Long-Term Effects of Microfiltered Seawater and Resistance Training with Elastic Bands on Hepatic Parameters, Inflammation, Oxidative Stress, and Blood Pressure of Older Women: A 32-Week, Double-Blinded, Randomized, Placebo-Controlled Trial
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Participants
2.3. Training Intervention
2.4. Supplementation Protocol
2.5. Biochemical Blood Parameters Measurement
2.6. Blood Pressure Measurement
2.7. Statistical Analysis
3. Results
3.1. Participants
3.2. Hepatic Biomarkers
3.3. Oxidative Stress, Inflammatory State, and Related Parameters (Vitamin D Status)
3.4. Systolic and Diastolic Blood Pressure
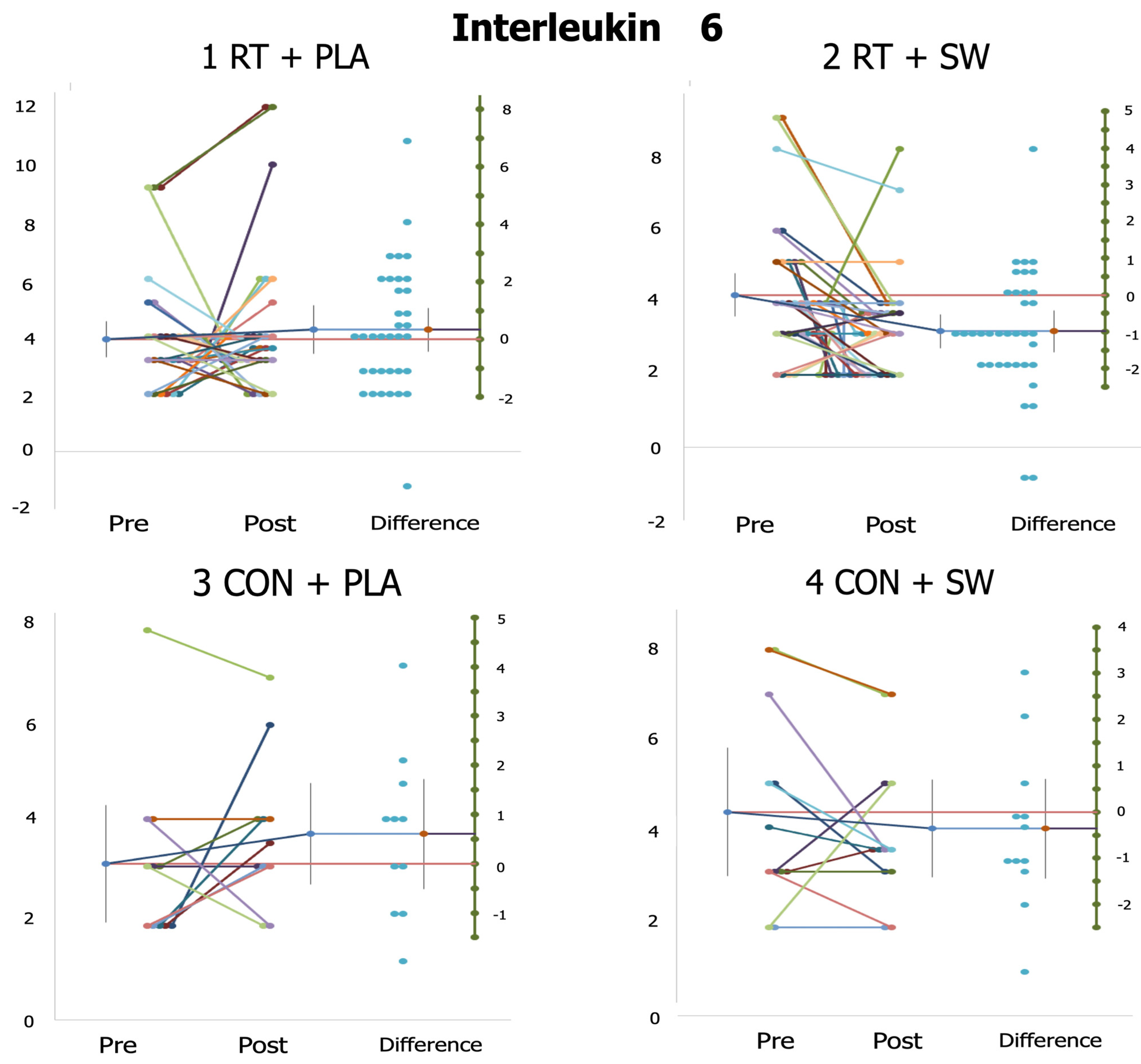
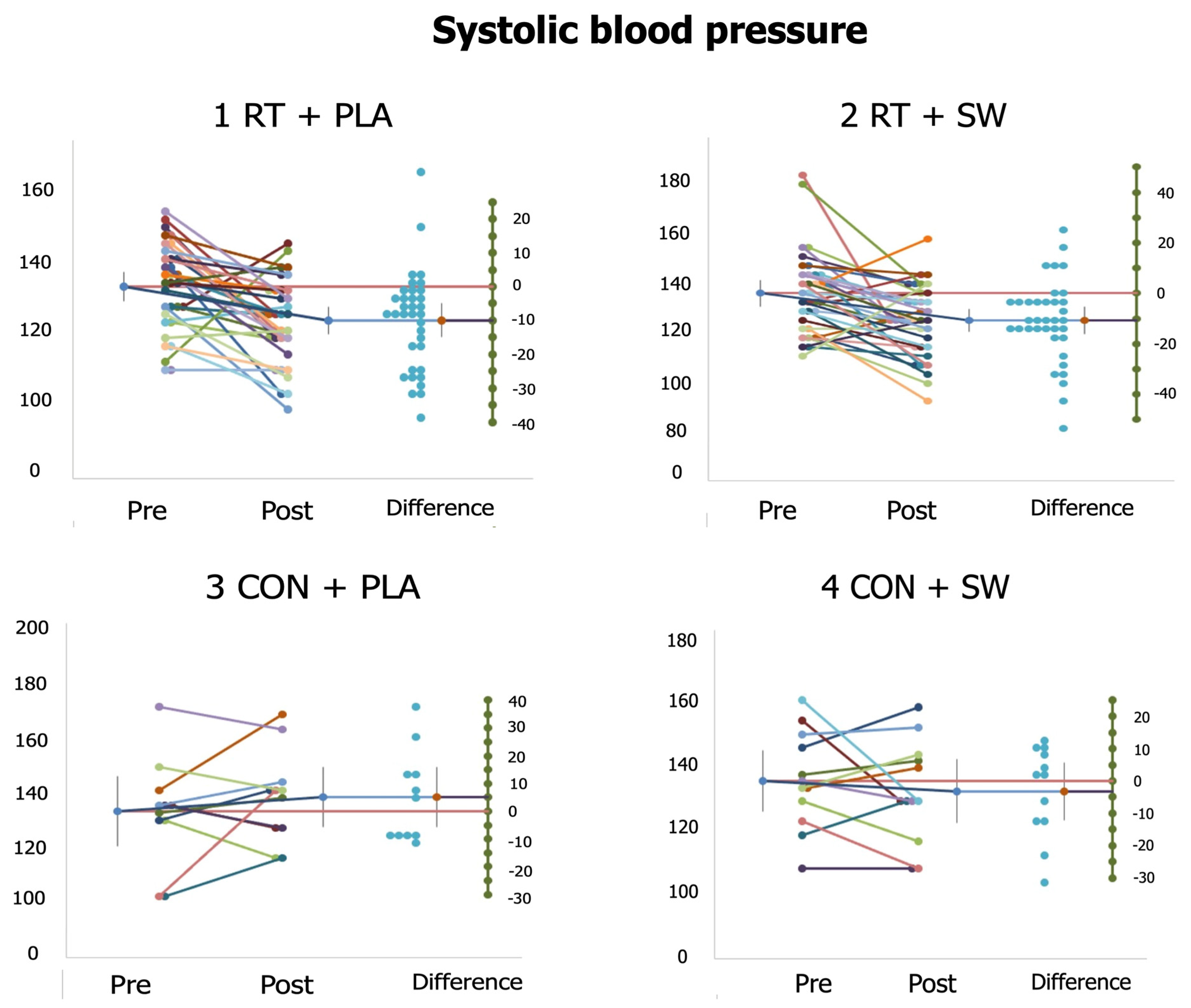
3.5. Individual Responses
3.6. Adverse Events
4. Discussion
4.1. Hepatic Responses
4.2. Oxidative Stress, Inflammatory Responses, and Related Parameters
4.3. Systolic and Diastolic Blood Pressure Responses
4.4. Limitations, Strengths, and Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moya-Nájera, D.; Moya-Herraiz, Á.; Compte-Torrero, L.; Hervás, D.; Borreani, S.; Calatayud, J.; Berenguer, M.; Colado, J.C. Combined resistance and endurance training at a moderate-to-high intensity improves physical condition and quality of life in liver transplant patients. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2017, 23, 1273–1281. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, L.; Li, S. Advances in nutritional supplementation for sarcopenia management. Front. Nutr. 2023, 10, 1189522. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Martínez, P.; Ramirez-Campillo, R.; Flandez, J.; Alix-Fages, C.; Baz-Valle, E.; Colado, J.C. Effects of oral capsaicinoids and capsinoids supplementation on resistance and high intensity in-terval training: A systematic review of randomized controlled trials. J. Hum. Sport. Exerc. 2022, 18, 375–389. [Google Scholar]
- Lee, S.R.; Directo, D. Fish Oil Supplementation with Resistance Exercise Training Enhances Physical Function and Cardiometabolic Health in Postmenopausal Women. Nutrients 2023, 15, 4516. [Google Scholar] [CrossRef]
- Nani, S.Z.M.; Zura, D.; Majid, F.A.A.; Jaafar, A.B.; Mahdzir, A.; Musa, M.N. Potential Health Benefits of Deep Sea Water: A Review. Evid.-Based Complement. Altern. Med. ECAM 2016, 2016, 6520475. [Google Scholar] [CrossRef]
- Al-Qurashi, T.M.; Aljaloud, K.S.; Aldayel, A.A.; Alsharif, Y.R.; Alaqil, A.I.; Alshuwaier, G.O. Effect of Rehydration with Mineral Water Following Exercise Induced Dehydration on Cardiorespiratory Fitness in Athletes. Preprints 2022, 2022030147. [Google Scholar] [CrossRef]
- Pérez-Turpin, J.A.; Trottini, M.; Chinchilla-Mira, J.J.; Cyganik, W. Effects of seawater ingestion on lactate response to exercise in runners. Biol. Sport. 2017, 34, 407–412. [Google Scholar] [CrossRef]
- Shiraishi, H.; Fujino, M.; Shirakawa, N.; Ishida, N.; Funato, H.; Hirata, A.; Abe, N.; Iizuka, M.; Jobu, K.; Yokota, J.; et al. Effect of Minerals on Intestinal IgA Production Using Deep Sea Water Drinks. Biol. Pharm. Bull. 2017, 40, 1700–1705. [Google Scholar] [CrossRef]
- Sowers, J.R. Hypertension in the elderly. Am. J. Med. 1987, 82, 1–8. [Google Scholar] [CrossRef]
- Malta, D.; Petersen, K.S.; Johnson, C.; Trieu, K.; Rae, S.; Jefferson, K.; Santos, J.A.; Wong, M.M.Y.; Raj, T.S.; Webster, J.; et al. High sodium intake increases blood pressure and risk of kidney disease. From the Science of Salt: A regularly updated systematic review of salt and health outcomes (August 2016 to March 2017). J. Clin. Hypertens. Greenwich Conn. 2018, 20, 1654–1665. [Google Scholar] [CrossRef]
- Babiloni-Lopez, C.; Gene-Morales, J.; Saez-Berlanga, A.; Ramirez-Campillo, R.; Moreno-Murcia, J.A.; Colado, J.C. The Use of Elastic Bands in Velocity-Based Training Allows Greater Acute External Training Stimulus and Lower Perceived Effort Compared to Weight Plates. Int. J. Environ. Res. Public Health 2022, 19, 16616. [Google Scholar] [CrossRef] [PubMed]
- Saez-Berlanga, A.; Gargallo, P.; Gene-Morales, J.; Babiloni, C.; Colado, J.C.; Juesas, A. Multicomponent elastic training improves short-term body composition and balance in older women. Sci. J. Sport Perform. 2022, 1, 4–13. [Google Scholar] [CrossRef]
- Gene-Morales, J.; Gené-Sampedro, A.; Salvador-Palmer, R.; Colado, J.C. Effects of squatting with elastic bands or conventional resistance—Training equipment at different effort levels in post-exercise intraocular pressure of healthy men. Biol. Sport 2021, 39, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; Triplett, N.T. Effects of a short-term resistance program using elastic bands versus weight machines for sedentary middle-aged women. J. Strength Cond. Res. 2008, 22, 1441–1448. [Google Scholar] [CrossRef] [PubMed]
- Niki, E. Oxidative stress and antioxidants: Distress or eustress? Arch. Biochem. Biophys. 2016, 595, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Gargallo, P.; Colado, J.C.; Juesas, A.; Hernando-Espinilla, A.; Estañ-Capell, N.; Monzó-Beltran, L.; García-Pérez, P.; Cauli, O.; Sáez, G.T. The Effect of Moderate- Versus High-Intensity Resistance Training on Systemic Redox State and DNA Damage in Healthy Older Women. Biol. Res. Nurs. 2018, 20, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.; Tan, Z.; Hua, Y.; Huang, X.; Gao, Y.; Wu, Y.; Liu, B.; Zhou, Y. Deep sea water improves exercise and inhibits oxidative stress in a physical fatigue mouse model. Biomed. Rep. 2016, 4, 751–757. [Google Scholar] [CrossRef]
- Wee, C.L.; Azemi, A.K.; Mokhtar, S.S.; Yahaya, S.; Yaacob, N.S.; Rasool, A.H.G. Vitamin D deficiency enhances vascular oxidative stress, inflammation, and angiotensin II levels in the microcirculation of diabetic patients. Microvasc. Res. 2023, 150, 104574. [Google Scholar] [CrossRef]
- Saovieng, S.; Wu, J.; Huang, C.-Y.; Kao, C.-L.; Higgins, M.F.; Chuanchaiyakul, R.; Kuo, C.-H. Deep Ocean Minerals Minimize Eccentric Exercise-Induced Inflammatory Response of Rat Skeletal Muscle. Front. Physiol. 2018, 9, 1351. [Google Scholar] [CrossRef]
- Yudkin, J.S.; Kumari, M.; Humphries, S.E.; Mohamed-Ali, V. Inflammation, obesity, stress and coronary heart disease: Is interleukin-6 the link? Atherosclerosis 2000, 148, 209–214. [Google Scholar] [CrossRef]
- Wimalawansa, S.J. Vitamin D Deficiency: Effects on Oxidative Stress, Epigenetics, Gene Regulation, and Aging. Biology 2019, 8, 30. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.M.; Nam, J.; Park, S.-Y.; Park, G.; Kim, B.G.; Jeong, G.-H.; Hurh, B.S.; Kim, J.Y. Effect of Mineral-Balanced Deep-Sea Water on Kidney Function and Renal Oxidative Stress Markers in Rats Fed a High-Salt Diet. Int. J. Mol. Sci. 2021, 22, 13415. [Google Scholar] [CrossRef] [PubMed]
- Uwitonze, A.M.; Razzaque, M.S. Role of Magnesium in Vitamin D Activation and Function. J. Am. Osteopat. Assoc. 2018, 118, 181–189. [Google Scholar] [CrossRef]
- Cornelissen, V.A.; Fagard, R.H.; Coeckelberghs, E.; Vanhees, L. Impact of resistance training on blood pressure and other cardiovascular risk factors: A meta-analysis of randomized, controlled trials. Hypertension 2011, 58, 950–958. [Google Scholar] [CrossRef] [PubMed]
- Juesas, A.; Gargallo, P.; Gene-Morales, J.; Babiloni-López, C.; Saez-Berlanga, A.; Jiménez-Martínez, P.; Casaña, J.; Benitez-Martinez, J.C.; Ramirez-Campillo, R.; Chulvi-Medrano, I.; et al. Effects of Microfiltered Seawater Intake and Variable Resistance Training on Strength, Bone Health, Body Composition, and Quality of Life in Older Women: A 32-Week Randomized, Double-Blinded, Placebo-Controlled Trial. Int. J. Environ. Res. Public Health 2023, 20, 4700. [Google Scholar] [CrossRef] [PubMed]
- Gargallo, P.; Tamayo, E.; Jiménez-Martínez, P.; Juesas, A.; Casaña, J.; Benitez-Martinez, J.C.; Gene-Morales, J.; Fernandez-Garrido, J.; Saez, G.T.; Colado, J.C. Multicomponent and power training with elastic bands improve metabolic and inflammatory parameters, body composition and anthropometry, and physical function in older women with metabolic syndrome: A 20-week randomized, controlled trial. Exp. Gerontol. 2024, 185, 112340. [Google Scholar] [CrossRef] [PubMed]
- Faul, F.; Erdfelder, E.; Lang, A.G.; Buchner, A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav. Res. Methods 2007, 39, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Colado, J.C.; García-Massó, X. Technique and safety aspects of resistance exercises: A systematic review of the literature. Physician Sportsmed. 2009, 37, 104–111. [Google Scholar] [CrossRef]
- Colado, J.C.; Pedrosa, F.M.; Juesas, A.; Gargallo, P.; Carrasco, J.J.; Flandez, J.; Chupel, M.U.; Teixeira, A.M.; Naclerio, F. Concurrent validation of the OMNI-Resistance Exercise Scale of perceived exertion with elastic bands in the elderly. Exp. Gerontol. 2018, 103, 11–16. [Google Scholar] [CrossRef]
- Page, P. Beyond statistical significance: Clinical interpretation of rehabilitation research literature. Int. J. Sports Phys. Ther. 2014, 9, 726–736. [Google Scholar]
- Rodriguez-Hernandez, H.; Cervantes-Huerta, M.; Rodriguez-Moran, M.; Guerrero-Romero, F. Oral magnesium supplementation decreases alanine aminotransferase levels in obese women. Magnes Res. 2010, 23, 90–96. [Google Scholar]
- Daneshyar, F.; Moradi, F.; Atashak, S. Effect of yoga with and without elastic band resistance training on visfatin, liver enzymes and body composition in postmenopausal women. Comp. Exerc. Physiol. 2020, 16, 347–355. [Google Scholar] [CrossRef]
- Pal, S.; Chaki, B.; Chattopadhyay, S.; Bandyopadhyay, A. High-Intensity Exercise Induced Oxidative Stress and Skeletal Muscle Damage in Postpubertal Boys and Girls: A Comparative Study. J. Strength Cond. Res. 2018, 32, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Hernando-Espinilla, A. Oxidative Stress and Aging Biomarkers. Controlled Physical Exercise on the Levels of the Oxidative Parameters of Older Women. A Special Study of the Redox State and Oxidative Modification of the DNA. Ph.D. Dissertation, University of Valencia, Valencia, Spain, 2020. Available online: https://roderic.uv.es/items/504a34d9-ab32-4608-97cd-82df43462b33 (accessed on 20 November 2023).
- Vincent, K.R.; Vincent, H.K.; Braith, R.W.; Lennon, S.L.; Lowenthal, D.T. Resistance exercise training attenuates exercise-induced lipid peroxidation in the elderly. Eur. J. Appl. Physiol. 2002, 87, 416–423. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Li, J.; Liu, Z.; Chuang, C.-C.; Yang, W.; Zuo, L. Redox Mechanism of Reactive Oxygen Species in Exercise. Front. Physiol. 2016, 7, 486. [Google Scholar] [CrossRef] [PubMed]
- Peters, P.G.; Alessio, H.M.; Hagerman, A.E.; Ashton, T.; Nagy, S.; Wiley, R.L. Short-term isometric exercise reduces systolic blood pressure in hypertensive adults: Possible role of reactive oxygen species. Int. J. Cardiol. 2006, 110, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Cakir-Atabek, H.; Demir, S.; PinarbaŞili, R.D.; Gündüz, N. Effects of different resistance training intensity on indices of oxidative stress. J. Strength Cond. Res. 2010, 24, 2491–2497. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Rubio, P.; Trapero, I. The Beneficial Effect of Physical Exercise on Inflammatory Makers in Older Individuals. Endocr. Metab. Immune Disord. Drug Targets 2021, 21, 1008–1016. [Google Scholar] [CrossRef]
- Dai, Q.; Zhu, X.; Manson, J.E.; Song, Y.; Li, X.; Franke, A.A.; Costello, R.B.; Rosanoff, A.; Nian, H.; Fan, L.; et al. Magnesium status and supplementation influence vitamin D status and metabolism: Results from a randomized trial. Am. J. Clin. Nutr. 2018, 108, 1249–1258. [Google Scholar] [CrossRef]
- Kalvandi, F.; Azarbayjani, M.A.; Azizbeigi, R.; Azizbeigi, K. Elastic resistance training is more effective than vitamin D3 supplementation in reducing oxidative stress and strengthen antioxidant enzymes in healthy men. Eur. J. Clin. Nutr. 2022, 76, 610–615. [Google Scholar] [CrossRef]
- Katsuda, S.-I.; Yasukawa, T.; Nakagawa, K.; Miyake, M.; Yamasaki, M.; Katahira, K.; Mohri, M.; Shimizu, T.; Hazama, A. Deep-Sea water improves cardiovascular hemodynamics in Kurosawa and Kusanagi-Hypercholesterolemic (KHC) rabbits. Biol. Pharm. Bull. 2008, 31, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Liu, C.; Kuang, X.; Deng, Q.; Zhao, F.; Li, D. Effects of Multivitamin and Multimineral Supplementation on Blood Pressure: A Meta-Analysis of 12 Randomized Controlled Trials. Nutrients 2018, 10, 1018. [Google Scholar] [CrossRef] [PubMed]
- Flack, J.M.; Adekola, B. Blood pressure and the new ACC/AHA hypertension guidelines. Trends Cardiovasc. Med. 2020, 30, 160–164. [Google Scholar] [CrossRef] [PubMed]
- Alfredsson, L.; Armstrong, B.K.; Butterfield, D.A.; Chowdhury, R.; de Gruijl, F.R.; Feelisch, M.; Garland, C.F.; Hart, P.H.; Hoel, D.G.; Jacobsen, R.; et al. Insufficient Sun Exposure Has Become a Real Public Health Problem. Int. J. Environ. Res. Public Health 2020, 17, 5014. [Google Scholar] [CrossRef]
- Zhou, Y.; Hua, J.; Huang, Z. Effects of beer, wine, and baijiu consumption on non-alcoholic fatty liver disease: Potential implications of the flavor compounds in the alcoholic beverages. Front. Nutr. 2023, 9, 1022977. [Google Scholar] [CrossRef]
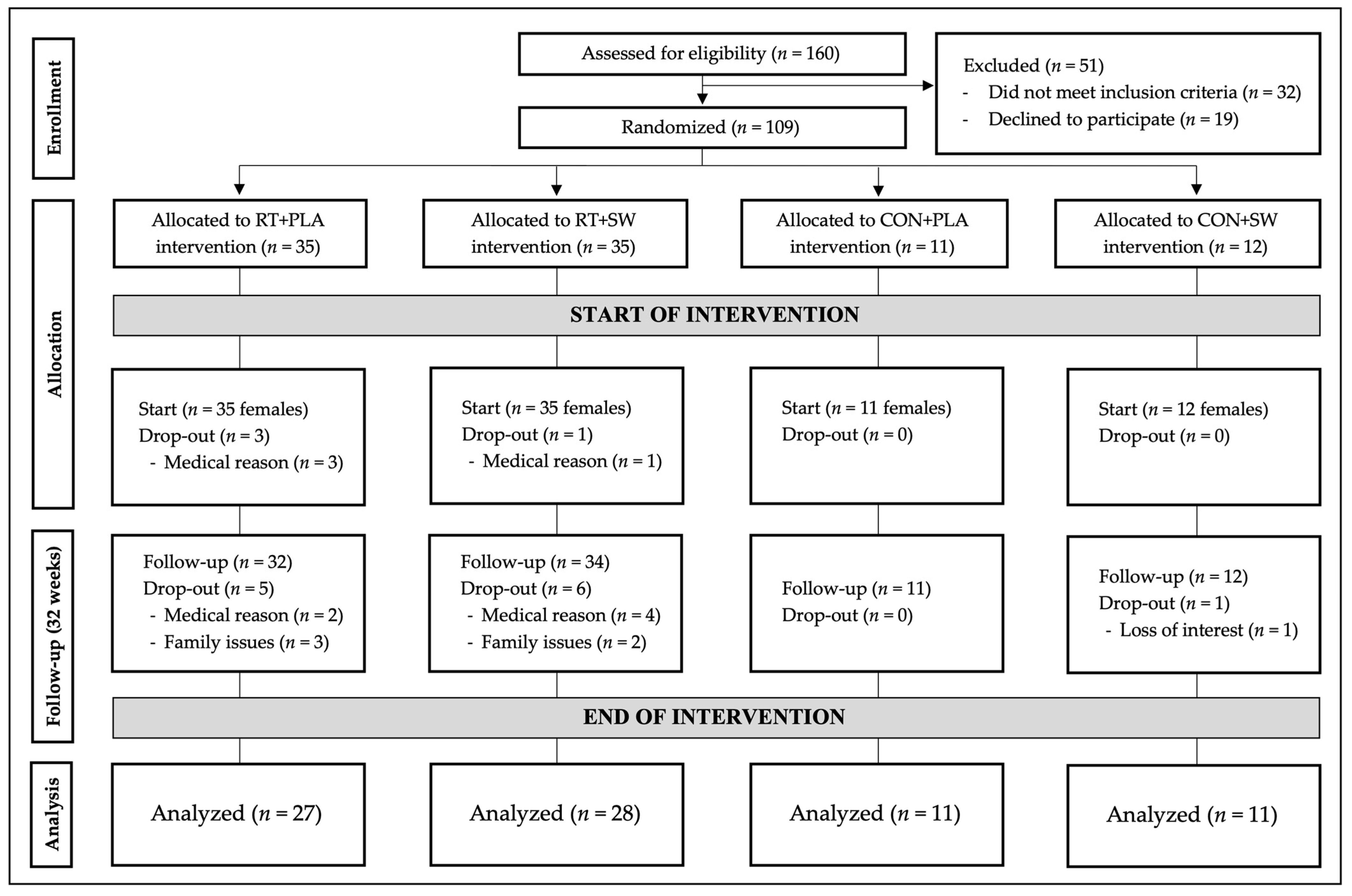
| 1 RT + PLA (n = 35) | 2 RT + SW (n = 35) | 3 CON + PLA (n = 11) | 4 CON + SW (n = 12) | |
|---|---|---|---|---|
| Age (years) | 69.17 ± 5.71 | 70.80 ± 5.86 | 67.90 ± 8.60 | 72.00 ± 7.07 |
| Weight (kg) | 66.25 ± 9.41 | 68.25 ± 11.41 | 64.36 ± 6.80 | 71.67 ± 10.46 |
| Height (cm) | 152.63 ± 4.54 | 153.46 ± 5.97 | 150.95 ± 5.19 | 153.24 ± 5.35 |
| BMI (kg/m2) | 28.41 ± 3.99 | 28.83 ± 4.73 | 28.50 ± 3.57 | 29.88 ± 4.69 |
| Fat mass (%) | 43.15 ± 1.39 | 43.94 ± 4.56 | 41.66 ± 3.26 | 45.91 ± 5.43 |
| UGT (s) | 6.74 ± 1.00 | 6.79 ± 0.86 | 6.39 ± 1.61 | 6.38 ± 0.87 |
| 1 RT + PLA (n = 35) | 2 RT + SW (n = 35) | 3 CON + PLA (n = 11) | 4 CON + SW (n = 12) | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| GOT | 22.23 ± 6.24 | 21.42 ± 4.77 | 21.20 ± 5.76 | 19.81 ± 3.76 | 19.80 ± 3.58 | 20.39 ± 3.69 | 24.09 ± 4.30 | 22.79 ± 6.27 |
| GPT | 19.68 ± 8.54 | 17.02 ± 6.23 * d = 0.36 | 19.44 ± 7.57 | 16.20 ± 4.68 * d = 0.51 | 17.70 ± 7.79 | 18.40 ± 6.02 | 18.73 ± 6.31 | 17.90 ± 4.18 |
| GGT | 21.26 ± 13.98 | 22.36 ± 13.11 | 20.09 ± 11.19 | 18.84 ± 7.50 | 24.00 ± 15.89 | 23.79 ± 12.90 | 20.36 ± 8.90 | 19.98 ± 5.40 |
| ALP | 73.97 ± 17.67 | 73.36 ± 13.63 | 66.32 ± 16.43 | 65.46 ± 11.97 | 71.30 ± 15.23 | 71.61 ± 12.81 | 68.64 ± 17.69 | 72.32 ± 12.95 |
| 1 RT + PLA (n = 35) | 2 RT + SW (n = 35) | 3 CON + PLA (n = 11) | 4 CON + SW (n = 12) | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| MDA (nmol/mg) | 0.19 ± 0.08 4 | 0.19 ± 0.04 4 | 0.20 ± 0.06 | 0.17 ± 0.06 4 | 0.18 ± 0.07 | 0.18 ± 0.07 4 | 0.26 ± 0.05 | 0.26 ± 0.05 |
| GSH/GSSG (%) | 1.18 ± 0.44 | 1.21 ± 0.46 | 1.08 ± 0.37 | 1.21 ± 0.46 | 1.25 ± 0.75 | 1.25 ± 0.75 | 0.97 ± 0.24 | 0.98 ± 0.25 |
| Vitamin D (ng/mL) | 26.31 ± 11.66 | 28.63 ± 13.59 | 25.56 ± 13.56 | 29.06 ± 13.99 * d = 0.25 | 23.10 ± 7.92 | 22.40 ± 7.37 | 17.73 ± 11.15 | 20.27 ± 10.19 |
| IL-6 (pg/mL) | 3.91 ± 1.84 | 4.25 ± 2.45 | 4.03 ± 1.64 | 3.03 ± 1.22 *,1 d = 0.69 | 2.70 ± 0.82 | 3.47 ± 1.17 | 4.09 ± 1.97 | 3.78 ± 1.45 |
| 1 RT + PLA (n = 35) | 2 RT + SW (n = 35) | 3 CON + PLA (n = 11) | 4 CON + SW (n = 12) | |||||
|---|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | |
| SBP (mmHg) | 134.01 ± 12.75 | 124.11 ± 12.31 *,3 d = 0.79 | 136.10 ± 16.07 | 125.43 ± 13.69 *,3 d = 0.71 | 132.55 ± 19.99 | 139.40 ± 15.29 | 133.77 ± 15.70 | 131.41 ± 15.57 |
| DBP (mmHg) | 75.60 ± 7.02 | 73.46 ± 8.65 | 76.23 ± 7.91 | 71.91 ± 9.04 *,3 d = 0.51 | 78.95 ± 10.87 | 81.40 ± 9.10 | 82.09 ± 11.64 | 76.82 ± 9.10 * d = 0.50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Babiloni-Lopez, C.; Gargallo, P.; Juesas, A.; Gene-Morales, J.; Saez-Berlanga, A.; Jiménez-Martínez, P.; Casaña, J.; Benitez-Martinez, J.C.; Sáez, G.T.; Fernández-Garrido, J.; et al. Long-Term Effects of Microfiltered Seawater and Resistance Training with Elastic Bands on Hepatic Parameters, Inflammation, Oxidative Stress, and Blood Pressure of Older Women: A 32-Week, Double-Blinded, Randomized, Placebo-Controlled Trial. Healthcare 2024, 12, 204. https://doi.org/10.3390/healthcare12020204
Babiloni-Lopez C, Gargallo P, Juesas A, Gene-Morales J, Saez-Berlanga A, Jiménez-Martínez P, Casaña J, Benitez-Martinez JC, Sáez GT, Fernández-Garrido J, et al. Long-Term Effects of Microfiltered Seawater and Resistance Training with Elastic Bands on Hepatic Parameters, Inflammation, Oxidative Stress, and Blood Pressure of Older Women: A 32-Week, Double-Blinded, Randomized, Placebo-Controlled Trial. Healthcare. 2024; 12(2):204. https://doi.org/10.3390/healthcare12020204
Chicago/Turabian StyleBabiloni-Lopez, Carlos, Pedro Gargallo, Alvaro Juesas, Javier Gene-Morales, Angel Saez-Berlanga, Pablo Jiménez-Martínez, Jose Casaña, Josep C. Benitez-Martinez, Guillermo T. Sáez, Julio Fernández-Garrido, and et al. 2024. "Long-Term Effects of Microfiltered Seawater and Resistance Training with Elastic Bands on Hepatic Parameters, Inflammation, Oxidative Stress, and Blood Pressure of Older Women: A 32-Week, Double-Blinded, Randomized, Placebo-Controlled Trial" Healthcare 12, no. 2: 204. https://doi.org/10.3390/healthcare12020204
APA StyleBabiloni-Lopez, C., Gargallo, P., Juesas, A., Gene-Morales, J., Saez-Berlanga, A., Jiménez-Martínez, P., Casaña, J., Benitez-Martinez, J. C., Sáez, G. T., Fernández-Garrido, J., Alix-Fages, C., & Colado, J. C. (2024). Long-Term Effects of Microfiltered Seawater and Resistance Training with Elastic Bands on Hepatic Parameters, Inflammation, Oxidative Stress, and Blood Pressure of Older Women: A 32-Week, Double-Blinded, Randomized, Placebo-Controlled Trial. Healthcare, 12(2), 204. https://doi.org/10.3390/healthcare12020204












