Survive and Thrive: Outcomes of Children Enrolled in a Follow-Up Clinic for Small and Sick Newborns in Rural Rwanda
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Setting
2.2. The PDC Intervention Description
2.3. Household Surveys
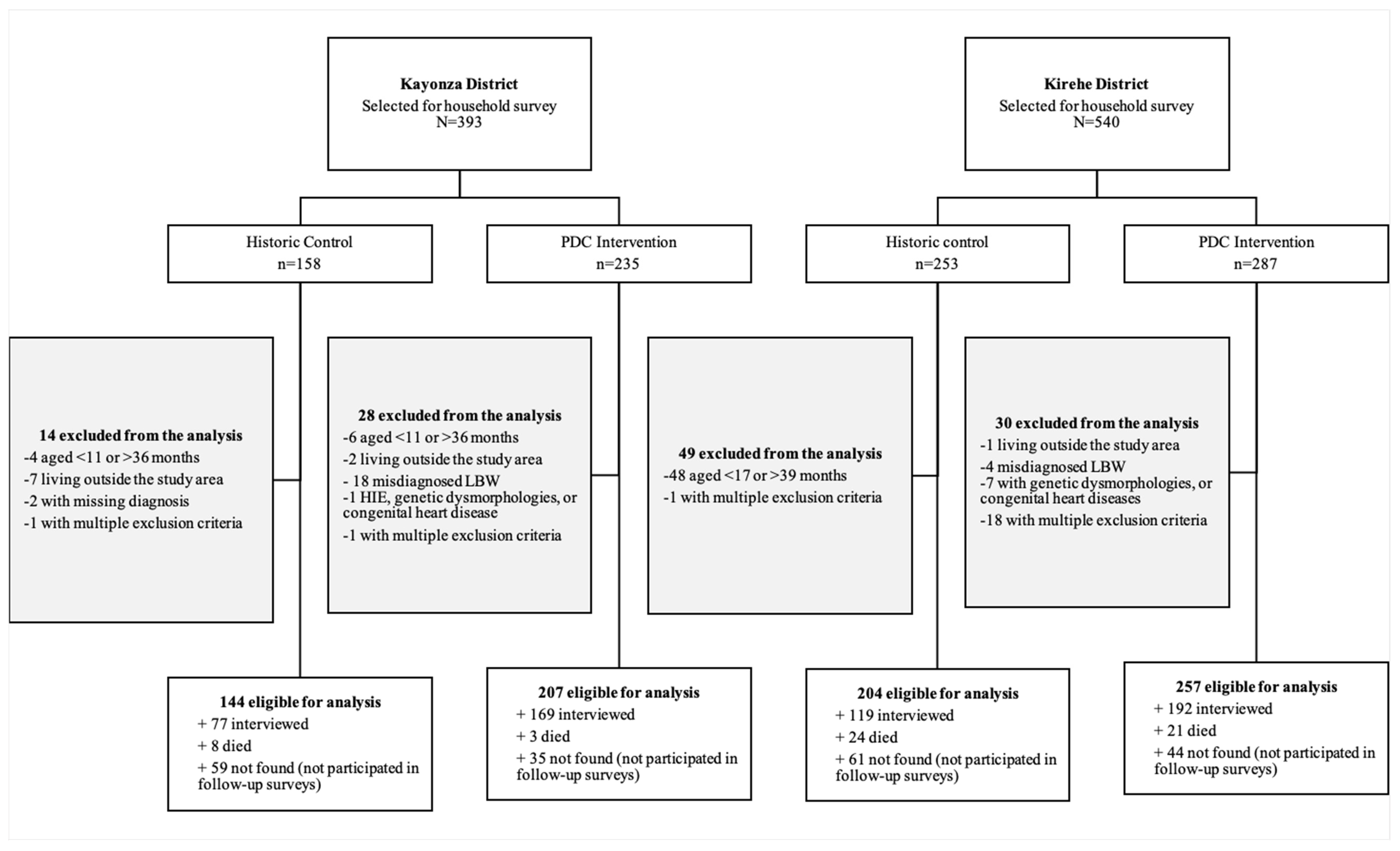
2.4. Study Participants
2.5. Outcome Measures
2.6. Data Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blencowe, H.; Krasevec, J.; de Onis, M.; Black, R.E.; An, X.; Stevens, G.A.; Borghi, E.; Hayashi, C.; Estevez, D.; Cegolon, L.; et al. National, regional, and worldwide estimates of low birthweight in 2015, with trends from 2000: A systematic analysis. Lancet Glob. Health 2019, 7, e849–e860. [Google Scholar] [CrossRef] [PubMed]
- Chawanpaiboon, S.; Vogel, J.P.; Moller, A.; Lumbiganon, P.; Petzold, M.; Hogan, D.; Landoulsi, S. Articles Global, regional, and national estimates of levels of preterm birth in 2014: A systematic review and modelling analysis. Lancet Glob. Health 2018, 7, e37–e46. [Google Scholar] [CrossRef] [PubMed]
- Kleinhout, M.Y.; Stevens, M.M.; Osman, K.A.; Adu-Bonsaffoh, K.; Groenendaal, F.; Biza Zepro, N.; Rijken, M.J.; Browne, J.L. Evidence-based interventions to reduce mortality among preterm and low-birthweight neonates in low-income and middle-income countries: A systematic review and meta-analysis. BMJ Glob. Health 2021, 6, e003618. [Google Scholar] [CrossRef] [PubMed]
- Christian, P.; Lee, S.E.; Angel, M.D.; Adair, L.S.; Arifeen, S.E.; Ashorn, P.; Barros, F.C.; Fall, C.H.D.; Fawzi, W.W.; Hao, W.; et al. Risk of childhood undernutrition related to small-for-gestational age and preterm birth in low- and middle-income countries. Int. J. Epidemiol. 2013, 42, 1340–1355. [Google Scholar] [CrossRef] [PubMed]
- Sania, A.; Spiegelman, D.; Rich-Edwards, J.; Okuma, J.; Kisenge, R.; Msamanga, G.; Urassa, W.; Fawzi, W.W. The Contribution of Preterm Birth and Intrauterine Growth Restriction to Infant Mortality in Tanzania. Pediatr. Perinat. Epidemiol. 2014, 28, 23–31. [Google Scholar] [CrossRef]
- Namazzi, G.; Tumwine, J.K.; Hildenwall, H.; Ndeezi, G.; Mubiri, P.; Hanson, C.; Kakooza-Mwesige, A.; Waiswa, P. Neurodevelopmental outcomes of preterm babies during infancy in Eastern Uganda: A prospective cohort study. Glob. Health Action 2020, 13, 1820714. [Google Scholar] [CrossRef]
- Gladstone, M.; White, S.; Kafulafula, G.; Neilson, J.P.; Van Den Broek, N. Post-neonatal Mortality, Morbidity, and Developmental Outcome after Ultrasound-Dated Preterm Birth in Rural Malawi: A Community-Based Cohort Study. PLoS Med. 2011, 8, e1001121. [Google Scholar] [CrossRef]
- Lawn, J.E.; Blencowe, H.; Oza, S.; You, D.; Lee, A.C.; Waiswa, P.; Lalli, M.; Bhutta, Z.; Barros, A.J.D.; Christian, P.; et al. Every Newborn: Progress, priorities, and potential beyond survival. Lancet 2014, 384, 189–205. [Google Scholar] [CrossRef]
- Milner, K.M.; Neal, E.F.G.; Roberts, G.; Steer, A.C.; Duke, T. Long-term neurodevelopmental outcome in high-risk newborns in resource-limited settings: A systematic review of the literature. Paediatr. Int. Child Health 2015, 35, 227–242. [Google Scholar] [CrossRef]
- WHO-UNICEF Expert and Country Consultation on Small and/or Sick Newborn Care Group. A comprehensive model for scaling up care for small and/or sick newborns at district level-based on country experiences presented at a WHO-UNICEF expert consultation. J. Glob. Health 2023, 13, 03023. [Google Scholar] [CrossRef]
- Lipner, H.S.; Huron, R.F. Developmental and Interprofessional Care of the Preterm Infant: Neonatal Intensive Care Unit Through High-Risk Infant Follow-up. Pediatr. Clin. 2018, 65, 135–141. [Google Scholar] [CrossRef]
- Yousafzai, A.K.; Lynch, P.; Gladstone, M. Moving beyond prevalence studies: Screening and interventions for children with disabilities in low-income and middle-income countries. Arch. Dis. Child. 2014, 99, 840–848. [Google Scholar] [CrossRef] [PubMed]
- Wainaina, J.; Lee, E.; Irimu, G.; Aluvaala, J.; English, M.; The Clinical Information Network Author Group. Identifying and quantifying initial post-discharge needs for clinical review of sick, newborns in Kenya based on a large multi-site, retrospective cohort study. Front. Pediatr. 2024, 12, 1374629. [Google Scholar] [CrossRef] [PubMed]
- Kabugo, D.; Nakamura, H.; Magnusson, B.; Vaughan, M.; Niyonshaba, B.; Nakiganda, C.; Otai, C.; Haddix-McKay, K.; Seela, M.; Nankabala, J.; et al. Mixed-method study to assess the feasibility, acceptability and early effectiveness of the Hospital to Home programme for follow-up of high-risk newborns in a rural district of Central Uganda: A study protocol. BMJ Open 2021, 11, e043773. [Google Scholar] [CrossRef] [PubMed]
- Doyle, L.W.; Anderson, P.J.; Battin, M.; Bowen, J.R.; Brown, N.; Callanan, C.; Campbell, C.; Chandler, S.; Cheong, J.; Darlow, B.; et al. Long term follow up of high risk children: Who, why and how? BMC Pediatr. 2014, 14, 279. [Google Scholar] [CrossRef]
- Hansen, A.; Magge, H.; Labrecque, M.; Munyaneza, R.B.M.; Nahimana, E.; Nyishime, M.; Mwali, A. The development and implementation of a newborn medicine program in a resource-limited setting. Public Health Action 2015, 5, 17–22. [Google Scholar] [CrossRef]
- National Institute of Statistics of Rwanda (NISR) [Rwanda]; Ministry of Health (MOH) [Rwanda]; ICF. Rwanda Demographic and Health Survey 2019–2020 Final Report; NISR: Kigali, Rwanda; ICF: Rockville, MD, USA, 2021; Available online: https://www.dhsprogram.com/pubs/pdf/FR370/FR370.pdf (accessed on 19 November 2024).
- Thukral, A.; Sankar, M.J.; Chandrasekaran, A.; Agarwal, R.; Paul, V.K. Efficacy and safety of CPAP in low- and middle-income countries. J. Perinatol. 2016, 36 (Suppl. S1), S21–S28. [Google Scholar] [CrossRef]
- Ntigurirwa, P.; Mellor, K.; Langer, D.; Evans, M.; Robertson, E.; Tuyisenge, L.; Groves, A.; Lissauer, T. A health partnership to reduce neonatal mortality in four hospitals in Rwanda. Glob. Health 2017, 13, 28. [Google Scholar] [CrossRef]
- Prullage, G.S.; Kenner, C.; Uwingabire, F.; Ndayambaje, A.; Boykova, M.; Walker, K. Survey of neonatal nursing: Staffing, education, and equipment availability in Rwanda. J. Neonatal Nurs. 2022, 28, 192–199. [Google Scholar] [CrossRef]
- Ngabireyimana, E.; Mutaganzwa, C.; Kirk, C.M.; Miller, A.C.; Wilson, K.; Dushimimana, E.; Bigirumwami, O.; Mukakabano, E.S.; Nkikabahizi, F.; Magge, H. A retrospective review of the Pediatric Development Clinic implementation: A model to improve medical, nutritional and developmental outcomes of at-risk under-five children in rural Rwanda. Matern. Health Neonatol. Perinatol. 2017, 3, 13. [Google Scholar] [CrossRef]
- National Institute of Statistics. Fourth Population and Housing Census, Rwanda, 2012: Final Results Main Indicators Report; Government of Rwanda: Kigali, Rwanda, 2012.
- UNHCR. Operational Update August 2019: Rwanda; UNHCR: Kigali, Rwanda, 2019; Available online: https://data.unhcr.org/en/documents/details/83957 (accessed on 19 November 2024).
- Nyishime, M.; Borg, R.; Ingabire, W.; Hedt-Gauthier, B.; Nahimana, E.; Gupta, N.; Hansen, A.; Labrecque, M.; Nkikabahizi, F.; Mutaganzwa, C.; et al. A retrospective study of neonatal case management and outcomes in rural Rwanda post implementation of a national neonatal care package for sick and small infants. BMC Pediatr. 2018, 18, 353. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Survive and Thrive: Transforming Care for Every Small and Sick Newborn; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Mutaganzwa, C. Understanding Factors Associated with Adherence to the Follow Up Visits in the Pediatric Development Clinic in the Eastern Province of Rwanda from 2014–2019. Master’s Thesis, Harvard Medical School, Boston, MA, USA, 2020. Available online: https://nrs.harvard.edu/URN-3:HUL.INSTREPOS:37365192 (accessed on 19 November 2024).
- Bradford, J.; Beck, K.; Nshimyiryo, A.; Wilson, K.; Mutaganzwa, C.; Havugarurema, S.; Ngamije, P.; Uwamahoro, A.; Kirk, C.M. Nutritional evaluation and growth of infants in a Rwandan neonatal follow-up clinic. Matern. Child. Nutr. 2020, 16, e13026. [Google Scholar] [CrossRef] [PubMed]
- Kirk, C.M.; Uwamungu, J.C.; Wilson, K.; Hedt-Gauthier, B.L.; Tapela, N.; Niyigena, P.; Rusangwa, C.; Nyishime, M.; Nahimana, E.; Nkikabahizi, F.; et al. Health, nutrition, and development of children born preterm and low birth weight in rural Rwanda: A cross-sectional study. BMC Pediatr. 2017, 17, 191. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. WHO Child Growth Standards: Length/Height-for-Age, Weight-for-Age, Weight-for-Length, Weight-for-Height and Body Mass Index-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- World Health Organization. WHO Child Growth Standards: Head Circumference-for-Age, Arm Circumference-for-Age, Triceps Skinfold-for-Age and Subscapular Skinfold-for-Age: Methods and Development; World Health Organization: Geneva, Switzerland, 2007. [Google Scholar]
- Squires, J.; Twombly, E.; Bricker, D.; Potter, L. ASQ-3 User’s Guide; Paul H. Brookes Publishing Company: Baltimore, MD, USA, 2009. [Google Scholar]
- Hsiao, C.; Richter, L.; Makusha, T.; Matafwali, B.; van Heerden, A.; Mabaso, M. Use of the ages and stages questionnaire adapted for South Africa and Zambia. Child Care Health Dev. 2017, 43, 59–66. [Google Scholar] [CrossRef]
- McCoy, D.C.; Waldman, M.; CREDI Field Team; Fink, G. Measuring early childhood development at a global scale: Evidence from the Caregiver-Reported Early Development Instruments. Early Child. Res. Q. 2018, 45, 58–68. [Google Scholar] [CrossRef]
- Piedvache, A.; Van Buuren, S.; Barros, H.; Ribeiro, A.I.; Draper, E.; Zeitlin, J.; the EPICE Research group; Martens, E.; Martens, G.; Van Reempts, P.; et al. Strategies for assessing the impact of loss to follow-up on estimates of neurodevelopmental impairment in a very preterm cohort at 2 years of age. BMC Med. Res. Methodol. 2021, 21, 118. [Google Scholar] [CrossRef]
- Olds, D.L.; Kitzman, H.; Knudtson, M.D.; Anson, E.; Smith, J.A.; Cole, R. Effect of Home Visiting by Nurses on Maternal and Child Mortality: Results of a 2-Decade Follow-up of a Randomized Clinical Trial. JAMA Pediatr. 2014, 168, 800. [Google Scholar] [CrossRef]
- Britto, P.R.; Lye, S.J.; Proulx, K.; Yousafzai, A.K.; Matthews, S.G.; Vaivada, T.; Perez-Escamilla, R.; Rao, N.; Ip, P.; Fernald, L.C.H.; et al. Nurturing care: Promoting early childhood development. Lancet 2017, 389, 91–102. [Google Scholar] [CrossRef]
- Fernald, L.C.H.; Prado, E.; Kariger, P.; Raikes, A. A Toolkit for Measuring Early Childhood Development in Low and Middle-Income Countries; World Bank: Washington, DC, USA, 2017. [Google Scholar]
- Ertem, I.O.; Dogan, D.G.; Gok, C.G.; Kizilates, S.U.; Caliskan, A.; Atay, G.; Vatandas, N.; Karaaslan, T.; Baskan, S.G.; Cicchetti, D.V. A Guide for Monitoring Child Development in Low- and Middle-Income Countries. Pediatrics 2008, 121, e581–e589. [Google Scholar] [CrossRef]
- Boggs, D.; Milner, K.M.; Chandna, J.; Black, M.; Cavallera, V.; Dua, T.; Fink, G.; Kc, A.; Grantham-McGregor, S.; Hamadani, J.; et al. Rating early child development outcome measurement tools for routine health programme use. Arch. Dis. Child. 2019, 104, S22–S33. [Google Scholar] [CrossRef]
- Debebe, Z.Y.; Raju, D. Covariate Shocks and Child Undernutrition: A Review of Evidence from Low- and Middle-Income Countries; World Bank: Washington, DC, USA, 2020. [Google Scholar]
- Schoenbuchner, S.M.; Dolan, C.; Mwangome, M.; Hall, A.; Richard, S.A.; Wells, J.C.; Khara, T.; Sonko, B.; Prentice, A.M.; Moore, S.E. The relationship between wasting and stunting: A retrospective cohort analysis of longitudinal data in Gambian children from 1976 to 2016. Am. J. Clin. Nutr. 2019, 110, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Rwanda 2018|Comprehensive Food Security and Vulnerability Analysis; National Institute of Statistics of Rwanda: Kigali, Rwanda, 2018.
- Children Aged Under 5 Years Who Are Underweight—MEASURE Evaluation. Available online: https://www.measureevaluation.org/rbf/indicator-collections/health-outcome-impact-indicators/children-aged-under-5-years-who-are-underweight.html (accessed on 5 June 2022).
- Emergency Nutrition Network; GOAL; London School of Hygiene and Tropical Medicine. Save the Children C-MAMI Tool. Version 2. 2018. Available online: https://resourcecentre.savethechildren.net/document/c-mami-tool-version-2/ (accessed on 19 November 2024).
- Beck, K.; Kirk, C.M.; Mutaganzwa, C.; Bradford, J.; Bigirumwami, O.; Nahimana, E. The Paediatric Development Clinic: A model to improve outcomes for high-risk children aged under-five in rural Rwanda. Emerg. Nutr. Netw. Field Exch. 2018, 58, 51–53. [Google Scholar]
- National Institute of Statistics of Rwanda (NISR) [Rwanda]; Ministry of Health (MOH) [Rwanda]; ICF International. Rwanda Demographic and Health Survey 2014–2015; NISR: Kigali, Rwanda; MOH: Kigali, Rwanda; ICF International: Rockville, MD, USA, 2015.
- Magge, H.; Nahimana, E.; Mugunga, J.C.; Nkikabahizi, F.; Tadiri, E.; Sayinzoga, F.; Manzi, A.; Nyishime, M.; Biziyaremye, F.; Iyer, H.; et al. The All Babies Count Initiative: Impact of a Health System Improvement Approach on Neonatal Care and Outcomes in Rwanda. Glob. Health Sci. Pract. 2020, 8, 505–517. [Google Scholar] [CrossRef] [PubMed]

| Variable | Kayonza District, n = 351 | Kirehe District, n = 461 | All, n = 812 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Historical Control 144 (41.0%) | PDC Intervention 207 (59.0%) | p-Value | Historical Control 204 (44.2%) | PDC 1 Intervention 257 (55.8%) | p-Value | Historical Control 348 (42.9%) | PDC Intervention 464 (57.1%) | p-Value | |
| Child sex, n (%) | 0.938 | 0.345 | 0.406 | ||||||
| Male | 66 (45.8) | 94 (45.4) | 113 (55.4) | 131 (51.0) | 179 (51.4) | 225 (48.5) | |||
| Female | 78 (54.2) | 113 (54.6) | 91 (44.6) | 126 (49.0) | 169 (48.6) | 239 (51.5) | |||
| Birth weight (grams), n (%) | 0.478 | 0.001 | 0.003 | ||||||
| ≥1500 | 104 (72.2) | 141 (68.1) | 178 (87.2) | 192 (74.7) | 282 (81.0) | 333 (71.8) | |||
| <1500 | 40 (27.8) | 66 (31.9) | 26 (12.8) | 62 (24.1) | 66 (19.0) | 128 (27.6) | |||
| Missing data | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (1.2) | 0 (0.0) | 3 (0.7) | |||
| Diagnosis, n (%) | NA | 0.104 | 0.082 | ||||||
| Preterm/LBW 2 | 144 (100.0) | 207 (100.0) | 145 (71.1) | 190 (73.9) | 289 (83.0) | 397 (85.6) | |||
| HIE 3 | NA | NA | 57 (27.9) | 58 (22.6) | 57 (16.4) | 58 (12.5) | |||
| HIE 3 & Preterm/LBW 2 | NA | NA | 2 (1.0) | 9 (3.5) | 2 (0.6) | 9 (1.9) | |||
| Child’s expected age on survey date (months), median [IQR 4] | 22.4 [17.8–28.2] | 25.7 [18.9–29.5] | 0.162 | 34.6 [31.1–37.0] | 26.7 [23.6–32.0] | <0.001 | 31.1 [25.7–35.4] | 26.2 [22.0–30.4] | <0.001 |
| Primary caregiver’s education 5, n (%) | 0.262 | 0.684 | 0.620 | ||||||
| None | 19 (24.4) | 28 (16.8) | 19 (16.1) | 37 (19.3) | 38 (19.4) | 65 (18.1) | |||
| Primary | 52 (66.7) | 115 (68.9) | 82 (69.5) | 124 (64.6) | 134 (68.4) | 239 (66.6) | |||
| Secondary/higher | 7 (9.0) | 24 (14.4) | 17 (14.4) | 31 (16.1) | 24 (12.2) | 55 (15.3) | |||
| Primary caregiver’s marital status 5, n (%) | 0.301 | 0.290 | 0.937 | ||||||
| Married | 34 (43.6) | 56 (33.5) | 53 (44.9) | 101 (52.6) | 87 (44.4) | 157 (43.7) | |||
| Cohabitating | 29 (37.2) | 77 (46.1) | 47 (39.8) | 60 (31.2) | 76 (38.8) | 137 (38.2) | |||
| Single/Widowed/Divorced | 15 (19.2) | 34 (20.4) | 18 (15.3) | 31 (16.2) | 33 (16.8) | 65 (18.1) | |||
| Variable | Kayonza District, n = 351 | Kirehe District, n = 461 | All, n = 812 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Historical Control | PDC Intervention | p-Value | Historical Control | PDC Intervention | p-Value | Historical Control | PDC Intervention | p-Value | |
| Death, n = 613, n (%) | 0.007 | 0.073 | 0.002 | ||||||
| No | 77 (90.6) | 169 (98.3) | 119 (83.2) | 192 (90.1) | 196 (86.0) | 361 (93.8) | |||
| Yes | 8 (9.4) | 3 (1.7) | 24 (16.8) | 21 (9.9) | 32 (14.0) | 24 (6.2) | |||
| Stunting, n = 547 1, n (%) | 0.048 | 0.903 | 0.199 | ||||||
| No | 23 (30.7) | 75 (44.6) | 44 (37.0) | 67 (36.2) | 67 (34.5) | 142 (40.2) | |||
| Yes | 52 (69.3) | 93 (55.4) | 75 (63.0) | 118 (63.8) | 127 (65.5) | 211 (59.8) | |||
| Underweight, n = 556 1, n (%) | 0.178 | 0.708 | 0.214 | ||||||
| No | 48 (63.2) | 122 (72.2) | 79 (66.4) | 132 (68.8) | 127 (65.1) | 254 (70.4) | |||
| Yes | 28 (36.8) | 47 (27.8) | 40 (33.6) | 60 (31.2) | 68 (34.9) | 107 (29.6) | |||
| Wasting, n = 540 1, n (%) | 0.621 | 0.610 | 0.483 | ||||||
| No | 65 (90.3) | 151 (92.1) | 111 (93.3) | 176 (95.1) | 176 (92.1) | 327 (93.7) | |||
| Yes | 7 (9.7) | 13 (7.9) | 8 (6.7) | 9 (4.9) | 15 (7.9) | 22 (6.3) | |||
| ASQ-3 total, n = 549 1, n (%) | <0.001 | 0.007 | 0.072 | ||||||
| Typical | 5 (6.6) | 64 (39.0) | 31 (26.3) | 26 (13.6) | 36 (18.6) | 90 (25.3) | |||
| Potential delay | 71 (93.4) | 100 (61.0) | 87 (73.7) | 165 (86.4) | 158 (81.4) | 265 (74.7) | |||
| Anthropometric z-scores 2 | |||||||||
| Height-for-age, n = 547, median [IQR] | −2.61 [−3.44, −1.79] | −2.19 [−2.99, −1.29] | 0.005 | −2.52 [−3.09, −1.70] | −2.44 [−3.09, −1.53] | 0.816 | −2.56 [−3.23, −1.77] | −2.36 [−3.04, −1.37] | 0.037 |
| Weight-for-age, n = 556, median [IQR] | −1.40 [−2.37, −0.54] | −1.31 [−2.15, −0.57] | 0.430 | −1.66 [−2.25, −0.80] | −1.48 [−2.31, −0.68] | 0.646 | −1.54 [−2.33, −0.66] | −1.37 [−2.22, −0.58] | 0.312 |
| Weight-for-height, n = 541, median [IQR] | 0.01 [−0.93, 0.61] | −0.18 [−1.11, 0.39] | 0.319 | −0.15 [−0.93, 0.50] | −0.28 [−0.91, 0.50] | 0.915 | −0.12 [−0.93, 0.57] | −0.22 [−1.00, 0.43] | 0.576 |
| ASQ-3 z-scores by domain 3 | |||||||||
| Fine Motor, n = 536, median [IQR] | −2.68 [−3.10, −1.79] | −1.33 [−2.06, −0.34] | <0.001 | −2.51 [−3.47, −1.31] | −1.80 [−2.91, −1.04] | 0.024 | −2.55 [−3.24, −1.51] | −1.49 [−2.70, −0.75] | <0.001 |
| Gross Motor, n = 554, median [IQR] | −2.09 [−3.65, −1.06] | −1.15 [−2.03, 0.03] | <0.001 | −2.23 [−3.63, −0.62] | −1.35 [−3.45, −0.35] | 0.092 | −2.21 [−3.63, −0.77] | −1.24 [−2.58, −0.09] | <0.001 |
| Communication, n = 554, median [IQR] | −2.24 [−3.24, −1.59] | −1.38 [−2.61, −0.13] | <0.001 | −1.96 [−3.79, −0.66] | −2.52 [−4.06, −0.98] | 0.297 | −2.10 [−3.34, −0.98] | −2.03 [−3.14, −0.54] | 0.056 |
| Problem solving, n = 551, median [IQR] | −1.80 [−3.07, −0.64] | −0.51 [−1.86, 0.25] | <0.001 | −0.26 [−1.64, 0.13] | −1.41 [−2.75, −0.51] | <0.001 | −0.93 [−2.27, 0.13] | −0.96 [−2.32, −0.13] | 0.296 |
| Personal social, n = 554, median [IQR] | −1.81 [−3.17, −0.77] | −0.65 [−1.48, 0.34] | <0.001 | −1.62 [−2.70, −0.73] | −1.32 [−2.71, −0.37] | 0.046 | −1.75 [−3.11, −0.73] | −0.86 [−2.04, 0.02] | <0.001 |
| CREDI 4 Overall score, median [IQR], n = 262 | - | - | - | 51.54 [51.15, 51.95] | 50.96 [50.36, 51.51] | <0.001 | 51.54 [51.15, 51.95] | 50.96 [50.36, 51.51] | <0.001 |
| Kayonza District | Kirehe District | All | ||||
|---|---|---|---|---|---|---|
| Binary Outcomes | aOR (95% CI) | p-value | aOR (95% CI) | p-value | aOR (95% CI) | p-value |
| Death 1 (n = 613) | 0.12 (0.03, 0.50) | 0.003 | 0.62 (0.25, 1.55) | 0.309 | 0.49 (0.26, 0.92) | 0.025 |
| Stunting 2 (n = 544) | 0.52 (0.28, 0.98) | 0.044 | 1.17 (0.62, 2.19) | 0.624 | 0.87 (0.59, 1.28) | 0.465 |
| Underweight 2 (n = 553) | 0.76 (0.41, 1.39) | 0.370 | 1.19 (0.61, 2.34) | 0.605 | 0.77 (0.52, 1.15) | 0.209 |
| Wasting 2 (n = 537) | 1.25 (0.41, 3.84) | 0.699 | 0.35 (0.07, 1.78) | 0.207 | 0.72 (0.35, 1.48) | 0.377 |
| ASQ-3 Total 2 (n = 546) | 0.10 (0.04, 0.29) | <0.001 | 1.31 (0.60, 2.85) | 0.497 | 0.48 (0.30, 0.77) | 0.002 |
| Continuous Outcomes | β (95% CI) | p-value | β (95% CI) | p-value | β (95% CI) | p-value |
| Nutrition indicators | ||||||
| Height-for-age z-score (n = 544) | 0.52 (0.19, 0.86) | 0.002 | 0.04 (−0.35, 0.42) | 0.856 | 0.22 (−0.001, 0.44) | 0.051 |
| Weight-for-age z-score (n = 553) | 0.12 (−0.24, 0.49) | 0.511 | −0.03 (−0.41, 0.35) | 0.885 | 0.12 (−0.11, 0.34) | 0.306 |
| Weight-for-height z-score (n = 537) | −0.27 (−0.61, 0.07) | 0.123 | 0.17 (−0.20, 0.54) | 0.377 | 0.05 (−0.17, 0.27) | 0.664 |
| Development indicators | ||||||
| ASQ-3 Fine Motor z-score (n = 533) | 1.09 (0.77, 1.42) | <0.001 | −0.16 (−0.55, 0.23) | 0.416 | 0.68 (0.45, 0.92) | <0.001 |
| ASQ-3 Gross Motor z-score (n = 551) | 1.25 (0.74, 1.75) | <0.001 | −0.39 (−1.18, 0.39) | 0.326 | 0.51 (0.13, 0.89) | 0.008 |
| ASQ-3 Communication z-score (n = 551) | 0.80 (0.42, 1.18) | <0.001 | −0.69 (−1.33, −0.04) | 0.037 | 0.31 (−0.02, 0.63) | 0.064 |
| ASQ-3 Problem Solving z-score (n = 548) | 0.78 (0.44, 1.11) | <0.001 | −0.83 (−1.20, −0.45) | <0.001 | 0.21 (−0.03, 0.45) | 0.081 |
| ASQ-3 Personal Social z-score (n = 551) | 1.10 (0.64, 1.57) | <0.001 | −0.14 (−0.63, 0.34) | 0.557 | 0.77 (0.47, 1.07) | <0.001 |
| CREDI Overall score (n = 261) | - | - | −0.04 (−0.28, 0.21) | 0.778 | −0.04 (−0.28, 0.21) | 0.778 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nshimyiryo, A.; Barnhart, D.A.; Nemerimana, M.; Beck, K.; Wilson, K.; Mutaganzwa, C.; Bigirumwami, O.; Shema, E.; Uwamahoro, A.; Itangishaka, C.; et al. Survive and Thrive: Outcomes of Children Enrolled in a Follow-Up Clinic for Small and Sick Newborns in Rural Rwanda. Healthcare 2024, 12, 2368. https://doi.org/10.3390/healthcare12232368
Nshimyiryo A, Barnhart DA, Nemerimana M, Beck K, Wilson K, Mutaganzwa C, Bigirumwami O, Shema E, Uwamahoro A, Itangishaka C, et al. Survive and Thrive: Outcomes of Children Enrolled in a Follow-Up Clinic for Small and Sick Newborns in Rural Rwanda. Healthcare. 2024; 12(23):2368. https://doi.org/10.3390/healthcare12232368
Chicago/Turabian StyleNshimyiryo, Alphonse, Dale A. Barnhart, Mathieu Nemerimana, Kathryn Beck, Kim Wilson, Christine Mutaganzwa, Olivier Bigirumwami, Evelyne Shema, Alphonsine Uwamahoro, Cécile Itangishaka, and et al. 2024. "Survive and Thrive: Outcomes of Children Enrolled in a Follow-Up Clinic for Small and Sick Newborns in Rural Rwanda" Healthcare 12, no. 23: 2368. https://doi.org/10.3390/healthcare12232368
APA StyleNshimyiryo, A., Barnhart, D. A., Nemerimana, M., Beck, K., Wilson, K., Mutaganzwa, C., Bigirumwami, O., Shema, E., Uwamahoro, A., Itangishaka, C., Havugarurema, S., Sayinzoga, F., Baganizi, E., Magge, H., & Kirk, C. M. (2024). Survive and Thrive: Outcomes of Children Enrolled in a Follow-Up Clinic for Small and Sick Newborns in Rural Rwanda. Healthcare, 12(23), 2368. https://doi.org/10.3390/healthcare12232368






