Using Myofascial Therapy to Improve Psychological Outcomes, Quality of Life, and Sexual Function in Women with Chronic Pelvic Pain—A Case Series
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Participants
2.3. Outcomes
2.4. Intervention
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
- Participant has signed and dated the appropriate Informed Consent document. Participant is a woman and 18 years of age or older.
- Participant has a clinical diagnosis of CPP in the opinion of the investigator. Chronic pelvic pain (CPP) is defined as intermittent or continuous pain in the lower abdomen, pelvis or intrapelvic structures, lasting at least 3–6 months, and in women not exclusively associated with the menstrual cycle or pregnancy.
- Participant has had symptoms of discomfort or pain in the pelvic region for at least a 3-month period within the last 6 months. Current symptoms have been present for less than 5 years.
- The presence of tenderness/pain to palpation found by the physician in 1 of the pelvic floor musculature domains during the first baseline screening visit physical examination which is confirmed by the physical therapist at screening visit 2. The presence of tenderness/pain is defined as a mild, moderate, or severe finding by the physician at visit 1 and the physical therapist at visit 2. The pelvic floor musculature domains are defined as anterior or posterior levator muscles, obturator internus muscles and urogenital diaphragm (bulbospongiosus, superficial transverse perinei, ischiocavernosus, central tendon/perineal body). Also evaluated were the gluteus (maximus, medius, and minimus) and pyramidal muscles. The assessments of tenderness/pain at visits 1 and 2 did not need to be identical in severity or location in order for the participant to be eligible.
- Participant has relevant, painful scars on lower abdominal wall that, in the opinion of the study physician or physical therapist, are unlikely to respond to physical therapy without adjuvant therapy such as injection/needling.
- A positive urine culture (defined as greater than 100,000 cfu/mL). A negative urine culture within 1 month of study enrollment is acceptable.
- Participant had prior course of physical therapy that included manual therapy with connective tissue manipulation by physical therapist for the same symptoms. Prior treatment by a therapist with biofeedback, electrical stimulation or pelvic floor exercises is not exclusionary.
- Participant has a relevant neurologic disorder that affects bladder and/or neuromuscular function in the opinion of the investigator.
- Participant has active urethral or ureteral calculi, urethral diverticulum, history of pelvic radiation therapy, tuberculous cystitis, bladder cancer, carcinoma in situ or urethral cancer.
- Participant has/reports any severe, debilitating, or urgent concurrent medical condition.
- Participant has a potentially significant pelvic pathology or abnormalities on examination or prior imaging, including prolapse beyond the hymenal ring, pelvic mass, etc. that could cause or contribute to the clinical symptoms, or require treatment.
- Pregnancy or refusal of medically approved/reliable birth control in women of childbearing potential.
References
- Montenegro, M.L.L.S.; Vasconcelos, E.C.L.M.; Candido Dos Reis, F.J.; Nogueira, A.A.; Poli-Neto, O.B. Physical Therapy in the Management of Women with Chronic Pelvic Pain. Int. J. Clin. Pract. 2007, 62, 263–269. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Kennedy, S. Causes of Chronic Pelvic Pain. Best Pract. Res. Clin. Obstet. Gynaecol. 2000, 14, 389–402. [Google Scholar] [CrossRef] [PubMed]
- Bartoletti, R.; Cai, T.; Mondaini, N.; Dinelli, N.; Pinzi, N.; Pavone, C.; Gontero, P.; Gavazzi, A.; Giubilei, G.; Prezioso, D.; et al. Prevalence, Incidence Estimation, Risk Factors and Characterization of Chronic Prostatitis/Chronic Pelvic Pain Syndrome in Urological Hospital Outpatients in Italy: Results of a Multicenter Case-Control Observational Study. J. Urol. 2007, 178, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Grace, V.M.; Zondervan, K.T. Chronic Pelvic Pain in New Zealand: Prevalence, Pain Severity, Diagnoses and Use of the Health Services. Aust. N. Z. J. Public Health 2004, 28, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gyang, A.; Hartman, M.; Lamvu, G. Musculoskeletal Causes of Chronic Pelvic Pain: What a Gynecologist Should Know. Obstet. Gynecol. 2013, 121, 645–650. [Google Scholar] [CrossRef] [PubMed]
- Muhammad, Y.Y.; Nossier, S.A.; El-Dawaiaty, A.A. Prevalence and Characteristics of Chronic Pelvic Pain among Women in Alexandria, Egypt. J. Egypt. Public Health Assoc. 2011, 86, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Unruh, A.M. Gender Variations in Clinical Pain Experience. Pain 1996, 65, 123–167. [Google Scholar] [CrossRef]
- Da Luz, R.A.; Rodrigues, F.M.; Vila, V.S.C.; de Deus, J.M.; Lima, K.P. Depressive symptoms in women with chronic pelvic pain. Rev. Bras. Ginecol. Obstet. 2014, 36, 79–83. [Google Scholar]
- Poleshuck, E.L.; Gamble, S.A.; Bellenger, K.; Lu, N.; Tu, X.; Sörensen, S.; Giles, D.E.; Talbot, N.L. Randomized Controlled Trial of Interpersonal Psychotherapy versus Enhanced Treatment as Usual for Women with Co-Occurring Depression and Pelvic Pain. J. Psychosom. Res. 2014, 77, 264–272. [Google Scholar] [CrossRef]
- Steele, A. Opioid Use and Depression in Chronic Pelvic Pain. Obstet. Gynecol. Clin. N. Am. 2014, 41, 491–501. [Google Scholar] [CrossRef]
- Davis, S.N.P.; Binik, Y.M.; Amsel, R.; Carrier, S. Is a Sexual Dysfunction Domain Important for Quality of Life in Men with Urological Chronic Pelvic Pain Syndrome? Signs “UPOINT” to Yes. J. Urol. 2013, 189, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, D.; Sarton, J. Chronic Pelvic Floor Dysfunction. Best Pract. Res. Clin. Obstet. Gynaecol. 2014, 28, 977–990. [Google Scholar] [CrossRef] [PubMed]
- Pink, L.; Rancourt, V.; Gordon, A. Persistent Genital Arousal in Women with Pelvic and Genital Pain. J. Obstet. Gynaecol. Can. 2014, 36, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Romão, A.P.M.S.; Gorayeb, R.; Romão, G.S.; Poli-Neto, O.B.; Nogueira, A.A. Impact of Chronic Pelvic Pain on Female Sexual Function. Int. J. Clin. Med. 2013, 4, 178–182. [Google Scholar] [CrossRef][Green Version]
- Anderson, R.U.; Wise, D.; Sawyer, T.; Chan, C. Integration of Myofascial Trigger Point Release and Paradoxical Relaxation Training Treatment of Chronic Pelvic Pain in Men. J. Urol. 2005, 174, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Berger, R.E.; Ciol, M.A.; Rothman, I.; Turner, J.A. Pelvic Tenderness Is Not Limited to the Prostate in Chronic Prostatitis/Chronic Pelvic Pain Syndrome (CPPS) Type IIIA and IIIB: Comparison of Men with and without CP/CPPS. BMC Urol. 2007, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- FitzGerald, M.P.; Kotarinos, R. Rehabilitation of the Short Pelvic Floor. II: Treatment of the Patient with the Short Pelvic Floor. Int. Urogynecol. J. 2003, 14, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.M.; O’Dougherty, E. Pelvic Floor Physical Therapy for Patients with Prostatitis. Curr. Urol. Rep. 2000, 1, 155–158. [Google Scholar] [CrossRef]
- Pastore, E.A.; Katzman, W.B. Recognizing Myofascial Pelvic Pain in the Female Patient with Chronic Pelvic Pain. J. Obstet. Gynecol. Neonatal Nurs. 2012, 41, 680–691. [Google Scholar] [CrossRef]
- Rosenbaum, T.Y. Reviews: Pelvic Floor Involvement in Male and Female Sexual Dysfunction and the Role of Pelvic Floor Rehabilitation in Treatment: A Literature Review. J. Sex. Med. 2007, 4, 4–13. [Google Scholar] [CrossRef]
- Tu, F.F.; As-Sanie, S.; Steege, J.F. Prevalence of Pelvic Musculoskeletal Disorders in a Female Chronic Pelvic Pain Clinic. J. Reprod. Med. 2006, 51, 185–189. [Google Scholar] [PubMed]
- Anderson, R.U.; Sawyer, T.; Wise, D.; Morey, A.; Nathanson, B.H. Painful Myofascial Trigger Points and Pain Sites in Men with Chronic Prostatitis/Chronic Pelvic Pain Syndrome. J. Urol. 2009, 182, 2753–2758. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, D. Understanding Multisymptom Presentations in Chronic Pelvic Pain: The Inter-Relationships Between the Viscera and Myofascial Pelvic Floor Dysfunction. Curr. Pain Headache Rep. 2011, 15, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Clemens, J.Q.; Nadler, R.B.; Schaeffer, A.J.; Belani, J.; Albaugh, J.; Bushman, W. Biofeedback, Pelvic Floor Re-Education, and Bladder Training for Male Chronic Pelvic Pain Syndrome. Urology 2000, 56, 951–955. [Google Scholar] [CrossRef] [PubMed]
- Oyama, I.A.; Rejba, A.; Lukban, J.C.; Fletcher, E.; Kellogg-Spadt, S.; Holzberg, A.S.; Whitmore, K.E. Modified Thiele Massage as Therapeutic Intervention for Female Patients with Interstitial Cystitis and High-Tone Pelvic Floor Dysfunction. Urology 2004, 64, 862–865. [Google Scholar] [CrossRef]
- Shoskes, D.A.; Katz, E. Multimodal Therapy for Chronic Prostatitis/Chronic Pelvic Pain Syndrome. Curr. Urol. Rep. 2005, 6, 296–299. [Google Scholar] [CrossRef] [PubMed]
- Weiss, J.M. Pelvic Floor Myofascial Trigger Points: Manual Therapy for the Interstitial Cystitis and the Urgency-Frequency Syndrome. J. Urol. 2001, 166, 2226–2231. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Bernardes, N.; Bahamondes, L. Intravaginal Electrical Stimulation for the Treatment of Chronic Pelvic Pain. J. Reprod. Med. 2005, 50, 267–272. [Google Scholar]
- Schleip, R.; Duerselen, L.; Vleeming, A.; Naylor, I.L.; Lehmann-Horn, F.; Zorn, A.; Jaeger, H.; Klingler, W. Strain Hardening of Fascia: Static Stretching of Dense Fibrous Connective Tissues Can Induce a Temporary Stiffness Increase Accompanied by Enhanced Matrix Hydration. J. Bodyw. Mov. Ther. 2012, 16, 94–100. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The Hospital Anxiety and Depression Scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef]
- Olssøn, I.; Mykletun, A.; Dahl, A.A. The Hospital Anxiety and Depression Rating Scale: A Cross-Sectional Study of Psychometrics and Case Finding Abilities in General Practice. BMC Psychiatry 2005, 5, 46. [Google Scholar] [CrossRef] [PubMed]
- Ware, J.E.; Kosinski, M.; Keller, S.D. A 12-Item Short-Form Health Survey: Construction of Scales and Preliminary Tests of Reliability and Validity. Med. Care 1996, 34, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Rosen, C.; Brown, J.; Heiman, S.; Leiblum, S.; Meston, C.; Shabsigh, R.; Ferguson, D.; D’Agostino, R. The Female Sexual Function Index (FSFI): A Multidimensional Self-Report Instrument for the Assessment of Female Sexual Function. J. Sex Marital. Ther. 2000, 26, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Wiegel, M.; Meston, C.; Rosen, R. The Female Sexual Function Index (FSFI): Cross-Validation and Development of Clinical Cutoff Scores. J. Sex Marital. Ther. 2005, 31, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Facchin, F.; Barbara, G.; Saita, E.; Mosconi, P.; Roberto, A.; Fedele, L.; Vercellini, P. Impact of Endometriosis on Quality of Life and Mental Health: Pelvic Pain Makes the Difference. J. Psychosom. Obstet. Gynecol. 2015, 36, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Tripp, D.A.; Nickel, J.C.; Shoskes, D.; Koljuskov, A. A 2-Year Follow-up of Quality of Life, Pain, and Psychosocial Factors in Patients with Chronic Prostatitis/Chronic Pelvic Pain Syndrome and Their Spouses. World J. Urol. 2013, 31, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Poleshuck, E.L.; Bair, M.J.; Kroenke, K.; Watts, A.; Tu, X.; Giles, D.E. Pain and Depression in Gynecology Patients. Psychosomatics 2009, 50, 270–276. [Google Scholar] [CrossRef][Green Version]
- Berna, C.; Vincent, K.; Moore, J.; Tracey, I.; Goodwin, G.M.; Holmes, E.A. Presence of Mental Imagery Associated with Chronic Pelvic Pain: A Pilot Study. Pain Med. 2011, 12, 1086–1093. [Google Scholar] [CrossRef]
- Stratton, P.; Khachikyan, I.; Sinaii, N.; Ortiz, R.; Shah, J. Association of Chronic Pelvic Pain and Endometriosis with Signs of Sensitization and Myofascial Pain. Obstet. Gynecol. 2015, 125, 719–728. [Google Scholar] [CrossRef]
- Itza, F.; Teba, F.; Zarza, D.; Salinas, J.; Gomes, M. Clinical profile of patients with chronic pelvic pain: A descriptive analysis. Arch. Esp. Urol. 2014, 67, 692–698. [Google Scholar]
- Rod, K. Observing the Effects of Mindfulness-Based Meditation on Anxiety and Depression in Chronic Pain Patients. Psychiatr. Danub. 2015, 27 (Suppl. 1), S209–S211. [Google Scholar] [PubMed]
- Cardin, F.; Ambrosio, F.; Amodio, P.; Minazzato, L.; Bombonato, G.; Schiff, S.; Finotti, K.; Giuliani, D.; Bianco, T.; Terranova, C.; et al. Quality of Life and Depression in a Cohort of Female Patients with Chronic Disease. BMC Surg. 2012, 12, S10. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; De Cicco Nardone, F.; De Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. Impact of Endometriosis on Quality of Life and Work Productivity: A Multicenter Study across Ten Countries. Fertil. Steril. 2011, 96, 366–373.e8. [Google Scholar] [CrossRef] [PubMed]
- Culley, L.; Law, C.; Hudson, N.; Denny, E.; Mitchell, H.; Baumgarten, M.; Raine-Fenning, N. The Social and Psychological Impact of Endometriosis on Women’s Lives: A Critical Narrative Review. Hum. Reprod. Update 2013, 19, 625–639. [Google Scholar] [CrossRef] [PubMed]
- Jia, S.; Leng, J.; Sun, P.; Lang, J. Prevalence and Associated Factors of Female Sexual Dysfunction in Women with Endometriosis. Obstet. Gynecol. 2013, 121, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Verit, F.F.; Verit, A.; Yeni, E. The Prevalence of Sexual Dysfunction and Associated Risk Factors in Women with Chronic Pelvic Pain: A Cross-Sectional Study. Arch. Gynecol. Obstet. 2006, 274, 297–302. [Google Scholar] [CrossRef]
- Rosenbaum, T.Y.; Owens, A. Continuing Medical Education: The Role of Pelvic Floor Physical Therapy in the Treatment of Pelvic and Genital Pain-Related Sexual Dysfunction (CME). J. Sex. Med. 2008, 5, 513–523. [Google Scholar] [CrossRef]
- Fry, R.P.W.; Crisp, A.H.; Beard, R.W. Sociopsychological Factors in Chronic Pelvic Pain: A Review. J. Psychosom. Res. 1997, 42, 1–15. [Google Scholar] [CrossRef]
- Brotto, L.A.; Yong, P.; Smith, K.B.; Sadownik, L.A. Impact of a Multidisciplinary Vulvodynia Program on Sexual Functioning and Dyspareunia. J. Sex. Med. 2015, 12, 238–247. [Google Scholar] [CrossRef]
- Doumouchtsis, S.K.; Boama, V.; Gorti, M.; Tosson, S.; Fynes, M.M. Prospective Evaluation of Combined Local Bupivacaine and Steroid Injections for the Management of Chronic Vaginal and Perineal Pain. Arch. Gynecol. Obstet. 2011, 284, 681–685. [Google Scholar] [CrossRef]
- Masheb, R.M.; Kerns, R.D.; Lozano, C.; Minkin, M.J.; Richman, S. A Randomized Clinical Trial for Women with Vulvodynia: Cognitive-Behavioral Therapy vs. Supportive Psychotherapy. Pain 2009, 141, 31–40. [Google Scholar] [CrossRef]
- Silva, A.P.M.D.; Montenegro, M.L.; Gurian, M.B.F.; Mitidieri, A.M.D.S.; Lara, L.A.D.S.; Poli-Neto, O.B.; Rosa, E.; Silva, J.C. Perineal Massage Improves the Dyspareunia Caused by Tenderness of the Pelvic Floor Muscles. Rev. Bras. Ginecol. Obstet. 2017, 39, 26–30. [Google Scholar] [CrossRef]
- Goldfinger, C.; Pukall, C.F.; Gentilcore-Saulnier, E.; McLean, L.; Chamberlain, S. A Prospective Study of Pelvic Floor Physical Therapy: Pain and Psychosexual Outcomes in Provoked Vestibulodynia. J. Sex. Med. 2009, 6, 1955–1968. [Google Scholar] [CrossRef]
- Tuttle, L.J.; Nguyen, O.T.; Cook, M.S.; Alperin, M.; Shah, S.B.; Ward, S.R.; Lieber, R.L. Architectural Design of the Pelvic Floor Is Consistent with Muscle Functional Subspecialization. Int. Urogynecol. J. 2014, 25, 205–212. [Google Scholar] [CrossRef]
- Spitznagle, T.M.; McCurdy Robinson, C. Myofascial Pelvic Pain. Obstet. Gynecol. Clin. N. Am. 2014, 41, 409–432. [Google Scholar] [CrossRef]
- Van Der Velde, J.; Everaerd, W. The Relationship between Involuntary Pelvic Floor Muscle Activity, Muscle Awareness and Experienced Threat in Women with and without Vaginismus. Behav. Res. Ther. 2001, 39, 395–408. [Google Scholar] [CrossRef]
- Anderson, R.; Wise, D.; Sawyer, T.; Nathanson, B.H. Safety and Effectiveness of an Internal Pelvic Myofascial Trigger Point Wand for Urologic Chronic Pelvic Pain Syndrome. Clin. J. Pain 2011, 27, 764–768. [Google Scholar] [CrossRef]
- FitzGerald, M.P.; Anderson, R.U.; Potts, J.; Payne, C.K.; Peters, K.M.; Clemens, J.Q.; Kotarinos, R.; Fraser, L.; Cosby, A.; Fortman, C.; et al. Randomized Multicenter Feasibility Trial of Myofascial Physical Therapy for the Treatment of Urological Chronic Pelvic Pain Syndromes. J. Urol. 2013, 189, S75–S85. [Google Scholar] [CrossRef]
- Heyman, J.; Öhrvik, J.; Leppert, J. Distension of Painful Structures in the Treatment for Chronic Pelvic Pain in Women. Acta Obstet. Gynecol. Scand. 2006, 85, 599–603. [Google Scholar] [CrossRef]
- Lukban, J.C.; Parkin, J.V.; Holzberg, A.S.; Caraballo, R.; Kellogg-Spadt, S.; Whitmore, K.E. Interstitial Cystitis and Pelvic Floor Dysfunction: A Comprehensive Review. Pain Med. 2001, 2, 60–71. [Google Scholar] [CrossRef]
- Wurn, L.J.; Wurn, B.F.; King, C.R.; Roscow, A.S.; Scharf, E.S.; Shuster, J.J. Increasing Orgasm and Decreasing Dyspareunia by a Manual Physical Therapy Technique. MedGenMed 2004, 6, 47. [Google Scholar]
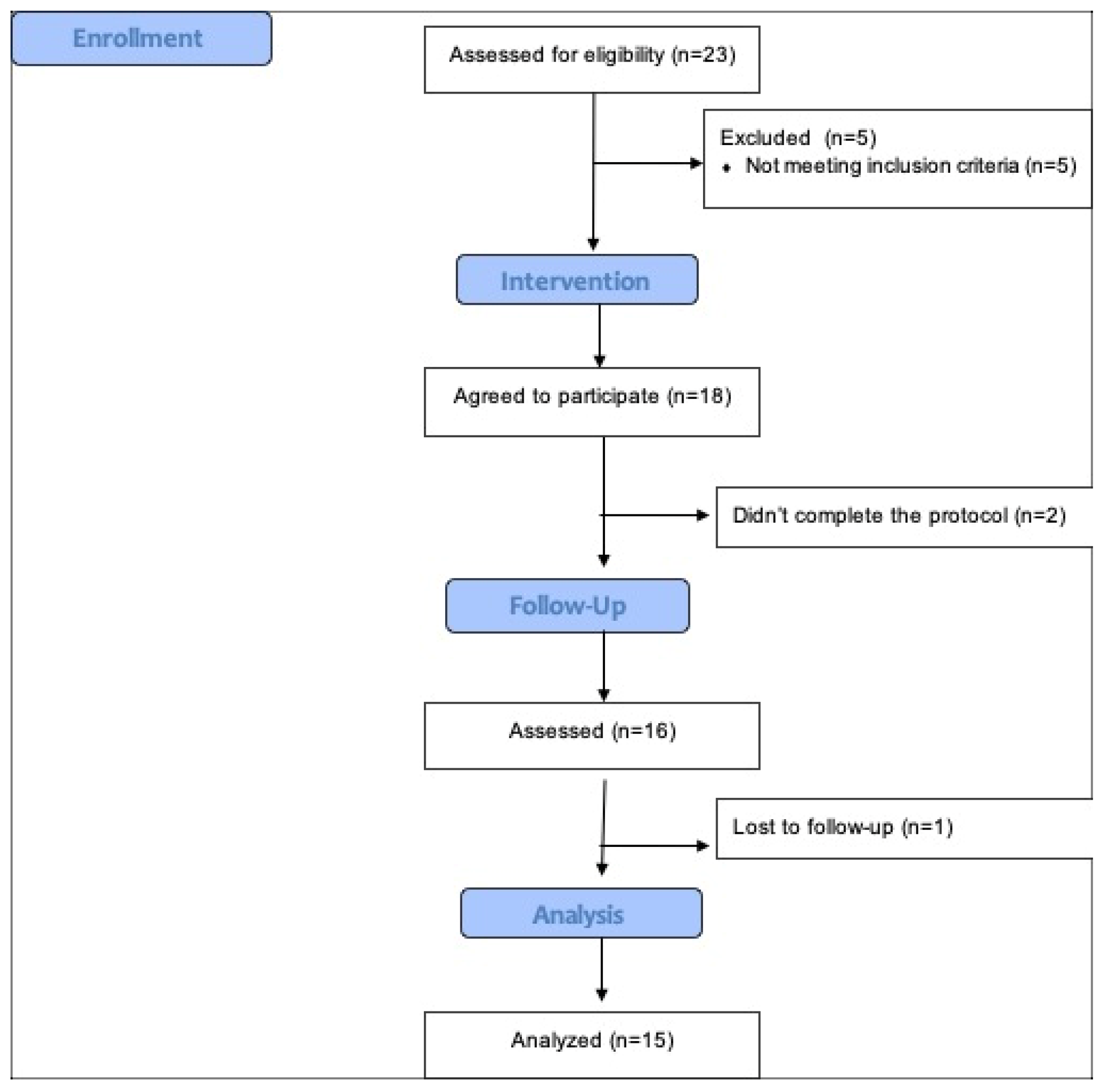


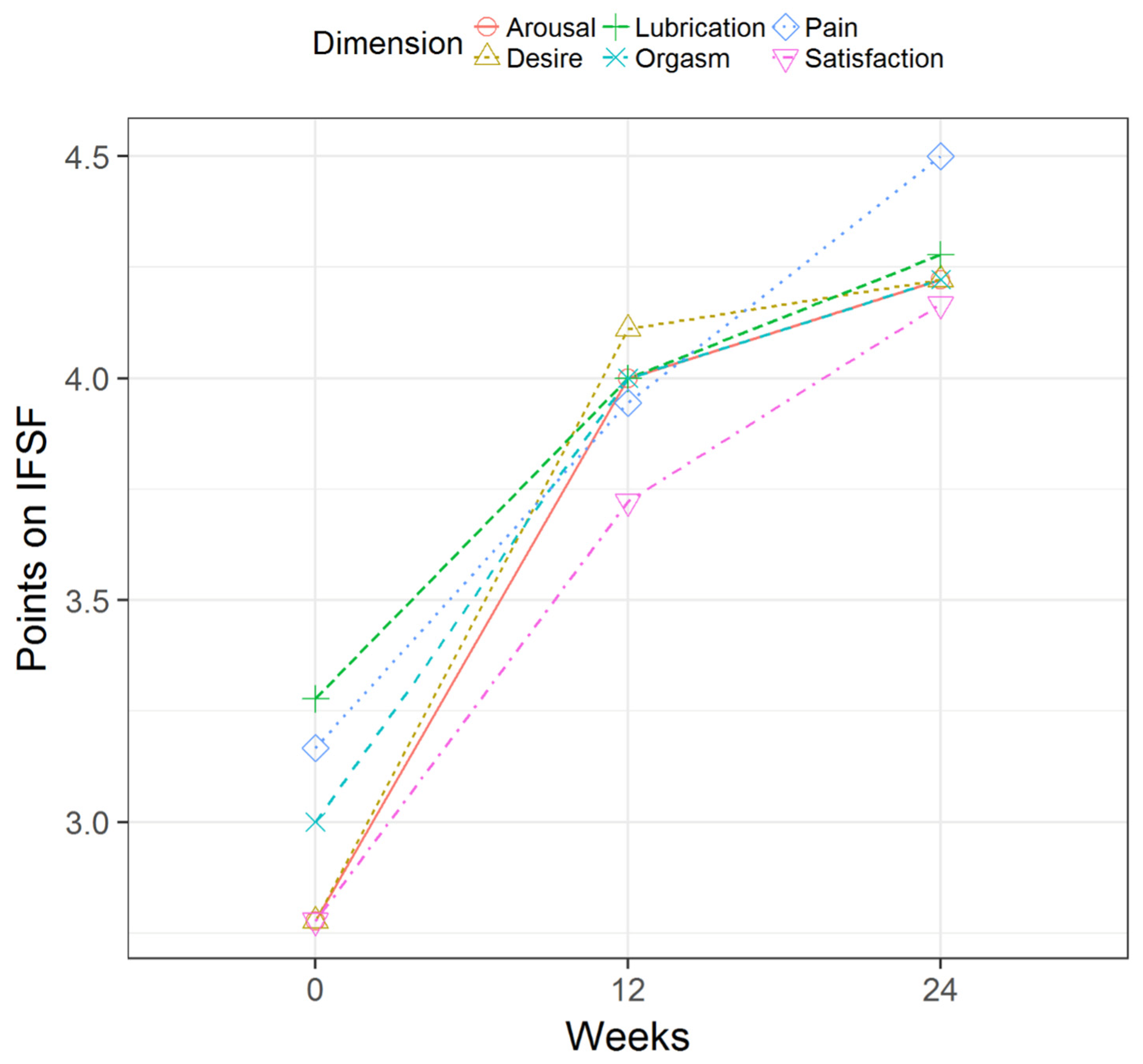
| Measures | Mean ± SD | Range | Median ± IR |
|---|---|---|---|
| HADS: Anxiety dimension | 11.3 ± 4.0 | 5.0–17.0 | 11.5 ± 6.8 |
| HADS: Depression dimension | 10.2 ± 5.1 | 3.0–20.0 | 9.0 ± 6.8 |
| SF-12 (Physical component) | 43.8 ± 5.0 | 34.3–51.0 | 44.0 ± 6.0 |
| SF-12 (Mental component) | 38.3 ± 10.1 | 25.6–54.0 | 36.7 ± 18.9 |
| FSFI | 17.8 ± 7.8 | 6.0–29.0 | 17.5 ± 14.6 |
| FSFI Domain: Desire | 2.8 ± 1.7 | 0.0–5.0 | 2.5 ± 2.8 |
| FSFI Domain: Arousal | 2.8 ± 1.4 | 0.0–5.0 | 3.0 ± 1.8 |
| FSFI Domain: Lubrication | 3.3 ± 1.2 | 1.0–5.0 | 3.0 ± 1.8 |
| FSFI Domain: Orgasm | 3.0 ± 1.6 | 0.0–5.0 | 3.0 ± 2.0 |
| FSFI Domain: Satisfaction | 2.8 ± 1.5 | 0.0–5.0 | 3.0 ± 2.0 |
| FSFI Domain: Pain | 3.2 ± 1.7 | 0.0–6.0 | 3.0 ± 2.0 |
| Type of Analysis | Parametric Analysis | Non-Parametric Analysis | ||||
|---|---|---|---|---|---|---|
| Measures | Time | Mean ± SD | p | Median ± IR | p | Range |
| HADS: Anxiety dimension | 12 weeks | −3.1 ± 2.2 | <0.001 * | −3.0 ± 1.8 | 0.001 * | −8.0–0.0 |
| 24 weeks | −4.0 ± 3.2 | <0.001 * | −3.0 ± 4.0 | 0.001 * | −12.0–0.0 | |
| HADS: Depression dimension | 12 weeks | −3.1 ± 2.5 | <0.001 * | −2.5 ± 4.0 | 0.008 * | −8.0–0.0 |
| 24 weeks | −3.4 ± 3.3 | <0.001 * | −3.5 ± 4.0 | 0.031 * | −11.0–2.0 | |
| SF-12 (Physical component) | 12 weeks | −2.0 ± 4.5 | 0.090 | −0.7 ± 6.1 | 0.815 | −12.4–4.0 |
| 24 weeks | −1.0 ± 5.6 | 0.488 | −0.7 ± 5.8 | 0.815 | −10.5–8.3 | |
| SF-12 (Mental component) | 12 weeks | 8.0 ± 9.3 | 0.003 * | 6.9 ± 15.0 | 0.008 * | −9.7–25.3 |
| 24 weeks | 8.8 ± 10.9 | 0.004 * | 5.0 ± 19.5 | 0.031 * | −4.4–27.3 | |
| FSFI | 12 weeks | 5.4 ± 4.7 | <0.001 * | 4.0 ± 2.0 | <0.001 * | −2.0–20.0 |
| 24 weeks | 7.8 ± 6.4 | <0.001 * | 6.5 ± 6.6 | <0.001 * | −2.0–21.0 | |
| FSFI Dimension: Desire | 12 weeks | 1.3 ± 1.1 | <0.001 * | 1.5 ± 1.8 | <0.001 * | 0.0–4.0 |
| 24 weeks | 1.4 ± 1.4 | <0.001 * | 2.0 ± 2.0 | <0.001 * | −1.0–4.0 | |
| FSFI Dimension: Arousal | 12 weeks | 1.2 ± 1.4 | 0.002 * | 1.0 ± 1.8 | <0.001 * | 0.0–5.0 |
| 24 weeks | 1.4 ± 1.2 | <0.001 * | 1.0 ± 1.0 | <0.001 * | 0.0–5.0 | |
| FSFI Dimension: Lubrication | 12 weeks | 0.7 ± 1.0 | 0.009 * | 0.5 ± 1.0 | <0.001 * | −1.0–3.0 |
| 24 weeks | 1.0 ± 1.3 | 0.006 * | 1.0 ± 2.0 | 0.001 * | −2.0–3.0 | |
| FSFI Dimension: Orgasm | 12 weeks | 1.0 ± 1.0 | <0.001 * | 1.0 ± 1.6 | <0.001 * | −1.0–3.0 |
| 24 weeks | 1.2 ± 1.4 | 0.002 * | 1.0 ± 1.0 | <0.001 * | 0.0–4.0 | |
| FSFI Dimension: Satisfaction | 12 weeks | 0.9 ± 1.3 | 0.008 * | 1.0 ± 1.0 | <0.001 * | −1.0–4.0 |
| 24 weeks | 1.4 ± 1.5 | 0.001 * | 1.0 ± 2.0 | <0.001 * | 0.0–5.0 | |
| FSFI Dimension: Pain | 12 weeks | 0.8 ± 1.4 | 0.035 * | 1.0 ± 2.0 | 0.001 * | −2.0–3.0 |
| 24 weeks | 1.3 ± 1.7 | 0.005 * | 1.0 ± 2.0 | <0.001 * | −1.0–5.0 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaz-Mohedo, E.; Hita-Contreras, F.; Castro-Martin, E.; Pilat, A.; Perez-Dominguez, B.; Valenza-Peña, G. Using Myofascial Therapy to Improve Psychological Outcomes, Quality of Life, and Sexual Function in Women with Chronic Pelvic Pain—A Case Series. Healthcare 2024, 12, 304. https://doi.org/10.3390/healthcare12030304
Diaz-Mohedo E, Hita-Contreras F, Castro-Martin E, Pilat A, Perez-Dominguez B, Valenza-Peña G. Using Myofascial Therapy to Improve Psychological Outcomes, Quality of Life, and Sexual Function in Women with Chronic Pelvic Pain—A Case Series. Healthcare. 2024; 12(3):304. https://doi.org/10.3390/healthcare12030304
Chicago/Turabian StyleDiaz-Mohedo, Esther, Fidel Hita-Contreras, Eduardo Castro-Martin, Andrzej Pilat, Borja Perez-Dominguez, and Geraldine Valenza-Peña. 2024. "Using Myofascial Therapy to Improve Psychological Outcomes, Quality of Life, and Sexual Function in Women with Chronic Pelvic Pain—A Case Series" Healthcare 12, no. 3: 304. https://doi.org/10.3390/healthcare12030304
APA StyleDiaz-Mohedo, E., Hita-Contreras, F., Castro-Martin, E., Pilat, A., Perez-Dominguez, B., & Valenza-Peña, G. (2024). Using Myofascial Therapy to Improve Psychological Outcomes, Quality of Life, and Sexual Function in Women with Chronic Pelvic Pain—A Case Series. Healthcare, 12(3), 304. https://doi.org/10.3390/healthcare12030304







