Do Preoperative Corticosteroid Injections Increase the Risk of Infection after Shoulder Arthroscopy or Shoulder Arthroplasty? A Systematic Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Selection
2.2. Inclusion and Exclusion Criteria
2.3. Risk of Bias Assessment
3. Results
3.1. Included Studies
3.2. Articles
3.2.1. Shoulder Arthroplasty
3.2.2. Shoulder Arthroscopy
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tallia, A.F.; Cardone, D.A. Diagnostic and therapeutic injection of the shoulder region. Am. Fam. Physician 2003, 67, 1271–1278. [Google Scholar] [PubMed]
- Coombes, B.K.; Bisset, L.; Vicenzino, B. Efficacy and safety of cortico-steroid injections and other injections for management of tendinopathy: A systematic review of randomised controlled trials. Lancet 2010, 376, 1751–1767. [Google Scholar] [CrossRef] [PubMed]
- Stitik, T.P.; Foye, P.M.; Fossati, J. Shoulder injections for osteoarthritis and other disorders. Phys. Med. Rehabil. Clin. N. Am. 2004, 15, 407–446. [Google Scholar] [CrossRef] [PubMed]
- Lutsky, K.F.; Lucenti, L.; Banner, L.; Matzon, J.; Beredjiklian, P.K. The Effect of Intraoperative Corticosteroid Injections on the Risk of Surgical Site Infections for Hand Procedures. J. Hand Surg. Am. 2019, 44, 840–845. [Google Scholar] [CrossRef]
- Bedard, N.A.; Pugely, A.J.; Elkins, J.M.; Duchman, K.R.; Westermann, R.W.; Liu, S.S.; Gao, Y.; Callaghan, J.J. The John N. Insall Award: Do Intraarticular Injections Increase the Risk of Infection After TKA? Clin. Orthop. Relat. Res. 2017, 475, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Schairer, W.W.; Nwachukwu, B.U.; Mayman, D.J.; Lyman, S.; Jerabek, S.A. Preoperative Hip Injections Increase the Rate of Periprosthetic Infection After Total Hip Arthroplasty. J. Arthroplast. 2016, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Papavasiliou, A.V.; Isaac, D.L.; Marimuthu, R.; Skyrme, A.; Armitage, A. Infection in knee replacements after previous injection of intra- articular steroid. J. Bone Jt. Surg. Br. 2006, 88, 321–323. [Google Scholar] [CrossRef] [PubMed]
- Cancienne, J.M.; Werner, B.C.; Luetkemeyer, L.M.; Browne, J.A. Does Timing of Previous Intra-Articular Steroid Injection Affect the Post-Operative Rate of Infection in Total Knee Arthroplasty? J. Arthroplast. 2015, 30, 1879–1882. [Google Scholar] [CrossRef]
- Schick, S.; Elphingstone, J.; Murali, S.; Carter, K.; Davis, W.; McGwin, G.; Evely, T.; Ponce, B.; Momaya, A.; Brabston, E. The Incidence of Shoulder Arthroplasty Infection Presents a Substantial Economic Burden in the United States: A Predictive Model. JSES Int. 2023, 7, 636–641. [Google Scholar] [CrossRef]
- Richards, J.; Inacio, M.C.S.; Beckett, M.; Navarro, R.A.; Singh, A.; Dillon, M.T.; Sodl, J.F.; Yian, E.H. Patient and Procedure-Specific Risk Factors for Deep Infection After Primary Shoulder Arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 2809–2815. [Google Scholar] [CrossRef]
- Pottinger, P.; Butler-Wu, S.; Neradilek, M.B.; Merritt, A.; Bertelsen, A.; Jette, J.L.; Warme, W.J.; AMatsen, F. Prognostic Factors for Bacterial Cultures Positive for Propionibacterium Acnes and Other Organisms in a Large Series of Revision Shoulder Arthroplasties Performed for Stiffness, Pain, or Loosenin. J. Bone Jt. Surg. Am. 2012, 94, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Vopat, B.G.; Lee, B.J.; DeStefano, S.; Waryasz, G.R.; Kane, P.M.; Gallacher, S.E.; Fava, J.; Green, A.G. Risk Factors for Infection After Rotator Cuff Repair. Arthrosc. J. Arthrosc. Relat. Surg. 2016, 32, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Cancienne, J.M.; Brockmeier, S.F.; Carson, E.W.; Werner, B.C. Risk Factors for Infection After Shoulder Arthroscopy in a Large Medicare Population. Am. J. Sports Med. 2018, 46, 809–814. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 151, 264–269. [Google Scholar]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Livesey, M.G.; Bains, S.S.; Weir, T.B.; Kolakowski, L.C.; Remily, E.A.; Sax, O.C.; Gilotra, M.N.; Hasan, S.A. Does needle penetration of the shoulder joint prior to arthroscopy increase infection risk? The effect of preoperative magnetic resonance arthrogram or corticosteroid injection. J. Shoulder Elb. Surg. 2023, 32, e305–e310. [Google Scholar] [CrossRef] [PubMed]
- Agarwalla, A.; Puzzitiello, R.N.; Mascarenhas, R.; Sumner, S.; Romeo, A.A.; Forsythe, B. Preoperative Injections May Be an Iatrogenic Cause of Reoperation After Arthroscopic Rotator Cuff Repair. J. Arthrosc. Relat. Surg. 2019, 35, 325–331. [Google Scholar] [CrossRef]
- Baksh, N.; Etcheson, J.I.; Liu, S.; Ikwuazom, C.P.; Chen, Z.; Dubin, J.A.; Hameed, D.; Naziri, Q. Pre-operative corticosteroid injection within 1 month of total shoulder arthroplasty is associated with increased risk of periprosthetic joint infection. Arch. Orthop. Trauma Surg. 2023, 143, 5609–5614. [Google Scholar] [CrossRef]
- Baksh, N.; Nadarajah, V.; Connors, K.M.; Bains, S.S.; Chen, Z.; Dubin, J.A.; Naziri, Q. Does preoperative corticosteroid injection increase the risk of periprosthetic joint infection after reverse shoulder arthroplasty. J. Shoulder Elb. Surg. 2023, 32, 1459–1464. [Google Scholar] [CrossRef]
- Bhattacharjee, S.; Lee, W.; Lee, M.J.; Shi, L.L. Preoperative corticosteroid joint injections within 2 weeks of shoulder arthroscopies increase postoperative infection risk. J. Shoulder Elb. Surg. 2019, 28, 2098–2102. [Google Scholar] [CrossRef]
- Forsythe, B.; Agarwalla, A.; Puzzitiello, R.N.; Sumner, S.; Romeo, A.A.; Mascarenhas, R. The Timing of Injections Prior to Arthroscopic Rotator Cuff Repair Impacts the Risk of Surgical Site Infection. J. Bone Jt. Surg. 2019, 101, 682–687. [Google Scholar] [CrossRef]
- Rashid, A.; Kalson, N.; Jiwa, N.; Patel, A.; Irwin, A.; Corner, T. The effects of pre-operative intra-articular glenohumeral corticosteroid injection on infective complications after shoulder arthroplasty. Shoulder Elb. 2015, 7, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Remily, E.; Dubin, J.; Bains, S.S.; Monarrez, R.; Livesey, M.G.; Weir, T.B.; Hameed, D.; Ingari, J.V.; Gilotra, M.N.; Hasan, S.A. Preoperative Corticosteroid Injections Within 4 Weeks of Arthroscopic Shoulder Procedures Are Associated With Increased Postoperative Infection Rates. Arthrosc. J. Arthrosc. Relat. Surg. 2023, 40, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Stadecker, M.; Gu, A.; Ramamurti, P.; Fassihi, S.C.; Wei, C.; Agarwal, A.R.; Bovonratwet, P.; Srikumaran, U. Risk of revision based on timing of corticosteroid injection prior to shoulder arthroplasty. Bone Jt. J. 2022, 104, 620–626. [Google Scholar] [CrossRef]
- Weber, A.E.; Trasolini, N.A.; Mayer, E.N.; Essilfie, A.; Vangsness, C.T., Jr.; Gamradt, S.C.; Tibone, J.E.; Kang, H.P. Injections Prior to Rotator Cuff Repair Are Associated With Increased Rotator Cuff Revision Rates. J. Arthrosc. Relat. Surg. 2019, 35, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Werner, B.C.; Cancienne, J.M.; Burrus, M.T.; Griffin, J.W.; Gwathmey, F.W.; Brockmeier, S.F. The timing of elective shoulder surgery after shoulder injection affects postoperative infection risk in Medicare patients. J. Shoulder Elb. Surg. 2016, 25, 390–397. [Google Scholar] [CrossRef]
- Bohsali, K.I.; Wirth, M.A.; Rockwood, C.A., Jr. Complications of total shoulder arthroplasty. J. Bone Jt. Surg. Am. 2006, 88, 2279–2292. [Google Scholar]
- Waterman, B.R.; Dunn, J.C.; Bader, J.; Urrea, L.; Schoenfeld, A.J.; Belmont, P.J., Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: Patient-based and surgical risk factors. J. Shoulder Elb. Surg. 2015, 24, 24–30. [Google Scholar] [CrossRef]
- Fink, B.; Sevelda, F. Periprosthetic Joint Infection of Shoulder Arthroplasties: Diagnostic and Treatment Options. BioMed Res. Int. 2017, 2017, 4582756. [Google Scholar] [CrossRef]
- Matzon, J.L.; Lebowitz, C.; Graham, J.G.; Lucenti, L.; Lutsky, K.F.; Beredjiklian, P.K. Risk of Infection in Trigger Finger Release Surgery Following Corticosteroid Injection. J. Hand Surg. Am. 2020, 45, 310–316. [Google Scholar] [CrossRef]
- Sabesan, V.J.; Ho, J.C.; Kovacevic, D.; Iannotti, J.P. Two-stage reimplantation for treating prosthetic shoulder infections. Clin. Orthop. Relat. Res. 2011, 469, 2538–2543. [Google Scholar] [CrossRef] [PubMed]
- Saltzman, B.M.; Chalmers, P.N.; Gupta, A.K.; Romeo, A.A.; Nicholson, G.P. Complication rates comparing primary with revision reverse total shoulder arthroplasty. J. Shoulder Elb. Surg. 2014, 23, 1647–1654. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.H.; Omiyi, D.; Kuczynski, B.; Cushner, F.D.; Scuderi, G.R. The risk of a deep infection associated with intraarticular injections before a total knee arthroplasty. J. Arthroplast. 2016, 31, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.S.; Schairer, W.W.; Sculco, T.P.; Sculco, P.K. Comparison of infection risk with corticosteroid or hyaluronic acid injection prior to total knee arthroplasty. J. Bone Jt. Surg. Am. 2019, 101, 112–118. [Google Scholar] [CrossRef]
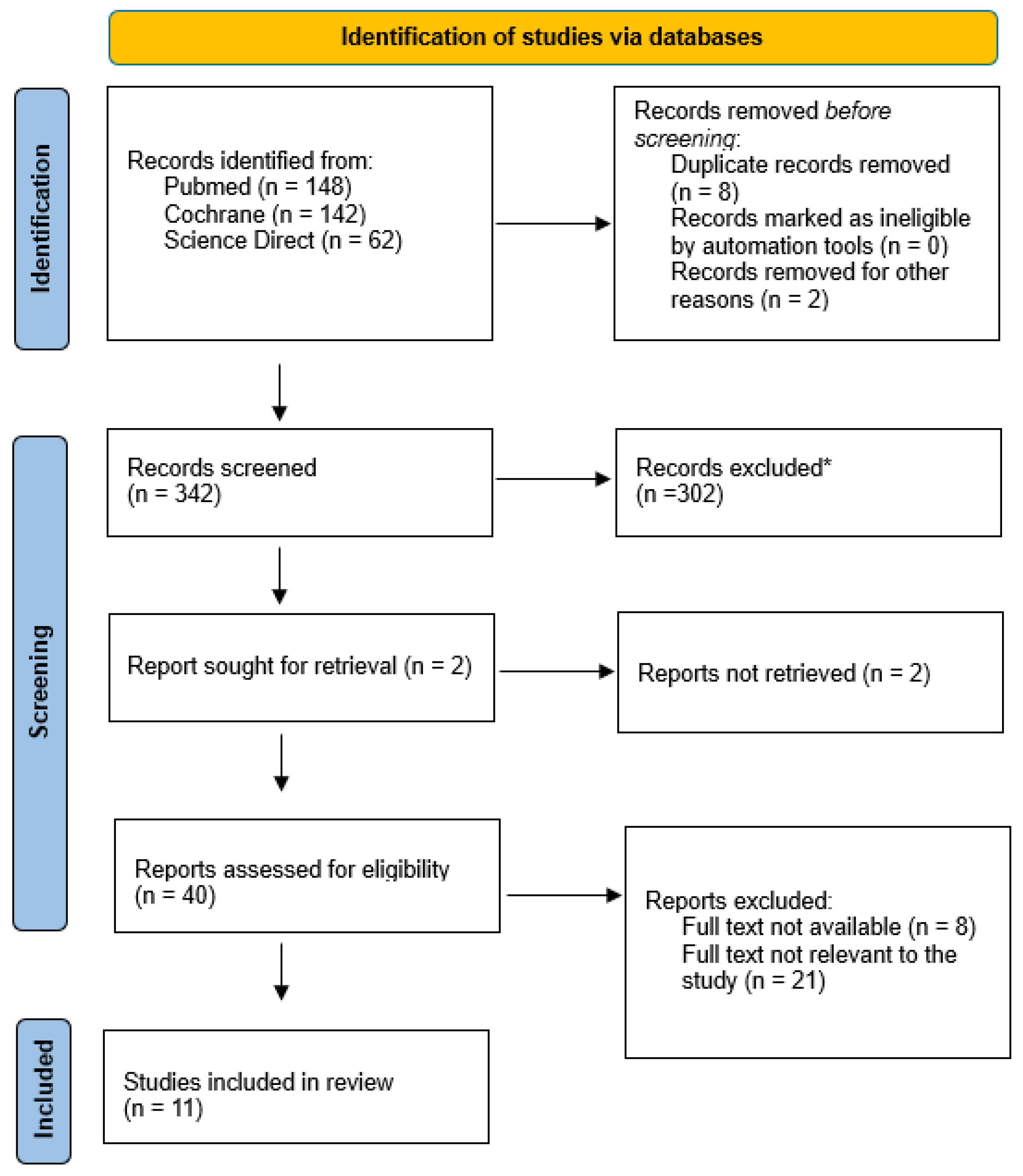
| Author | Publ. Year | Number of Patients | Type of Study | Follow-Up | Results | Main Topic |
|---|---|---|---|---|---|---|
| Agarwalla et al. [17] | 2019 | 12,054 | Retrospective cohort, comparative study | 3 m, 6 m, 9 m, 1 y | The rate of reoperation within 3 months of the index procedure was higher in the control group (3.7% vs. 3.1%, P 1⁄4 0.01); however, 3 to 6 months after the index procedure, the rate of reoperation was higher in patients who received an injection within 1 year of the index procedure (1.8% vs. 1.4%, P 1⁄4 0.03). The incidence of revision RCR (1.6% vs. 1.1%; odds ratio, 1.4; P 1⁄4 0.003) and incidence of subacromial decompression (1.5% vs. 1.1%; odds ratio, 1.3; P 1⁄4 0.01) 6 to 12 months after the index procedure were significantly higher in patients receiving an injection within 1 year before surgery. | Arthroscopy |
| Baksh et al. [18] | 2023 | 1291 | Retrospective cohort study | 90 d, 1 y, 2 y | A significant increase in PJI risk at 1 year (odds ratio [OR] = 2.29, 95% confidence interval [CI] = 1.19–3.99, p = 0.007) and 2 years (OR = 2.03, CI = 1.09–3.46, p = 0.016) in patients who received CSIs within 1 month of TSA was noted. PJI risk was not significantly increased at any time point for patients who received a CSI greater than 4 weeks prior to TSA (all p ≥ 0.396). | Arthroplasty |
| Baksh et al. [19] | 2023 | 20,898 | Retrospective cohort study | 3 m, 1 y | Significantly increased risk of PJIs at 90 days in patients who received CSIs within 1 month of rTSA (p < 0.001). Additionally, the PJI risk was increased at 1 year postoperatively in patients who received CSIs within 1 month of rTSA (p = 0.015). However, no significant increase in the PJI risk was noted at any time point for patients who received CSIs > 1 month before rTSA (all p ≥ 0.088). | Arthroplasty |
| Bhattacharjee et al. [20] | 2019 | 4115 | Retrospective cohort study | 6 m | Significant increase in both the overall infection rate (p < 0.0001) and severe infection rate (p < 0.0001) in patients who received injections within 2 weeks before surgery (n = 79; 8.86% and 6.33%, respectively). | Arthroscopy |
| Forsythe et al. [21] | 2019 | 12,060 | Retrospective cohort study | There was no significant difference in the incidence of surgical site infection in patients receiving a shoulder injection at 0.7% compared with the control cohort at 0.8% (odds ratio [OR], 0.9 [95% confidence interval (CI), 0.7 to 1.1]; p = 0.2). However, patients receiving an injection within 1 month prior to operative management had a significantly higher rate of surgical site infection overall at 1.3% compared to the control group at 0.8% (OR, 1.7 [95% CI, 1.0 to 2.9]; p = 0.04). | Arthroscopy | |
| Livesey et al. [16] | 2023 | 3630 | Retrospective cohort, prognostic study | 3 m, 1 y, 2 y | Two main groups: CSI swithin 2 and 2/4 weeks of surgery. They were associated with an increased risk of postoperative infection. CSIs for 2 weeks at 90 days (OR, 1.72; p = 0.022), 1 year (OR, 1.65; p = 0.005), and 2 years (OR, 1.63; p = 0.002) following surgery. CSIs for 2–4 weeks increased the risk of postoperative infection at 90 days (OR, 1.83; p < 0.001), 1 year (OR, 1.62; p < 0.001), and 2 years (OR, 1.79; p < 0.001). | Arthroscopy |
| Rashid et al. [22] | 2015 | 23 | Retrospective cohort study | 16.6 m | Patients received a CSI at approximately 11.4 months (range 2.5 months to 172.5 months) before their surgery. One patient developed a deep joint infection that warranted revision arthroplasty. | Arthroplasty |
| Remily et al. [23] | 2023 | 9860 | Retrospective cohort, prognostic study | 3 m, 1 y, 2 y | Postoperative infection in patients who received CSIs 0–2 weeks before shoulder arthroscopy at 90 days (3.10, 95% confidence interval [CI] 1.62–5.57, p < 0.001), 1 year (2.51, 95% CI 1.46–4.12, p < 0.001), and 2 years (2.08, 95% CI 1.27–3.28, p = 0.002) compared to the control group. Patients who received CSIs 2–4 weeks before shoulder arthroscopy had a greater OR for infection at 90 days (2.26, 95% CI 1.28–3.83, p = 0.03), 1 year (1.82, 95% CI 1.13–2,82, p = 0.01), and 2 years (1.62, 95% CI 1.10–2.47, p = 0.012). Patients who received CSI safter 4 weeks had similar ORs of infection at 90 days (OR 1.15, 95% CI 0.78–1.69, p = 0.48), 1 year (OR 1.18, 95% CI 0.85–1.63 p = 0.33), and 2 years (OR 1.09, 95% CI 0.83–1.42, p = 0.54), compared to the control cohort. | Arthroscopy |
| Stadecker et al. [24] | 2022 | 1632 | Retrospective cohort study | 2 y | On multivariate analysis, patients who received corticosteroid injection < three months prior to TSA or rTSA were at a significantly increased risk for revision (odds ratio (OR) 2.61 (95% confidence interval (CI) 1.77 to 3.28); p < 0.001) when compared to the control cohort. However, there was no significant increase in revision risk for all other timing interval cohorts. | Arthroplasty |
| Weber et al. [25] | 2018 | 21,796 | Therapeutic study | 1 y | Patients who received injections prior to RCR were more likely to undergo RCR revision than the matched controls (odds ratio [OR], 1.52; 95% confidence interval [CI], 1.38–1.68; p < 0.0001). Patients who received injections closer to the time of index RCR were more likely to undergo revision (p < 0.0001). Patients who received a single injection prior to RCR had a higher likelihood of revision (OR, 1.25; 95% CI, 1.10–1.43; P 1⁄4 0.001). Patients who received 2 or more injections prior to RCR had greater than 2-fold odds of revision (combined OR, 2.12; 95% CI, 1.82–2.47; p < 0.0001) versus the control group. | Arthroscopy |
| Werner et al. [26] | 2016 | 12,903 | Multicenter study | 3 m, 6 m | The incidence of infection after arthroscopy at 3 months (0.7%; odds ratio [OR], 2.2; p < 0.0001) and 6 months (1.1%; OR, 1.6; p = 0.003) was significantly higher in patients who underwent injection within 3 months before arthroscopy compared to the control group. The incidence of infection after arthroplasty at 3 months (3.0%; OR, 2.0; p = 0.007) and 6 months (4.6%; OR, 2.0; p = 0.001) was significantly higher in patients who underwent injection within 3 months before arthroplasty compared to the control group. | Arthroscopy and arthroplasty |
| (A) | ||||||||||
| 1 m | 1–2 m | 2–3 m | 3–6 m | 6–9 m | 1 y | 172.5 m | ||||
| Baksh et al. [18] (8) | 2023 | |||||||||
| Baksh et al. [19] (9) | 2023 | |||||||||
| Werner et al. [26] (17) | 2016 | |||||||||
| Rashid et al. [22] (13) | 2015 | |||||||||
| Stadecker et al. [24] (15) | 2022 | |||||||||
| (B) | ||||||||||
| 0–2 w | 2–4 w | 1–3 m | 4–6 m | 7–12 m | ||||||
| Werner et al. [26] (17) | 2016 | |||||||||
| Weber et al. [25] (16) | 2018 | |||||||||
| Remily et al. [23] (14) | 2023 | |||||||||
| Agarwalla et al. [17] (7) | 2019 | |||||||||
| Bhattacharjee et al. [20] (10) | 2019 | |||||||||
| Forsythe et al. [21] (11) | 2019 | |||||||||
| Livesey et al. [16] (12) | 2023 | |||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lucenti, L.; Panvini, F.M.C.; de Cristo, C.; Rapisarda, D.; Sapienza, M.; Testa, G.; Pavone, V. Do Preoperative Corticosteroid Injections Increase the Risk of Infection after Shoulder Arthroscopy or Shoulder Arthroplasty? A Systematic Review. Healthcare 2024, 12, 543. https://doi.org/10.3390/healthcare12050543
Lucenti L, Panvini FMC, de Cristo C, Rapisarda D, Sapienza M, Testa G, Pavone V. Do Preoperative Corticosteroid Injections Increase the Risk of Infection after Shoulder Arthroscopy or Shoulder Arthroplasty? A Systematic Review. Healthcare. 2024; 12(5):543. https://doi.org/10.3390/healthcare12050543
Chicago/Turabian StyleLucenti, Ludovico, Flora Maria Chiara Panvini, Claudia de Cristo, Damiano Rapisarda, Marco Sapienza, Gianluca Testa, and Vito Pavone. 2024. "Do Preoperative Corticosteroid Injections Increase the Risk of Infection after Shoulder Arthroscopy or Shoulder Arthroplasty? A Systematic Review" Healthcare 12, no. 5: 543. https://doi.org/10.3390/healthcare12050543
APA StyleLucenti, L., Panvini, F. M. C., de Cristo, C., Rapisarda, D., Sapienza, M., Testa, G., & Pavone, V. (2024). Do Preoperative Corticosteroid Injections Increase the Risk of Infection after Shoulder Arthroscopy or Shoulder Arthroplasty? A Systematic Review. Healthcare, 12(5), 543. https://doi.org/10.3390/healthcare12050543









