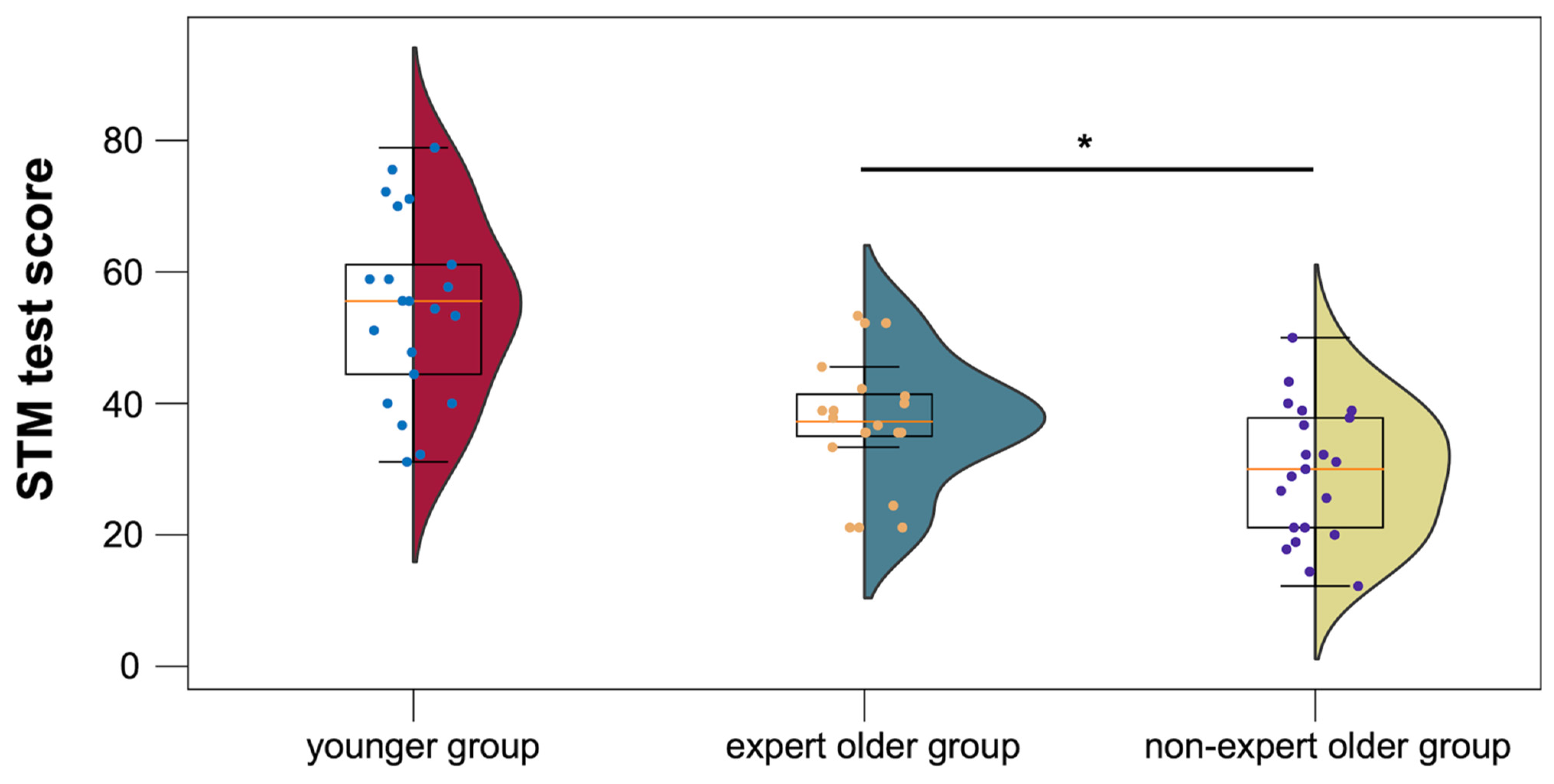
3.3.2. STM Test
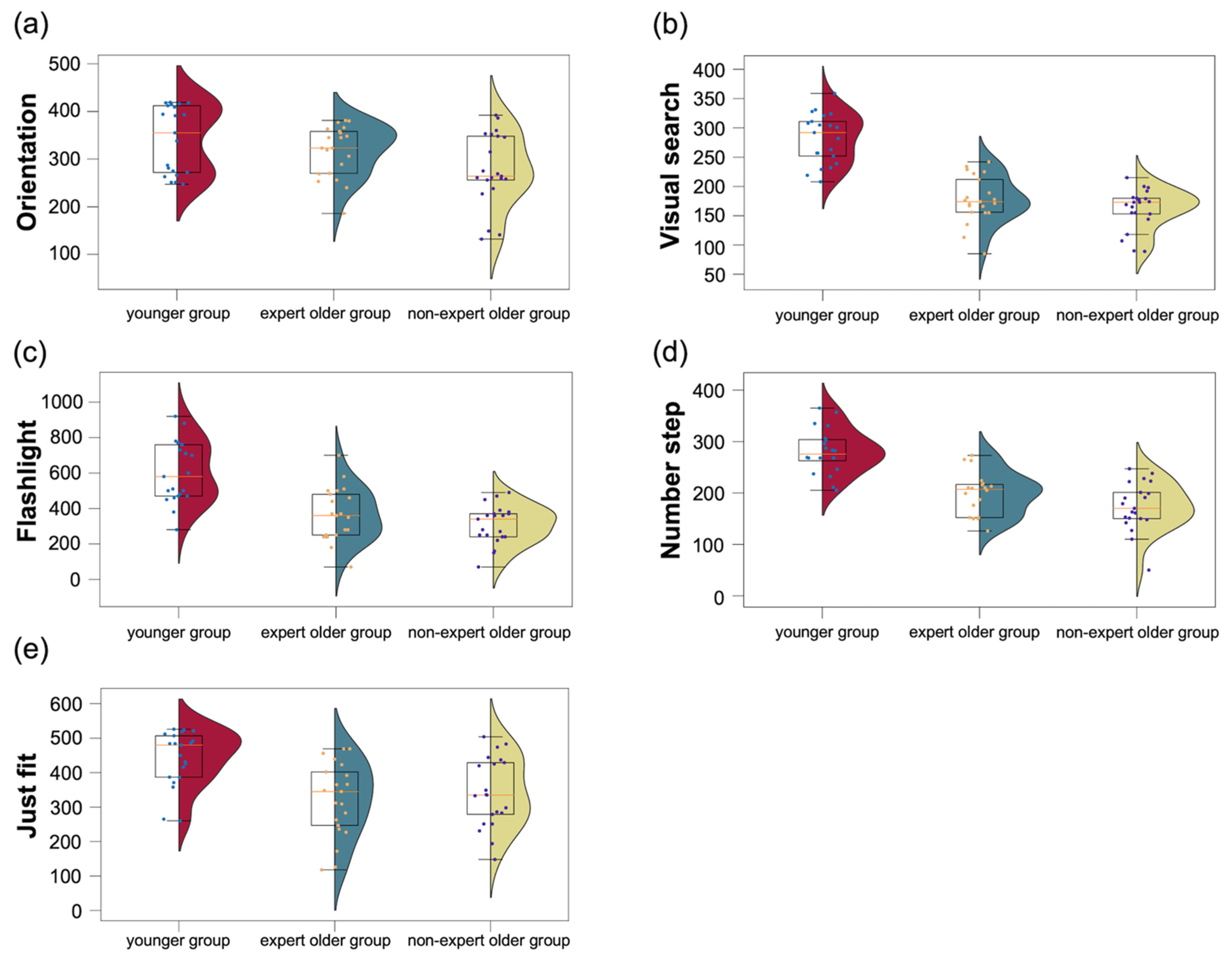
3.3.3. CogEvo
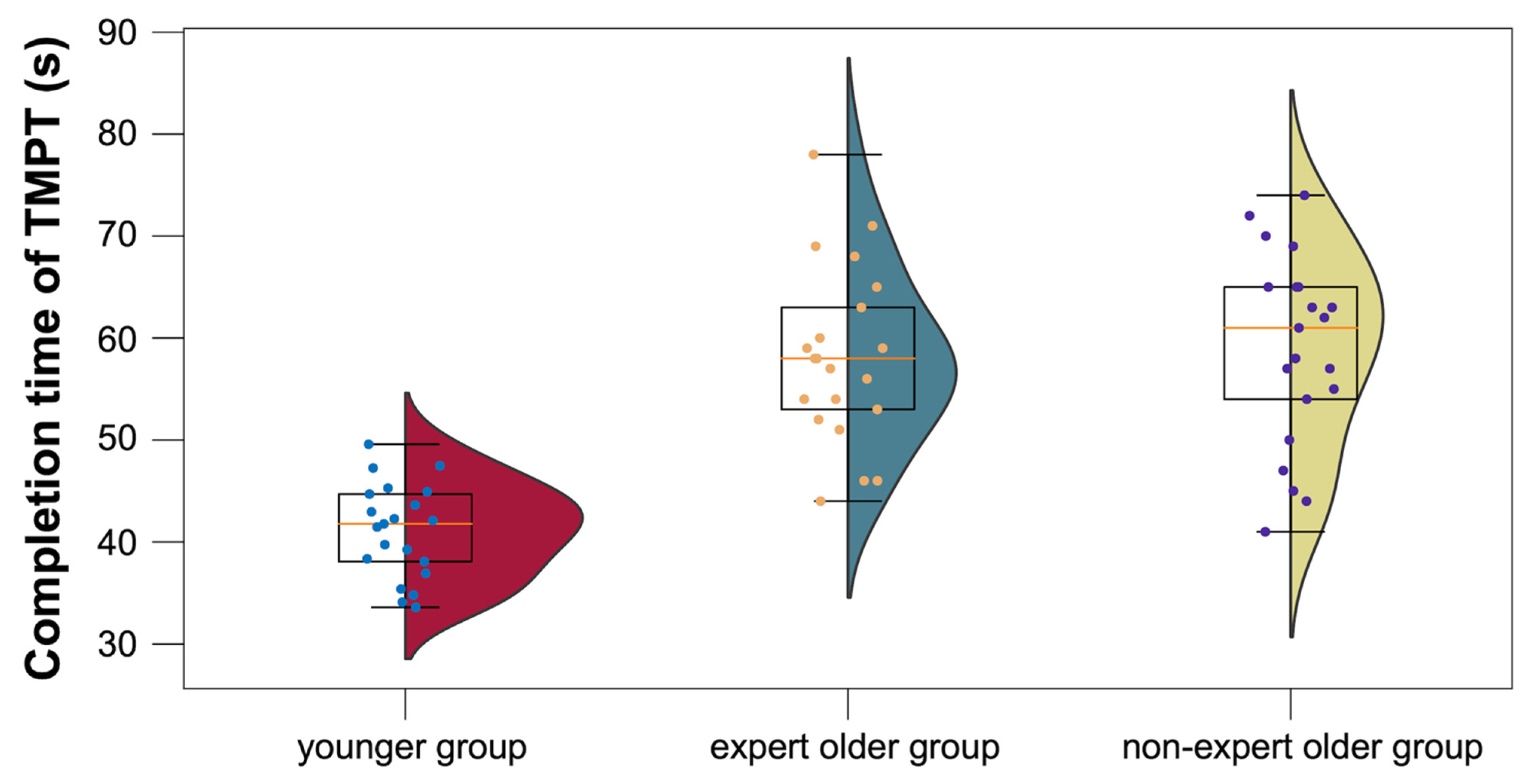
3.3.4. TMPT
4. Discussion
4.1. Hypothesis 1
4.2. Hypothesis 2
4.3. Hypothesis 3
4.4. Cognitive Tests
4.4.1. Computational Ability Test
4.4.2. STM Test
4.4.3. CogEvo
4.4.4. TMPT
4.4.5. Strengths of the Current Study, Limitations, and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Northey, J.M.; Cherbuin, N.; Pumpa, K.L.; Smee, D.J.; Rattray, B. Exercise interventions for cognitive function in adults older than 50: A systematic review with meta-analysis. Br. J. Sports Med. 2018, 52, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Bherer, L. Cognitive plasticity in older adults: Effects of cognitive training and physical exercise. Ann. N. Y. Acad. Sci. 2015, 1337, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Levin, O.; Netz, Y.; Ziv, G. The beneficial effects of different types of exercise interventions on motor and cognitive functions in older age: A systematic review. Eur. Rev. Aging Phys. Act. 2017, 14, 20. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Yi, Q.; Zheng, X.; Cui, S.; Chen, B.; Lu, L.; Tang, C. Effects of Mind-Body Exercises on Cognitive Function in Older Adults: A Meta-Analysis. J. Am. Geriatr. Soc. 2019, 67, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Babaei, P.; Azari, H.B. Exercise Training Improves Memory Performance in Older Adults: A Narrative Review of Evidence and Possible Mechanisms. Front. Hum. Neurosci. 2022, 15, 771553. [Google Scholar] [CrossRef] [PubMed]
- Gheysen, F.; Poppe, L.; DeSmet, A.; Swinnen, S.; Cardon, G.; De Bourdeaudhuij, I.; Chastin, S.; Fias, W. Physical activity to improve cognition in older adults: Can physical activity programs enriched with cognitive challenges enhance the effects? A systematic review and meta-analysis. Int. J. Behav. Nutr. Phys. Act. 2018, 15, 63. [Google Scholar] [CrossRef]
- Rieker, J.A.; Reales, J.M.; Muiños, M.; Ballesteros, S. The Effects of Combined Cognitive-Physical Interventions on Cognitive Functioning in Healthy Older Adults: A Systematic Review and Multilevel Meta-Analysis. Front. Hum. Neurosci. 2022, 16, 838968. [Google Scholar] [CrossRef] [PubMed]
- Kirk-Sanchez, N.J.; McGough, E.L. Physical exercise and cognitive performance in the elderly: Current perspectives. Clin. Interv. Aging 2014, 9, 51–62. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Min, L.; Liu, R.; Zhang, X.; Wu, M.; Di, Q.; Ma, X. The effect of exercise on cerebral blood flow and executive function among young adults: A double-blinded randomized controlled trial. Sci. Rep. 2023, 13, 8269. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M.; Yasuda, N.; Ito, S.; Abe, M.; Takamichi, Y.; Yanagisawa, K.; Radak, Z. Effects of habitual darts training on cognitive function in elderly people. Harris Sci. Rev. Doshisha Univ. 2017, 58, 53–60. [Google Scholar] [CrossRef]
- Hermand, E.; Tapie, B.; Dupuy, O.; Fraser, S.; Compagnat, M.; Salle, J.Y.; Daviet, J.C.; Perrochon, A. Prefrontal cortex activation during dual task with increasing cognitive load in subacute stroke patients: A pilot study. Front. Aging Neurosci. 2019, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R. Hemispheric asymmetry reduction in older adults: The harold model. Psychol. Aging 2002, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Anderson, N.D.; Houle, S.; Mangels, J.A.; Nyberg, L. Age-related differences in neural activity during item and temporal-order memory retrieval: A positron emission tomography study. J. Cogn. Neurosci. 2000, 12, 197–206. [Google Scholar] [CrossRef] [PubMed]
- Müller, L.D.; Guhn, A.; Zeller, J.B.M.; Biehl, S.C.; Dresler, T.; Hahn, T.; Fallgatter, A.J.; Polak, T.; Deckert, J.; Herrmann, M.J. Neural correlates of a standardized version of the trail making test in young and elderly adults: A functional near-infrared spectroscopy study. Neuropsychologia 2014, 56, 271–279. [Google Scholar] [CrossRef]
- Erickson, K.I.; Colcombe, S.J.; Wadhwa, R.; Bherer, L.; Peterson, M.S.; Scalf, P.E.; Kim, J.S.; Alvarado, M.; Kramer, A.F. Training-induced plasticity in older adults: Effects of training on hemispheric asymmetry. Neurobiol. Aging 2007, 28, 272–283. [Google Scholar] [CrossRef] [PubMed]
- Cabeza, R.; Anderson, N.D.; Locantore, J.K.; McIntosh, A.R. Aging gracefully: Compensatory brain activity in high-performing older adults. NeuroImage 2002, 17, 1394–1402. [Google Scholar] [CrossRef] [PubMed]
- Reuter-Lorenz, P.A.; Jonides, J.; Smith, E.E.; Hartley, A.; Miller, A.; Marshuetz, C.; Koeppe, R.A. Age differences in the frontal lateralization of verbal and spatial working memory revealed by pet. J. Cogn. Neurosci. 2000, 12, 174–187. [Google Scholar] [CrossRef] [PubMed]
- Blum, L.; Rosenbaum, D.; Röben, B.; Dehnen, K.; Maetzler, W.; Suenkel, U.; Fallgatter, A.J.; Ehlis, A.-C.; Metzger, F.G. Age-related deterioration of performance and increase of cortex activity comparing time-versus item-controlled fnirs measurement. Sci. Rep. 2021, 11, 6766. [Google Scholar] [CrossRef]
- Vermeij, A.; van Beek, A.H.E.; Reijs, B.L.R.; Claassen, J.A.H.; Kessels, R.P.C. An exploratory study of the effects of spatial working-memory load on prefrontal activation in low- and high-performing elderly. Front. Aging Neurosci. 2014, 6, 303. [Google Scholar] [CrossRef]
- Verner, M.; Herrmann, M.J.; Troche, S.J.; Roebers, C.M.; Rammsayer, T.H. Cortical oxygen consumption in mental arithmetic as a function of task difficulty: A near-infrared spectroscopy approach. Front. Hum. Neurosci. 2013, 7, 217. [Google Scholar] [CrossRef]
- Pfurtscheller, G.; Bauernfeind, G.; Wriessnegger, S.C.; Neuper, C. Focal frontal (de)oxyhemoglobin responses during simple arithmetic. Int. J. Psychophysiol. 2010, 76, 186–192. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Stein, T.; Maslowski, N.; Neumann, J. Neural correlates of alzheimer’s disease and mild cognitive impairment: A systematic and quantitative meta-analysis involving 1351 patients. NeuroImage 2009, 47, 1196–1206. [Google Scholar] [CrossRef] [PubMed]
- Yeung, M.K.; Sze, S.L.; Woo, J.; Kwok, T.; Shum, D.H.K.; Yu, R.; Chan, A.S. Altered Frontal Lateralization Underlies the Category Fluency Deficits in Older Adults with Mild Cognitive Impairment: A Near-Infrared Spectroscopy Study. Front. Aging Neurosci. 2016, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.J.; Linden, K.G.; Weinberg, H.S. Evaluation of uv irradiation for photolytic and oxidative degradation of pharmaceutical compounds in water. Water Res. 2007, 41, 4413–4423. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. Homer: A review of time-series analysis methods for near-infrared spectroscopy of the brain. Appl. Opt. 2009, 48, D280–D298. [Google Scholar] [CrossRef] [PubMed]
- Tachtsidis, I.; Scholkmann, F. False positives and false negatives in functional near-infrared spectroscopy: Issues, challenges, and the way forward. Neurophotonics 2016, 3, 031405. [Google Scholar] [CrossRef] [PubMed]
- von Lühmann, A.; Li, X.; Müller, K.-R.; Boas, D.A.; Yücel, M.A. Improved physiological noise regression in fnirs: A multimodal extension of the general linear model using temporally embedded canonical correlation analysis. NeuroImage 2020, 208, 116472. [Google Scholar] [CrossRef] [PubMed]
- Santosa, H.; Zhai, X.; Fishburn, F.; Huppert, T. The nirs brain analyzir toolbox. Algorithms 2018, 11, 73. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Joliot, M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Nagata, H.; Yamamoto, Y.; Sakaguchi, R. P2-123: Our newly developed Simple Cognitive test can be also used by computer or paper. Alzheimer’s Dement. 2010, 6, S349. [Google Scholar] [CrossRef]
- Ichii, S.; Nakamura, T.; Kawarabayashi, T.; Takatama, M.; Ohgami, T.; Ihara, K.; Shoji, M. Cogevo, a cognitive function balancer, is a sensitive and easy psychiatric test battery for age-related cognitive decline. Geriatr. Gerontol. Int. 2020, 20, 248–255. [Google Scholar] [CrossRef]
- Takechi, H.; Yoshino, H. Usefulness of cogevo, a computerized cognitive assessment and training tool, for distinguishing patients with mild alzheimer’s disease and mild cognitive impairment from cognitively normal older people. Geriatr. Gerontol. Int. 2021, 21, 192–196. [Google Scholar] [CrossRef]
- Sawada, Y.; Satoh, T.; Saba, H.; Kitamoto, H.; Kato, Y.; Shiozuka, Y.; Kuwada, T.; Murakami, K.; Sasaki, M.; Abe, Y.; et al. Validity and reliability of a computerized cognitive function evaluation battery (CogEvo) as a screening tool. Psychiatry Clin. Neurosci. Rep. 2023, 2, e67. [Google Scholar] [CrossRef]
- Yoon, J.; Isoda, H.; Okura, T. Evaluation of beneficial effect of a dual-task exercise based on japanese transitional games in older adults: A pilot study. Aging 2020, 12, 18957. [Google Scholar] [CrossRef] [PubMed]
- Llinàs-Reglà, J.; Vilalta-Franch, J.; López-Pousa, S.; Calvó-Perxas, L.; Rodas, D.T.; Garre-Olmo, J. The Trail Making Test. Assessment 2017, 24, 183–196. [Google Scholar] [CrossRef]
- Abe, T.; Jindo, T.; Soma, Y.; Tsunoda, K.; Kitano, N.; Yoon, J.Y.; Okura, T. Validity and reliability of the “Trail Making Peg” test as a performance measurement for evaluating the cognitive function. Nihon Ronen Igakkai Zasshi 2015, 52, 71–78. (In Japanese) [Google Scholar] [CrossRef] [PubMed][Green Version]
- Strangman, G.; Culver, J.P.; Thompson, J.H.; Boas, D.A. A quantitative comparison of simultaneous BOLD fMRI and NIRS recordings during functional brain activation. NeuroImage 2002, 17, 719–731. [Google Scholar] [CrossRef]
- Cui, X.; Bray, S.; Bryant, D.M.; Glover, G.H.; Reiss, A.L. A quantitative comparison of NIRS and fMRI across multiple cognitive tasks. NeuroImage 2011, 54, 2808–2821. [Google Scholar] [CrossRef] [PubMed]
- Huppert, T.J.; Hoge, R.D.; Diamond, S.G.; Franceschini, M.A.; Boas, D.A. A temporal comparison of bold, asl, and NIRS hemodynamic responses to motor stimuli in adult humans. NeuroImage 2006, 29, 368–382. [Google Scholar] [CrossRef]
- Sasai, S.; Homae, F.; Watanabe, H.; Sasaki, A.T.; Tanabe, H.C.; Sadato, N.; Taga, G. A NIRS–fMRI study of resting state network. NeuroImage 2012, 63, 179–193. [Google Scholar] [CrossRef]
- Huo, C.; Xu, G.; Li, W.; Xie, H.; Zhang, T.; Liu, Y.; Li, Z. A review on functional near-infrared spectroscopy and application in stroke rehabilitation. Med. Nov. Technol. Devices 2021, 11, 100064. [Google Scholar] [CrossRef]
- Santosa, H.; Zhai, X.; Fishburn, F.; Sparto, P.J.; Huppert, T.J. Quantitative comparison of correction techniques for removing systemic physiological signal in functional near-infrared spectroscopy studies. Neurophotonics 2020, 7, 035009. [Google Scholar] [CrossRef] [PubMed]
- Seghier, M.L. Laterality index in functional MRI: Methodological issues. Magn. Reson. Imaging 2008, 26, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, E.; Maki, A.; Kawaguchi, F.; Takashiro, K.; Yamashita, Y.; Koizumi, H.; Mayanagi, Y. Non-invasive assessment of language dominance with near-infrared spectroscopic mapping. Neurosci. Lett. 1998, 256, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Bierre, K.L.; Lucas, S.J.E.; Guiney, H.; Cotter, J.D.; Machado, L. Cognitive difficulty intensifies age-related changes in anterior frontal hemodynamics: Novel evidence from near-infrared spectroscopy. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2017, 72, 181–188. [Google Scholar] [CrossRef]
- Fandakova, Y.; Sander, M.C.; Werkle-Bergner, M.; Shing, Y.L. Age differences in short-term memory binding are related to working memory performance across the lifespan. Psychol. Aging 2014, 29, 140–149. [Google Scholar] [CrossRef]
- Fournet, N.; Roulin, J.-L.; Vallet, F.; Beaudoin, M.; Agrigoroaei, S.; Paignon, A.; Dantzer, C.; Desrichard, O. Evaluating short-term and working memory in older adults: French normative data. Aging Ment. Health 2012, 16, 922–930. [Google Scholar] [CrossRef]



| Characteristics | Expert Older Group | Non-Expert Older Group (Older Control) | Younger Group (Non-Expert Younger Control) | |
|---|---|---|---|---|
| Age (years) | 75.1 ± 4.3 | 74.1 ± 4.5 | 22.5 ± 0.8 | |
| Number of days of Wellness Darts experience (days) | 170.7 ± 145.6 | 0.0 | 0.0 | |
| Cognitive tests | SC test | 41.1 ± 7.8 | 44.5 ± 5.8 | 48.8 ± 2.5 |
| Computational ability test | 20.5 ± 3.8 | 17.1 ± 5.5 | 24.0 ± 3.8 | |
| STM test | 57.1 ± 9.5 | 49.4 ± 9.9 | 74.6 ± 13.6 | |
| TMPT | 58.1 ± 8.5 | 58.9 ± 9.2 | 41.1 ± 4.5 | |
| CogEvo: disorientation | 361.8 ± 52.7 | 278.2 ± 74.5 | 341.5 ± 68.8 | |
| CogEvo: visual search | 177.9 ± 39.0 | 161.3 ± 33.8 | 282.0 ± 41.3 | |
| CogEvo: flashlight | 364.3 ± 146.9 | 308.1 ± 106.4 | 604.3 ± 169.2 | |
| CogEvo: number step | 197.4 ± 40.8 | 173.5 ± 45.9 | 280.9 ± 42.8 | |
| CogEvo: just fit | 332.2 ± 104.9 | 342.4 ± 98.7 | 442.1 ± 78.2 | |
| Dart-throwing performance | 49.9 ± 5.5 | 31.9 ± 13.7 | 39.3 ± 13.4 | |
| Condition | Anatomical Region | t-Value | p-Value |
|---|---|---|---|
| (FDR-Corrected) | |||
| planning | IPL.L | 0.15 | 0.909 |
| MFG.L | 1.931 | 0.186 | |
| MFG.R | 3.321 | 0.022 | |
| SFGdor.L | 3.155 | 0.022 | |
| SFGdor.R | 3.822 | 0.012 | |
| calculation | IPL.L | 0.139 | 0.454 |
| MFG.L | 3.321 | 0.022 | |
| MFG.R | 3.321 | 0.022 | |
| SFGdor.L | 2.866 | 0.052 | |
| SFGdor.R | 5.847 | 5.72 × 10−5 |
| Condition | Anatomical Region | t-Value | p-Value |
|---|---|---|---|
| (FDR-Corrected) | |||
| planning | IPL.L | 0.127 | 0.988 |
| IPL.R | 1.706 | 0.548 | |
| MFG.L | −0.163 | 0.988 | |
| MFG.R | 1.238 | 0.709 | |
| SFGdor.L | 0.343 | 0.988 | |
| SFGdor.R | 1.040 | 0.817 | |
| calculation | IPL.L | 0.545 | 0.723 |
| IPL.R | 0.367 | 0.820 | |
| MFG.L | 0.999 | 0.495 | |
| MFG.R | 1.542 | 0.289 | |
| SFGdor.L | 1.115 | 0.272 | |
| SFGdor.R | 0.989 | 0.495 |
| Condition | Anatomical Region | t-Value | p-Value |
|---|---|---|---|
| (FDR-Corrected) | |||
| planning | IPL.L | 2.282 | 0.385 |
| MFG.L | 0.446 | 0.943 | |
| MFG.R | −0.928 | 0.794 | |
| SFGdor.L | −0.331 | 0.943 | |
| SFGdor.R | −0.684 | 0.904 | |
| calculation | IPL.L | 1.234 | 0.525 |
| MFG.L | 0.309 | 0.876 | |
| MFG.R | −1.118 | 0.540 | |
| SFGdor.L | −0.496 | 0.803 | |
| SFGdor.R | −0.469 | 0.815 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toyofuku, K.; Hiwa, S.; Tanioka, K.; Hiroyasu, T.; Takeda, M. Hemispheric Lateralization in Older Adults Who Habitually Play Darts: A Cross-Sectional Study Using Functional Near-Infrared Spectroscopy. Healthcare 2024, 12, 734. https://doi.org/10.3390/healthcare12070734
Toyofuku K, Hiwa S, Tanioka K, Hiroyasu T, Takeda M. Hemispheric Lateralization in Older Adults Who Habitually Play Darts: A Cross-Sectional Study Using Functional Near-Infrared Spectroscopy. Healthcare. 2024; 12(7):734. https://doi.org/10.3390/healthcare12070734
Chicago/Turabian StyleToyofuku, Koki, Satoru Hiwa, Kensuke Tanioka, Tomoyuki Hiroyasu, and Masaki Takeda. 2024. "Hemispheric Lateralization in Older Adults Who Habitually Play Darts: A Cross-Sectional Study Using Functional Near-Infrared Spectroscopy" Healthcare 12, no. 7: 734. https://doi.org/10.3390/healthcare12070734
APA StyleToyofuku, K., Hiwa, S., Tanioka, K., Hiroyasu, T., & Takeda, M. (2024). Hemispheric Lateralization in Older Adults Who Habitually Play Darts: A Cross-Sectional Study Using Functional Near-Infrared Spectroscopy. Healthcare, 12(7), 734. https://doi.org/10.3390/healthcare12070734






