Effects of Static Meditation Practice on Blood Lipid Levels: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
1.1. Background
1.2. Research Aim
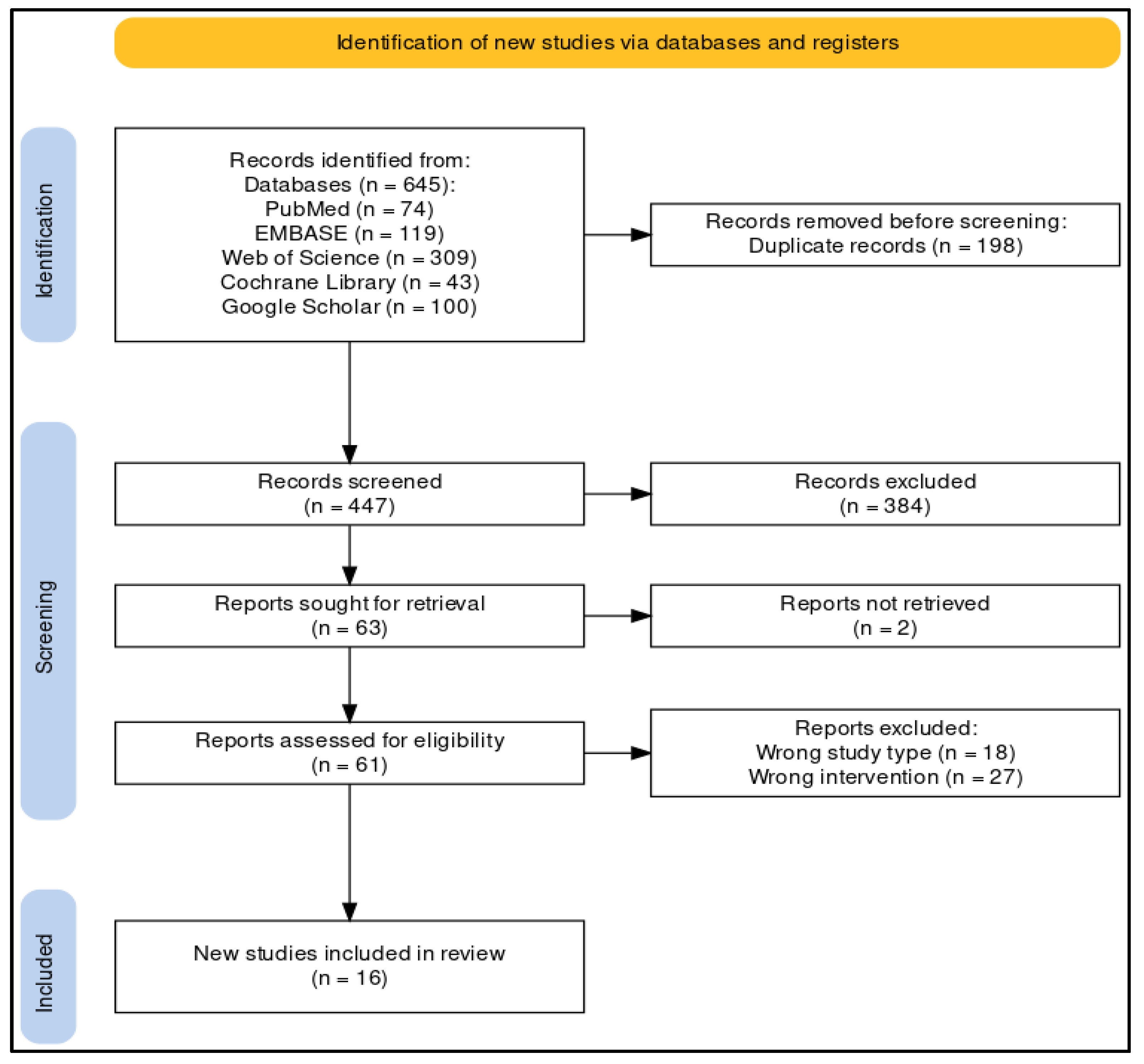
2. Methods
2.1. Eligibility Criteria
- P (population): individuals categorized as either healthy subjects or patients with specific disorders, with a particular emphasis on those with cardiometabolic risk factors or diseases.
- I (intervention): all forms of static meditation practiced for varying durations. Studies wherein the meditation intervention was combined with other approaches, such as dietary recommendations, massage, or aerobic physical exercises, were excluded to prevent confounding factors.
- C (comparison): the comparison category encompassed any type, including scenarios with no control.
- O (outcomes): significant alterations in blood lipid levels, covering total cholesterol, HDL cholesterol (HDL-C), LDL cholesterol (LDL-C), and triglycerides.
- S (study design): all clinical investigations comprising both trials and observational studies. Laboratory experiments conducted in vitro or in vivo with animal or cell models were intentionally excluded from the primary search.
2.2. Information Sources
2.3. Search Strategy
2.4. Selection Process
2.5. Data Collection Process
2.6. Data Items and Effect Measures
2.7. Study Risk of Bias Assessment
2.8. Synthesis Methods
- P (population): patients afflicted with chronic diseases or healthy individuals (considering only per-protocol and not intention-to-treat study populations).
- I (intervention): all forms of static meditation.
- C (comparison): no meditation.
- O (outcomes): end-of-study (or change-from-baseline) blood values of total cholesterol, HDL-C, LDL-C, and triglycerides. When necessary, data were converted from mmol/L to mg/dL.
3. Results
3.1. Qualitative Synthesis of the Available Evidence
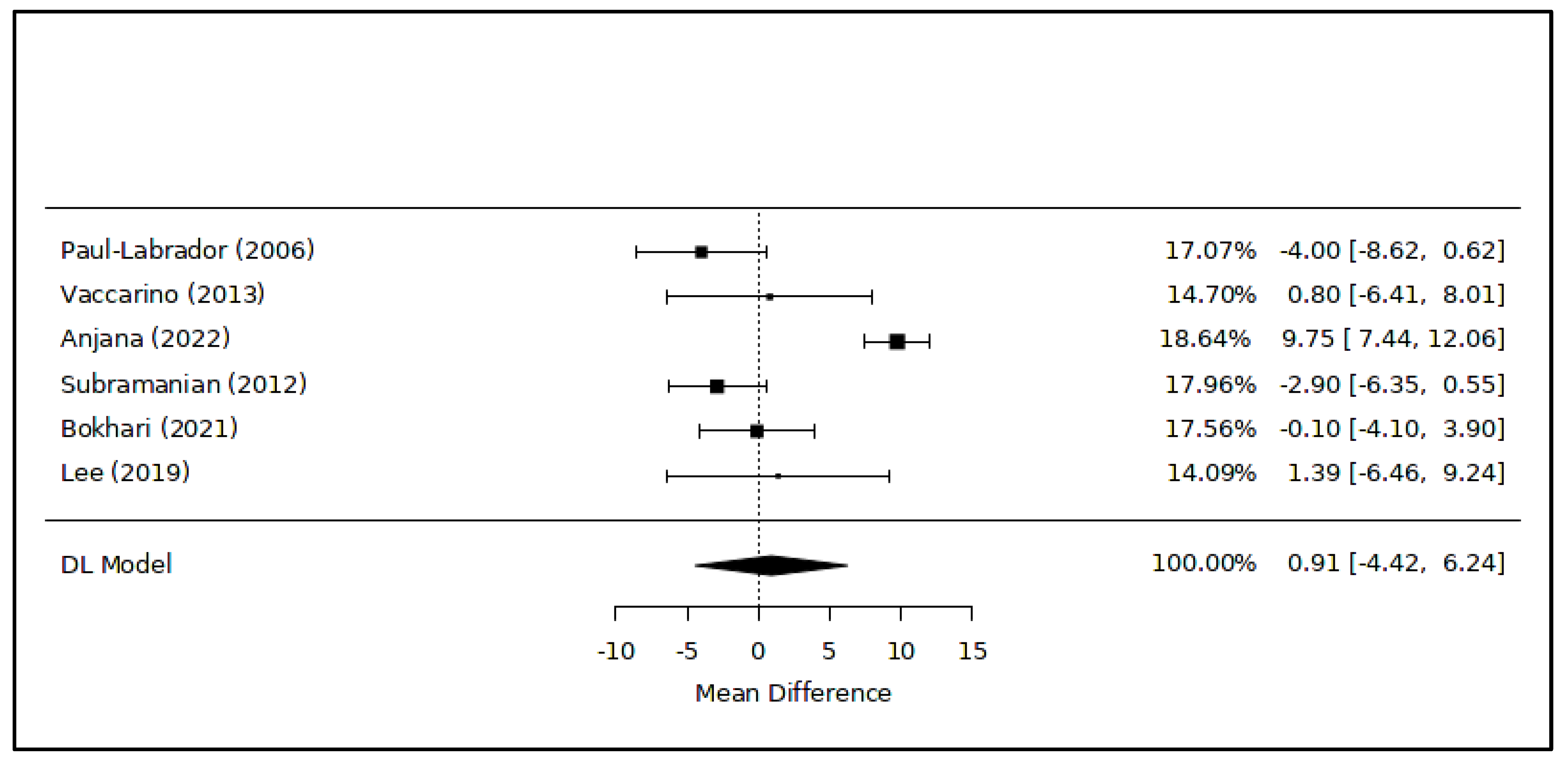
3.2. Meta-Analysis of Quantitative Results
4. Discussion
4.1. Brief Summary of the Available Evidence and Potential Explanations
4.2. Current Guidelines and Practical Implications of the Study Results
4.3. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sampaio, C.V.S.; Lima, M.G.; Ladeia, A.M. Meditation, Health and Scientific Investigations: Review of the Literature. J. Relig. Health 2017, 56, 411–427. [Google Scholar] [CrossRef] [PubMed]
- Sharma, H. Meditation: Process and Effects. AYU 2015, 36, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, R.; de Souza, E.; Camano, L.; Leite, J.R. Meditation in Health: An Operational Definition. Brain Res. Brain Res. Protoc. 2004, 14, 58–60. [Google Scholar] [CrossRef] [PubMed]
- Wallace, R.K.; Benson, H.; Wilson, A.F. A Wakeful Hypometabolic Physiologic State. Am. J. Physiol. 1971, 221, 795–799. [Google Scholar] [CrossRef]
- Goleman, D. Meditation and Consciousness: An Asian Approach to Mental Health. Am. J. Psychother. 1976, 30, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Craven, J.L. Meditation and Psychotherapy. Can. J. Psychiatry 1989, 34, 648–653. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, B. Issues and Perspectives in Meditation Research: In Search for a Definition. Front. Psychol. 2012, 3, 613. [Google Scholar] [CrossRef]
- Bachen, E.A.; Muldoon, M.F.; Matthews, K.A.; Manuck, S.B. Effects of Hemoconcentration and Sympathetic Activation on Serum Lipid Responses to Brief Mental Stress. Psychosom. Med. 2002, 64, 587–594. [Google Scholar] [CrossRef]
- Qi, Z.; Ding, S. Obesity-Associated Sympathetic Overactivity in Children and Adolescents: The Role of Catecholamine Resistance in Lipid Metabolism. J. Pediatr. Endocrinol. Metab. 2016, 29, 113–125. [Google Scholar] [CrossRef]
- Nestel, P.J.; Khan, A.A.; Straznicky, N.E.; Mellett, N.A.; Jayawardana, K.; Mundra, P.A.; Lambert, G.W.; Meikle, P.J. Markers of Sympathetic Nervous System Activity Associate with Complex Plasma Lipids in Metabolic Syndrome Subjects. Atherosclerosis 2017, 256, 21–28. [Google Scholar] [CrossRef]
- Muscella, A.; Stefàno, E.; Marsigliante, S. The Effects of Exercise Training on Lipid Metabolism and Coronary Heart Disease. Am. J. Physiol. Heart Circ. Physiol. 2020, 319, H76–H88. [Google Scholar] [CrossRef]
- Park, B.J.; Tsunetsugu, Y.; Kasetani, T.; Kagawa, T.; Miyazaki, Y. The Physiological Effects of Shinrin-Yoku (taking in the Forest Atmosphere or Forest Bathing): Evidence from Field Experiments in 24 Forests across Japan. Environ. Health Prev. Med. 2010, 15, 18–26. [Google Scholar] [CrossRef]
- Toneatto, T.; Nguyen, L. Does Mindfulness Meditation Improve Anxiety and Mood Symptoms? A Review of the Controlled Research. Can. J. Psychiatry 2007, 52, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Waechter, R.L.; Wekerle, C. Promoting Resilience among Maltreated Youth Using Meditation, Yoga, Tai Chi and Qigong: A Scoping Review of the Literature. Child Adolesc. Social Work J. 2015, 32, 17–31. [Google Scholar] [CrossRef]
- Goyal, M.; Singh, S.; Sibinga, E.M.S.; Gould, N.F.; Rowland-Seymour, A.; Sharma, R.; Berger, Z.; Sleicher, D.; Maron, D.D.; Shihab, H.M.; et al. Meditation Programs for Psychological Stress and Well-Being: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2014, 174, 357–368. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Zhang, L.; Zhuang, J.-H.; Xu, J.; Li, P.; Peng, H. The Effects of Different Meditation Exercises on Sleep Quality in Older People: A Network Meta-Analysis. Eur. Geriatr. Med. 2019, 10, 543–552. [Google Scholar] [CrossRef] [PubMed]
- Gard, T.; Hölzel, B.K.; Lazar, S.W. The Potential Effects of Meditation on Age-Related Cognitive Decline: A Systematic Review. Ann. N. Y. Acad. Sci. 2014, 1307, 89–103. [Google Scholar] [CrossRef]
- Nascimento, S.S.; Oliveira, L.R.; DeSantana, J.M. Correlations between Brain Changes and Pain Management after Cognitive and Meditative Therapies: A Systematic Review of Neuroimaging Studies. Complement. Ther. Med. 2018, 39, 137–145. [Google Scholar] [CrossRef]
- Black, D.S.; Slavich, G.M. Mindfulness Meditation and the Immune System: A Systematic Review of Randomized Controlled Trials. Ann. N. Y. Acad. Sci. 2016, 1373, 13–24. [Google Scholar] [CrossRef]
- Buric, I.; Farias, M.; Jong, J.; Mee, C.; Brazil, I.A. What Is the Molecular Signature of Mind-Body Interventions? A Systematic Review of Gene Expression Changes Induced by Meditation and Related Practices. Front. Immunol. 2017, 8, 670. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, D.; Wang, L.; Zhuang, J.; Cook, R.; Chen, L. Meditation and Blood Pressure: A Meta-Analysis of Randomized Clinical Trials. J. Hypertens. 2017, 35, 696–706. [Google Scholar] [CrossRef] [PubMed]
- Bai, Z.; Chang, J.; Chen, C.; Li, P.; Yang, K.; Chi, I. Investigating the Effect of Transcendental Meditation on Blood Pressure: A Systematic Review and Meta-Analysis. J. Hum. Hypertens. 2015, 29, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Klimecki, O.; Marchant, N.L.; Lutz, A.; Poisnel, G.; Chételat, G.; Collette, F. The Impact of Meditation on Healthy Ageing—The Current State of Knowledge and a Roadmap to Future Directions. Curr. Opin. Psychol. 2019, 28, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.K.; Loprinzi, P.D. Comparative Effects of Meditation and Exercise on Physical and Psychosocial Health Outcomes: A Review of Randomized Controlled Trials. Postgrad. Med. 2018, 130, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.H.; Grim, C.E.; Rainforth, M.V.; Kotchen, T.; Nidich, S.I.; Gaylord-King, C.; Salerno, J.W.; Kotchen, J.M.; Alexander, C.N. Stress Reduction in the Secondary Prevention of Cardiovascular Disease: Randomized, Controlled Trial of Transcendental Meditation and Health Education in Blacks. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 750–758. [Google Scholar] [CrossRef] [PubMed]
- Barnes, V.A.; Orme-Johnson, D.W. Prevention and Treatment of Cardiovascular Disease in Adolescents and Adults through the Transcendental Meditation(®) Program: A Research Review Update. Curr. Hypertens. Rev. 2012, 8, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Schneider, R.H.; Carr, T. Transcendental Meditation in the Prevention and Treatment of Cardiovascular Disease and Pathophysiological Mechanisms: An Evidence-Based Review. Adv. Integr. Med. 2014, 1, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, S.C.; Madan, K. Yoga and Meditation in Cardiovascular Disease. Clin. Res. Cardiol. 2014, 103, 675–680. [Google Scholar] [CrossRef]
- Loucks, E.B.; Schuman-Olivier, Z.; Britton, W.B.; Fresco, D.M.; Desbordes, G.; Brewer, J.A.; Fulwiler, C. Mindfulness and Cardiovascular Disease Risk: State of the Evidence, Plausible Mechanisms, and Theoretical Framework. Curr. Cardiol. Rep. 2015, 17, 112. [Google Scholar] [CrossRef]
- Ghazvineh, D.; Daneshvar, M.; Basirat, V.; Daneshzad, E. The Effect of Yoga on the Lipid Profile: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Front. Nutr. 2022, 9, 942702. [Google Scholar] [CrossRef]
- Vera, F.M.; Manzaneque, J.M.; Maldonado, E.F.; Carranque, G.A.; Cubero, V.M.; Blanca, M.J.; Morell, M. Biochemical Changes after a Qigong Program: Lipids, Serum Enzymes, Urea, and Creatinine in Healthy Subjects. Med. Sci. Monit. 2007, 13, CR560–CR566. [Google Scholar] [PubMed]
- Kormanovski, A.; Padilla, E.L.; Harasymowicz, J. Metabolic Effects of a Zen Meditation and Qigong Training Program in Experienced Meditation Instructors. Arch. Budo 2008, 4, 59–64. [Google Scholar]
- Kormanovski, A.; Padilla, E.L.; Campos-Rodríguez, R.; Harasymowicz, J. Metabolic Effects of a Zen Meditation and Qigong Training Program on Sedentary People. Arch. Budo 2009, 5, 15–19. [Google Scholar]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. Int. J. Surg. 2021, 88, 105906. [Google Scholar] [CrossRef] [PubMed]
- Bramer, W.M.; Rethlefsen, M.L.; Kleijnen, J.; Franco, O.H. Optimal Database Combinations for Literature Searches in Systematic Reviews: A Prospective Exploratory Study. Syst. Rev. 2017, 6, 245. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s Tool for Assessing Risk of Bias in Randomised Trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Jørgensen, L.; Paludan-Müller, A.S.; Laursen, D.R.T.; Savović, J.; Boutron, I.; Sterne, J.A.C.; Higgins, J.P.T.; Hróbjartsson, A. Evaluation of the Cochrane Tool for Assessing Risk of Bias in Randomized Clinical Trials: Overview of Published Comments and Analysis of User Practice in Cochrane and Non-Cochrane Reviews. Syst. Rev. 2016, 5, 80. [Google Scholar] [CrossRef] [PubMed]
- metaHUN: A Web Tool for Meta Analysis. Available online: http://softmed.hacettepe.edu.tr/metaHUN/ (accessed on 21 January 2024).
- Cuijpers, P.; Weitz, E.; Cristea, I.A.; Twisk, J. Pre-Post Effect Sizes Should Be Avoided in Meta-Analyses. Epidemiol. Psychiatr. Sci. 2017, 26, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons, Ltd: Chichester, UK, 2008; ISBN 9780470712184. [Google Scholar]
- IntHout, J.; Ioannidis, J.P.A.; Borm, G.F. The Hartung-Knapp-Sidik-Jonkman Method for Random Effects Meta-Analysis Is Straightforward and Considerably Outperforms the Standard DerSimonian-Laird Method. BMC Med. Res. Methodol. 2014, 14, 25. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.T. Cochrane Handbook for Systematic Reviews of Interventions, 2nd ed.; Wiley Cochrane Series; Higgins, J., Thomas, J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2019; ISBN 9781119536604. [Google Scholar]
- Paul-Labrador, M.; Polk, D.; Dwyer, J.H.; Velasquez, I.; Nidich, S.; Rainforth, M.; Schneider, R.; Merz, C.N.B. Effects of a Randomized Controlled Trial of Transcendental Meditation on Components of the Metabolic Syndrome in Subjects with Coronary Heart Disease. Arch. Intern. Med. 2006, 166, 1218–1224. [Google Scholar] [CrossRef]
- Vaccarino, V.; Kondwani, K.A.; Kelley, M.E.; Murrah, N.V.; Boyd, L.; Ahmed, Y.; Meng, Y.X.; Gibbons, G.H.; Hooper, W.C.; De Staercke, C.; et al. Effect of Meditation on Endothelial Function in Black Americans with Metabolic Syndrome: A Randomized Trial. Psychosom. Med. 2013, 75, 591–599. [Google Scholar] [CrossRef] [PubMed]
- Anjana, K.; Archana, R.; Mukkadan, J.K. Effect of Om Chanting and Yoga Nidra on Blood Pressure and Lipid Profile in Hypertension—A Randomized Controlled Trial. J. Ayurveda Integr. Med. 2022, 13, 100657. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, S.; Elango, T.; Malligarjunan, H.; Kochupillai, V.; Dayalan, H. Role of Sudarshan Kriya and Pranayam on Lipid Profile and Blood Cell Parameters during Exam Stress: A Randomized Controlled Trial. Int. J. Yoga 2012, 5, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Bokhari, S.; Schneider, R.H.; Salerno, J.W.; Rainforth, M.V.; Gaylord-King, C.; Nidich, S.I. Effects of Cardiac Rehabilitation with and without Meditation on Myocardial Blood Flow Using Quantitative Positron Emission Tomography: A Pilot Study. J. Nucl. Cardiol. 2021, 28, 1596–1607. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-H.; Hwang, S.-M.; Kang, D.-H.; Yang, H.-J. Brain Education-Based Meditation for Patients with Hypertension And/or Type 2 Diabetes: A Pilot Randomized Controlled Trial. Medicine 2019, 98, e15574. [Google Scholar] [CrossRef] [PubMed]
- Patel, C.; Carruthers, M. Coronary Risk Factor Reduction through Biofeedback-Aided Relaxation and Meditation. J. R. Coll. Gen. Pract. 1977, 27, 401–405. [Google Scholar] [PubMed]
- Bhatnagar, P.; Srivastava, M.; Vinay, A. Physiological and Biochemical Responses of Transcendental Meditation in Females. Int. J. Sci. Study 2015, 2, 18–23. [Google Scholar]
- Cooper, M.J.; Aygen, M.M. A Relaxation Technique in the Management of Hypercholesterolemia. J. Human Stress 1979, 5, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Li, H.; Wang, M.-T.; Shi, Y.; Shi, K.; Cheng, Y.; Cui, D.-H. Mindfulness Meditation Improves Metabolic Profiles in Healthy and Depressive Participants. CNS Neurosci. Ther. 2018, 24, 572–574. [Google Scholar] [CrossRef]
- Kumbukgolla, W.; Jayaweera, J.A.A.S.; Perera, P.; Hale, S. Detection of Serum High-Density Lipoprotein Cholesterol High Levels in Monks Practicing Samatha and Vipassana Meditation. Eur. J. Integr. Med. 2019, 28, 47–51. [Google Scholar] [CrossRef]
- Xue, T.; Chiao, B.; Xu, T.; Li, H.; Shi, K.; Cheng, Y.; Shi, Y.; Guo, X.; Tong, S.; Guo, M.; et al. The Heart-Brain Axis: A Proteomics Study of Meditation on the Cardiovascular System of Tibetan Monks. EBioMedicine 2022, 80, 104026. [Google Scholar] [CrossRef] [PubMed]
- Vyas, R.; Dikshit, N. Effect of Meditation on Respiratory System, Cardiovascular System and Lipid Profile. Indian J. Physiol. Pharmacol. 2002, 46, 487–491. [Google Scholar] [PubMed]
- Sung, M.-K.; Lee, U.S.; Ha, N.H.; Koh, E.; Yang, H.-J. A Potential Association of Meditation with Menopausal Symptoms and Blood Chemistry in Healthy Women: A Pilot Cross-Sectional Study. Medicine 2020, 99, e22048. [Google Scholar] [CrossRef]
- Vyas, R.; Raval, K.V.; Dikshit, N. Effect of Raja Yoga Meditation on the Lipid Profile of Post-Menopausal Women. Indian J. Physiol. Pharmacol. 2008, 52, 420–424. [Google Scholar] [PubMed]
- Naruka, J.S.; Mathur, R.; Mathur, A. Effect of Pranayama Practices on Fasting Blood Glucose and Serum Cholesterol. Indian J. Med. Sci. 1986, 40, 149–152. [Google Scholar] [PubMed]
- Cooper, M.; Aygen, M. [Effect of meditation on blood cholesterol and blood pressure]. Harefuah 1978, 95, 1–2. [Google Scholar] [PubMed]
- Haddaway, N.R.; Page, M.J.; Pritchard, C.C.; McGuinness, L.A. An R Package and Shiny App for Producing PRISMA 2020-Compliant Flow Diagrams, with Interactivity for Optimised Digital Transparency and Open Synthesis. Campbell Syst. Rev. 2022, 18, e1230. [Google Scholar] [CrossRef]
- Gerritsen, R.J.S.; Band, G.P.H. Breath of Life: The Respiratory Vagal Stimulation Model of Contemplative Activity. Front. Hum. Neurosci. 2018, 12, 397. [Google Scholar] [CrossRef]
- Ben-Soussan, T.D.; Srinivasan, N.; Glicksohn, J.; Beziau, J.-Y.; Carducci, F.; Berkovich-Ohana, A. Editorial: Neurophysiology of Silence: Neuroscientific, Psychological, Educational and Contemplative Perspectives. Front. Psychol. 2021, 12, 675614. [Google Scholar] [CrossRef]
- Pistorio, E.; Luca, M.; Luca, A.; Messina, V.; Calandra, C. Autonomic Nervous System and Lipid Metabolism: Findings in Anxious-Depressive Spectrum and Eating Disorders. Lipids Health Dis. 2011, 10, 192. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, D.; Cline, M.A.; Gilbert, E.R. Chronic Stress, Epigenetics, and Adipose Tissue Metabolism in the Obese State. Nutr. Metab. 2020, 17, 88. [Google Scholar] [CrossRef] [PubMed]
- Kyrou, I.; Tsigos, C. Stress Hormones: Physiological Stress and Regulation of Metabolism. Curr. Opin. Pharmacol. 2009, 9, 787–793. [Google Scholar] [CrossRef] [PubMed]
- Kissebah, A.H. “Stress” Hormones and Lipid Metabolism. Proc. R. Soc. Med. 1974, 67, 665–667. [Google Scholar] [CrossRef] [PubMed]
- Younge, J.O.; Leening, M.J.G.; Tiemeier, H.; Franco, O.H.; Kiefte-de Jong, J.; Hofman, A.; Roos-Hesselink, J.W.; Hunink, M.G.M. Association Between Mind-Body Practice and Cardiometabolic Risk Factors: The Rotterdam Study. Psychosom. Med. 2015, 77, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, S. Health Benefits of Meditation: What the Newest Research Shows. Altern. Complement. Ther. 2010, 16, 223–228. [Google Scholar] [CrossRef]
- Monin, J.K.; Sperduto, C.M.; Manigault, A.W.; Dutton, A.; Ali, A.; Clark, M.S.; Jastreboff, A.M. Mindfulnes-Based Stress Reduction for Older Couples with Metabolic Syndrome: A Pilot Randomized Controlled Trial. Mindfulness 2020, 11, 917–927. [Google Scholar] [CrossRef] [PubMed]
- Ray, I.B.; Menezes, A.R.; Malur, P.; Hiltbold, A.E.; Reilly, J.P.; Lavie, C.J. Meditation and Coronary Heart Disease: A Review of the Current Clinical Evidence. Ochsner J. 2014, 14, 696–703. [Google Scholar] [PubMed]
- Levine, G.N.; Lange, R.A.; Bairey-Merz, C.N.; Davidson, R.J.; Jamerson, K.; Mehta, P.K.; Michos, E.D.; Norris, K.; Ray, I.B.; Saban, K.L.; et al. Meditation and Cardiovascular Risk Reduction: A Scientific Statement From the American Heart Association. J. Am. Heart Assoc. 2017, 6, e002218. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Katcher, H.I.; Hill, A.M.; Lanford, J.L.G.; Yoo, J.S.; Kris-Etherton, P.M. Lifestyle Approaches and Dietary Strategies to Lower LDL-Cholesterol and Triglycerides and Raise HDL-Cholesterol. Endocrinol. Metab. Clin. N. Am. 2009, 38, 45–78. [Google Scholar] [CrossRef]
- Dattilo, A.M.; Kris-Etherton, P.M. Effects of Weight Reduction on Blood Lipids and Lipoproteins: A Meta-Analysis. Am. J. Clin. Nutr. 1992, 56, 320–328. [Google Scholar] [CrossRef]
- Ribeiro, S.M.L.; dos Santos Luz, S.; de Cássia Aquino, R. The Role of Nutrition and Physical Activity in Cholesterol and Aging. Clin. Geriatr. Med. 2015, 31, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Visseren, F.L.J.; Mach, F.; Smulders, Y.M.; Carballo, D.; Koskinas, K.C.; Bäck, M.; Benetos, A.; Biffi, A.; Boavida, J.-M.; Capodanno, D.; et al. 2021 ESC Guidelines on Cardiovascular Disease Prevention in Clinical Practice. Eur. Heart J. 2021, 42, 3227–3337. [Google Scholar] [CrossRef] [PubMed]
- Gawlik, K.S.; Melnyk, B.M.; Tan, A. Associations Between Stress and Cardiovascular Disease Risk Factors Among Million Hearts Priority Populations. Am. J. Health Promot. 2019, 33, 1063–1066. [Google Scholar] [CrossRef] [PubMed]
- Osborne, M.T.; Shin, L.M.; Mehta, N.N.; Pitman, R.K.; Fayad, Z.A.; Tawakol, A. Disentangling the Links Between Psychosocial Stress and Cardiovascular Disease. Circ. Cardiovasc. Imaging 2020, 13, e010931. [Google Scholar] [CrossRef]



| Population Characteristics | Intervention (n) | Comparison (n) | Outcomes (Mean ± SD) | Study Design | Reference | |
|---|---|---|---|---|---|---|
| Cholesterol [mg/dL] | Triglycerides [mg/dL] | |||||
| 103 patients with coronary heart disease (age range: 50–80 yo; 84 M—19 F) | Transcendental Meditation every day for 16 weeks, supported by personal lectures and group meetings (n = 52) | No meditation, only a health education program (n = 51) | TC (INT vs. CON): 158.6 ± 24.2 vs. 167.9 ± 28.6 (ns) HDL-C (INT vs. CON): 44.3 ± 8.3 vs. 48.3 ± 14.7 (ns) LDL-C (INT vs. CON): 89.0 ± 21.5 vs. 92.8 ± 30.8 (ns) | TG (INT vs. CON): 126.7 ± 56.2 vs. 152.8 ± 84.9 (ns) | RCT | [43] |
| 68 patients with risk factors for metabolic syndrome (age range: 30–65 yo; 14 M—54 F) | Consciously resting meditation, a mantra-based meditation (20 min) twice a day for 12 months (n = 33) | No meditation, only health education (n = 35) | HDL-C (INT vs. CON): 57.1 ± 17.5 vs. 56.3 ± 12.2 (ns) | TG (INT vs. CON): 109.6 ± 36.6 vs. 123.8 ± 90.1 (*) | RCT | [44] |
| 65 patients with hypertension (age range: 25–60 yo; 29 M—36 F) | Om chanting (5 min) + yoga nidra (20 min) 5 days a week for 2 months (n = 34) | No meditation (n = 31) | TC (INT vs. CON): 237.32 ± 35.07 vs. 250.44 ± 35.50 (*) HDL-C (INT vs. CON): 44.53 ± 3.86 vs. 34.78 ± 5.43 (*) LDL-C (INT vs. CON): 155.93 ± 34.39 vs. 166.66 ± 34.3 (*) | TG (INT vs. CON): 184.83 ± 72.67 vs. 245 ± 45.00 (*) | RCT | [45] |
| 40 students under exam stress (age range: 18–23 yo; 20 M—23 F) | Sudarshan kriya and pranayama meditation (~15 min) every day for 6 weeks (n = 21) | No meditation (n = 19) | TC (INT vs. CON): 146.7 ± 23.6 vs. 168.2 ± 18.2 (*) HDL-C (INT vs. CON): 42.5 ± 7.6 vs. 45.4 ± 2.6 (ns) LDL-C (INT vs. CON): 90.85 ± 27.0 vs. 102.4 ± 12.4 (ns) | TG (INT vs. CON): 71.0 ± 11.4 vs. 102.4 ± 15.1 (*) | RCT | [46] |
| 37 patients with history of coronary heart disease (age range: 50–80 yo; 22 M—15 F) | Transcendental Meditation twice a week + cardiac rehabilitation program (n = 19) | Cardiac rehabilitation program (n = 18) | Changes from baseline values after 12 weeks: TC (INT vs. CON): 0.0 ± 37.0 vs. +14.8 ± 34.0 (ns) HDL-C (INT vs. CON): +1.1 ± 6.0 vs. +1.2 ± 6.4 (ns) LDL-C (INT vs. CON): +0.3 ± 27.0 vs. +10.3 ± 28.1 (ns) | Changes from baseline values after 12 weeks: TG (INT vs. CON): −16.4 ± 76.8 vs. +16.6 ± 33.4 (ns) | RCT | [47] |
| 35 patients with hypertension and type 2 diabetes mellitus (age range: 60–80 yo; 24 M—19 F) | Brain Education Sangdahnjeon meditation twice a week for 8 weeks (n = 21) | No meditation, only health education advice (n = 14) | HDL-C (INT vs. CON): 49.10 ± 13.07 vs. 47.71 ± 10.51 (ns) LDL-C (INT vs. CON): 90.67 ± 35.54 vs. 97.36 ± 38.14 (ns) | N/A | RCT | [48] |
| 76 adults, including 18 smokers, 22 patients with hypertension, and 36 healthy subjects (18 allocated to INT and 18 to CON) (age range: 25–60 yo; 27 M—49 F) | Meditation with biofeedback-aided relaxation (30 min) every day for 6 weeks (n = 58) | No meditation (n = 18) | TC (INT in patients with hypertension vs. CON): 223 ± 44 vs. 234 ± 49 (*) Other comparisons between groups were not statistically significant. | TG (INT in patients with hypertension vs. CON): 132 ± 65 vs. 96 ± 28 Other comparisons between groups were not statistically significant too. | non-RCT | [49] |
| 30 healthy university students (age range: 17–22 yo; 0 M—30 F) | Transcendental Meditation (20 min) twice a day for 12 weeks (n = 15) | Relax with closed eyes (20 min) twice a day for 12 weeks (n = 15) | TC (INT: baseline—6 weeks—12 weeks): 162.5 ± 10.4; 161.2 ± 11.14 (*); 159 ± 11.11 (*) Comparisons between INT and CON values were not described. | N/A | non-RCT | [50] |
| 23 patients with hypercholesterolemia (age range: 40–50 yo; 14 M—9 F) | Transcendental Meditation (20 min) twice a day for 13 months (n = 12) | No meditation (n = 11) | TC (INT vs. CON): 225.0 ± 9.4 vs. 254.0 ± 11.3 (*) | N/A | non-RCT | [51] |
| 17 healthy subjects, including 10 experienced meditators (age range: 30–50 yo; 5 M—12 F) | Zen meditation (1.5 h) five days per week for 6 weeks (n = 7) | No meditation (n = 10) | HDL-C (INT) from 45.2 ± 8.9 to 53.0 ± 12.8 (*) | TG (INT): from 152.3 ± 125.8 to 221.4 ± 163.9 (ns) | non-RCT | [32] |
| 76 adults, including 61 healthy subjects and 15 patients with depression (age range: 18–65 yo; M/F ?) | Mindfulness-based meditation (20 min) every day for 2 months (n = 76) | N/A | TC (pre–post): significant reduction in healthy subjects (*), but not in patients with depression (ns). The same was observed for HDL-C levels (data were only visually displayed). | TG (pre–post): significant reduction in both healthy subjects (*) and patients with depression (*) (data were only visually displayed). | Pre–post study | [52] |
| 252 subjects from Sri Lanka, including 151 Buddhist monks and 101 laymen (age range: 40–60 yo; 252 M—0 F) | Buddhist monks meditating for >6 months according to the principles of Samatha and Vipassana meditation (n = 71) (INT-1) Buddhist monks meditating for <6 months (n = 44) (INT-2) | Buddhist monks not engaged in regular meditation (n = 36) (CON-1) Laymen without experience in meditation (n = 101) (CON-2) | TC (INT-1 vs. INT-2 vs. CON-1 vs. CON-2): 172.5 ± 23.4 vs. 173.3 ± 34.6 vs. 187.8 ± 45.2 vs. 174.6 ± 48.5 (ns) HDL-C (INT-1 vs. INT-2 vs. CON-1 vs. CON-2): 53.8 ± 5.3 (*) vs. 45.6 ± 5.8 vs. 42.8 ± 5.4 vs. 39.1 ± 7.4 LDL-C (INT-1 vs. INT-2 vs. CON-1 vs. CON-2): 93.26 ± 22.5 vs. 101.0 ± 32.4 vs. 114.9 ± 27.8 vs. 119.7 ± 34.9 (ns) | TG (INT-1 vs. INT-2 vs. CON-1 vs. CON-2): 136.5 ± 36.4 vs. 141.8 ± 42.4 vs. 126.1 ± 37.1 vs. 158.9 ± 56.7 (ns) | OS (case–control) | [53] |
| 106 Tibetan healthy subjects (age range: 30–50 yo; 106 M—0 F) | Tibetan monks of 3 sects (Gelug, Nyingma, Sakya) with at least 5 years of experience in meditation practice (n = 48) | Healthy controls with no experience in meditation and the same dietary habits as monks (n = 37) | TC Gelug (n = 29) vs. CON (n = 17): 185.2 ± 40.6 vs. 181.4 ± 38.3 (ns) Nyingma (n = 9) vs. CON (n = 10): 180.2 ± 46.8 vs. 207.7 ± 32.1 (*) Sakya (n = 10) vs. CON (n = 10): 192.2 ± 33.3 vs. 207.7 ± 32.1 (ns) LDL-C Gelug vs. CON: 120.7 ± 39.4 vs. 111.8 ± 33.3 (ns) Nyingma vs. CON: 114.1 ± 35.6 vs. 138.8 ± 31.3 (*) Sakya vs. CON: 119.1 ± 25.5 vs. 138.8 ± 31.3 (*) | N/A | OS (case–control) | [54] |
| 105 adult volunteers (age range: 30–70 yo; 42 M—63 F) | >5 years of experience in Raja Yoga meditation (INT-1) Up to 5 years of experience in Raja Yoga meditation (INT-2) | No experience in meditation (CON) | TC (CON vs. INT-2 vs. INT-1): 293.0 ± 67.9 vs. 240.6 ± 69.1 (*) vs. 235.4 ± 94.5 (*) HDL-C (CON vs. INT-2 vs. INT-1, median (min; max)): 46 (24; 120) vs. 44 (24; 99) vs. 52 (38; 76) (ns) | TG (CON vs. INT-2 vs. INT-1, median (min; max)): 141.5 (64.2; 435.3) vs. 105.0 (19.0; 409.4) vs. 123.0 (51.0; 212.6) (ns) | OS (case–control) | [55] |
| 65 women with menopausal symptoms (age range: 37–56 yo; 0 M—65 F) | Brain Education Sangdahnjeon meditation at least once a week for 6 months (n = 33) | No experience in meditation (n = 32) | Postmenopausal period: HDL-C (INT vs. CON): 65.87 ± 12.95 vs. 58.07 ± 13.53 Significant interaction between group and (pre–post) menopausal state (*) | Perimenopausal period: TG (INT vs. CON): 86.86 ± 43.30 vs. 104.42 ± 63.90 (ns) | OS (case–control) | [56] |
| 49 healthy women (age range: ?; 0 M—49 F) | Pre-(n = 8) and post-(n = 9) menopausal women with >5 years of experience in Raja Yoga meditation (INT-1) Pre-(n = 8) and post-(n = 9) menopausal women with up to 5 years of experience in Raja Yoga meditation (INT-2) | Pre- (n = 7) and post- (n = 8) menopausal women without experience in meditation (CON) | Premenopausal period: TC (CON vs. INT-2 vs. INT-1): 235.0 ± 61.2 vs. 202.5 ± 33.5 vs. 189.4 ± 47.6 (ns) HDL-C (CON vs. INT-2 vs. INT-1): 50.0 ± 26.8 vs. 42.5 ± 5.4 vs. 54.7 ± 11.7 (ns) LDL-C (CON vs. INT-2 vs. INT-1): 164.5 ± 41.4 vs. 137.0 ± 40.6 vs. 112.7 ± 47.0 (ns) Postmenopausal period: TC (CON vs. INT-2 vs. INT-1): 332.3 ± 74.5 vs. 238.8 ± 65.0 (*) vs. 232.2 ± 38.5 (*) HDL-C (CON vs. INT-2 vs. INT-1): 60.5 ± 29.6 vs. 56.6 ± 21.4 vs. 52.3 ± 12.8 (ns) LDL-C (CON vs. INT-2 vs. INT-1): 235.4 ± 68.0 vs. 149.6 ± 79.2 (*) vs. 151.3 ± 37.4 (*) | Premenopausal period: TG (CON vs. INT-2 vs. INT-1): 102.1 ± 43.4 vs. 114.8 ± 55.7 vs. 109.9 ± 40.2 (ns) Post-menopausal period: TG (CON vs. INT-2 vs. INT-1): 181.8 ± 86.0 vs. 163.3 ± 110.1 vs. 143.1 ± 42.0 (ns) | OS (case–control) | [57] |
| Meditation Technique and Duration of Study | Sample Size | CV Risk Factors | TC | HDL-C | LDL-C | TG | Study Design | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Ayurvedic-based meditation techniques | Transcendental Meditation | 16 weeks | 103 | Yes | = | = | = | = | ++++ | [43] |
| 12 weeks | 37 | Yes | = | = | = | = | ++++ | [47] | ||
| 12 weeks | 30 | No | ↓ | N/A | N/A | N/A | +++ | [50] | ||
| 13 months | 23 | Yes | ↓ | N/A | N/A | N/A | +++ | [51] | ||
| Yoga-based meditation techniques (without body movements) | 2 months | 65 | Yes | ↓ | ↑ | ↓ | ↓ | ++++ | [45] | |
| 6 weeks | 40 | No | ↓ | = | = | ↓ | ++++ | [46] | ||
| - | 105 | No | ↓ | = | N/A | = | + | [55] | ||
| - | 49 | No | ↓ | = | ↓ | = | + | [57] | ||
| Mindfulness-based meditation techniques | Mindfulness or consciously resting meditation | 12 months | 68 | Yes | N/A | = | N/A | ↓ | ++++ | [44] |
| 6 weeks | 76 | Yes | ↓ | N/A | N/A | = | +++ | [49] | ||
| 2 months | 76 | No | ↓ | ↓ | N/A | ↓ | ++ | [52] | ||
| Eastern meditation techniques with spiritual origin | Brain Education Sangdahnjeon meditation | 8 weeks | 35 | Yes | N/A | = | = | N/A | ++++ | [48] |
| - | 65 | No | N/A | ↑ | N/A | = | + | [56] | ||
| Spiritual (Zen, Buddhist, Tibetan) meditation | 6 weeks | 17 | No | N/A | ↑ | N/A | = | +++ | [32] | |
| - | 252 | ? | = | ↑ | = | = | + | [53] | ||
| - | 106 | No | ↓ | N/A | ↓ | N/A | + | [54] | ||
| First Author (Date of Publication) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Overall | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Paul-Labrador (2006) | (+) | (+) | / | / | (+) | (+) | (+) | (+) | [43] |
| Vaccarino (2013) | (+) | (+) | / | / | (+) | (+) | (+) | (+) | [44] |
| Anjana (2022) | (+) | (?) | / | / | (+) | (+) | (+) | (?) | [45] |
| Subramanian (2012) | (?) | (?) | / | / | (+) | (+) | (+) | (−) | [46] |
| Bokhari (2021) | (+) | (+) | / | / | (+) | (+) | (+) | (+) | [47] |
| Lee (2019) | (+) | (+) | / | / | (?) | (+) | (+) | (?) | [48] |
| First Author (Date of Publication) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | Study Design | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patel (1977) | N | / | ? | / | / | Y | Y | Y | Y | Y | Y | ? | ? | Y | non-RCT | [49] |
| Bhatnagar (2015) | N | / | ? | / | / | ? | Y | Y | Y | Y | Y | ? | ? | Y | [50] | |
| Cooper (1979) | N | / | ? | / | / | Y | Y | Y | Y | Y | Y | ? | ? | Y | [51] | |
| Kormanovski (2008) | N | / | ? | / | / | Y | Y | Y | Y | Y | Y | ? | ? | Y | [32] | |
| Xue (2018) | Y | Y | Y | Y | ? | Y | Y | / | N | Y | N | / | / | / | Pre–post study | [52] |
| Kumbukgolla (2019) | Y | Y | N | Y | ? | Y | N | ? | Y | Y | / | Y | / | / | OS (case–control) | [53] |
| Xue (2022) | Y | Y | ? | Y | Y | Y | N | Y | Y | Y | / | Y | / | / | [54] | |
| Vyas (2002) | Y | ? | N | Y | Y | Y | ? | ? | ? | ? | / | Y | / | / | [55] | |
| Sung (2020) | Y | Y | Y | Y | Y | Y | ? | Y | Y | Y | / | Y | / | / | [56] | |
| Vyas (2008) | Y | ? | N | Y | Y | Y | ? | ? | ? | ? | / | Y | / | / | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antonelli, M.; Donelli, D.; Gurgoglione, F.L.; Lazzeroni, D.; Halasz, G.; Niccoli, G. Effects of Static Meditation Practice on Blood Lipid Levels: A Systematic Review and Meta-Analysis. Healthcare 2024, 12, 655. https://doi.org/10.3390/healthcare12060655
Antonelli M, Donelli D, Gurgoglione FL, Lazzeroni D, Halasz G, Niccoli G. Effects of Static Meditation Practice on Blood Lipid Levels: A Systematic Review and Meta-Analysis. Healthcare. 2024; 12(6):655. https://doi.org/10.3390/healthcare12060655
Chicago/Turabian StyleAntonelli, Michele, Davide Donelli, Filippo Luca Gurgoglione, Davide Lazzeroni, Geza Halasz, and Giampaolo Niccoli. 2024. "Effects of Static Meditation Practice on Blood Lipid Levels: A Systematic Review and Meta-Analysis" Healthcare 12, no. 6: 655. https://doi.org/10.3390/healthcare12060655
APA StyleAntonelli, M., Donelli, D., Gurgoglione, F. L., Lazzeroni, D., Halasz, G., & Niccoli, G. (2024). Effects of Static Meditation Practice on Blood Lipid Levels: A Systematic Review and Meta-Analysis. Healthcare, 12(6), 655. https://doi.org/10.3390/healthcare12060655