Association between Subjective Cognitive Complaints and Sleep Disturbance among Community-Dwelling Elderly Individuals in Japan
Abstract
1. Introduction
2. Materials and Methods
2.1. General Cognitive Functioning (MMSE)
2.2. SCC (KCL-CF)
2.3. Insomnia Symptoms (AIS-J)
2.4. Depressive Symptoms (GDS-5)
2.5. Risk Factors for Dementia Development
2.6. Sample Size Calculation
2.7. Statistical Analysis
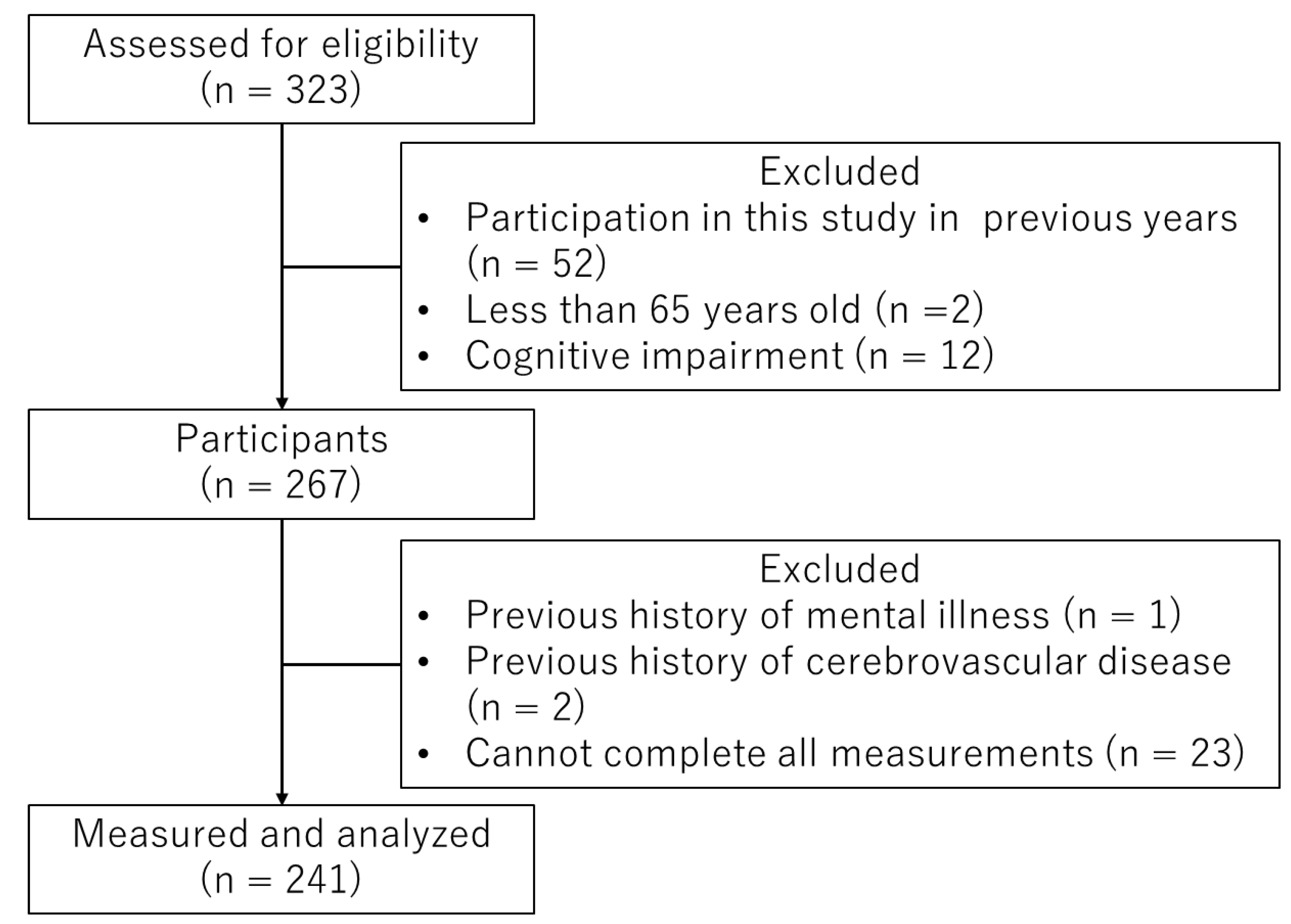
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Lancet Regional Health Europe. Challenges for addressing dementia. Lancet Reg. Health Eur. 2022, 20, 100504. [Google Scholar] [CrossRef]
- Nichols, E.; Steinmetz, J.D.; Vollset, S.E.; Fukutaki, K.; Chalek, J.; Abd-Allah, F.; Abdoli, A.; Abualhasan, A.; Abu-Gharbieh, E.; Akram, T.T.; et al. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: An analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022, 7, e105–e125. [Google Scholar] [CrossRef] [PubMed]
- Wimo, A.; Seeher, K.; Cataldi, R.; Cyhlarova, E.; Dielemann, J.L.; Frisell, O.; Guerchet, M.; Jönsson, L.; Malaha, A.K.; Nichols, E.; et al. The worldwide costs of dementia in 2019. Alzheimer’s Dement. 2023, 19, 2865–2873. [Google Scholar] [CrossRef]
- Niotis, K.; Akiyoshi, K.; Carlton, C.; Isaacson, R. Dementia prevention in clinical practice. Semin. Neurol. 2022, 42, 525–548. [Google Scholar] [CrossRef]
- 2022 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2022, 18, 700–789. [CrossRef] [PubMed]
- Jessen, F.; Amariglio, R.E.; van Boxtel, M.; Breteler, M.; Ceccaldi, M.; Chételat, G.; Dubois, B.; Dufouil, C.; Ellis, K.A.; van der Flier, W.M.; et al. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimer’s Dement. 2014, 10, 844–852. [Google Scholar] [CrossRef]
- Jacob, L.; Haro, J.M.; Koyanagi, A. Physical multimorbidity and subjective cognitive complaints among adults in the United Kingdom: A cross-sectional community-based study. Sci. Rep. 2019, 9, 12417. [Google Scholar] [CrossRef]
- Gómez-Ramírez, J.; Ávila-Villanueva, M.; Fernández-Blázquez, M.Á. Selecting the most important self-assessed features for predicting conversion to mild cognitive impairment with random forest and permutation-based methods. Sci. Rep. 2020, 10, 20630. [Google Scholar] [CrossRef] [PubMed]
- Parfenov, V.A.; Zakharov, V.V.; Kabaeva, A.R.; Vakhnina, N.V. Subjective cognitive decline as a predictor of future cognitive decline: A systematic review. Dement. Neuropsychol. 2020, 14, 248–257. [Google Scholar] [CrossRef]
- Gordon, B.A.; Blazey, T.M.; Su, Y.; Hari-Raj, A.; Dincer, A.; Flores, S.; Christensen, J.; McDade, E.; Wang, G.; Xiong, C.; et al. Spatial patterns of neuroimaging biomarker change in individuals from families with autosomal dominant Alzheimer’s disease: A longitudinal study. Lancet Neurol. 2018, 17, 241–250. [Google Scholar] [CrossRef]
- McDade, E.; Wang, G.; Gordon, B.A.; Hassenstab, J.; Benzinger, T.L.S.; Buckles, V.; Fagan, A.M.; Holtzman, D.M.; Cairns, N.J.; Goate, A.M.; et al. Longitudinal cognitive and biomarker changes in dominantly inherited Alzheimer disease. Neurology 2018, 91, e1295–e1306. [Google Scholar] [CrossRef]
- Yu, X.; Shao, K.; Wan, K.; Li, T.; Li, Y.; Zhu, X.; Han, Y. Progress in blood biomarkers of subjective cognitive decline in preclinical Alzheimer’s disease. Chin. Med. J. 2023, 136, 505–521. [Google Scholar] [CrossRef]
- Walsh, S.; Wallace, L.; Kuhn, I.; Mytton, O.; Lafortune, L.; Wills, W.; Mukadam, N.; Brayne, C. Population-level interventions for the primary prevention of dementia: A complex evidence review. Lancet 2023, 402 (Suppl. S1), S13. [Google Scholar] [CrossRef] [PubMed]
- Ismail, Z.; Black, S.E.; Camicioli, R.; Chertkow, H.; Herrmann, N.; Laforce, R., Jr.; Montero-Odasso, M.; Rockwood, K.; Rosa-Neto, P.; Seitz, D.; et al. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimer’s Dement. 2020, 16, 1182–1195. [Google Scholar] [CrossRef]
- Nakakubo, S.; Doi, T.; Makizako, H.; Tsutsumimoto, K.; Hotta, R.; Kurita, S.; Kim, M.; Suzuki, T.; Shimada, H. Sleep condition and cognitive decline in Japanese community-dwelling older people: Data from a 4-year longitudinal study. J. Sleep Res. 2019, 28, e12803. [Google Scholar] [CrossRef]
- Gaur, A.; Kaliappan, A.; Balan, Y.; Sakthivadivel, V.; Medala, K.; Umesh, M. Sleep and Alzheimer: The link. Maedica 2022, 17, 177–185. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, A.C.; Beath, A.P.; Naismith, S.L. Relationships between sleep quality, depressive symptoms and MCI diagnosis: A path analysis. J. Affect. Disord. 2019, 256, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.X.; Xie, X.; Xu, Y.; Wang, R.; Lei, X.; Yu, J. Older adults’ subjective cognitive decline correlated with subjective but not objective sleep: A mediator role of depression. Int. J. Aging Hum. Dev. 2022, 95, 42–56. [Google Scholar] [CrossRef]
- Ikeda, Y.; Tabira, T.; Ohshige, T.; Masumitsu, T.; Makizako, H.; Ku-Ohl Project Member. Association between sleep onset problem and subjective cognitive complaints among Japanese older adults during the coronavirus disease 2019 pandemic. Int. J. Environ. Res. Public Health 2022, 20, 156. [Google Scholar] [CrossRef]
- Röhr, S.; Pabst, A.; Riedel-Heller, S.G.; Jessen, F.; Turana, Y.; Handajani, Y.S.; Brayne, C.; Matthews, F.E.; Stephan, B.C.M.; Lipton, R.B.; et al. Estimating prevalence of subjective cognitive decline in and across international cohort studies of aging: A COSMIC study. Alzheimer’s Res. Ther. 2020, 12, 167. [Google Scholar] [CrossRef]
- Sugishita, M.; Hemmi, I.; Takeuchi, T. Reexamination of the validity and reliability of the Japanese version of the Mini-Mental State Examination (MMSE-J). Jpn. J. Cogn. Neurosci. 2016, 18, 168–183. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. ‘Mini-mental state’. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Ideno, Y.; Takayama, M.; Hayashi, K.; Takagi, H.; Sugai, Y. Evaluation of a Japanese version of the Mini-Mental State Examination in elderly persons. Geriatr. Gerontol. Int. 2012, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Hirose, J.; Taniwaki, T.; Usuku, K.; Oka, K.; Mizuta, H.; Nagata, T.; Tomiguchi, W.; Ogushi, M.; Koga, H.; Okamoto, N.; et al. Validation of each category of Kihon checklist for assessing physical functioning, nutrition and cognitive status in a community-dwelling older Japanese cohort. Epidemiology 2017, 7, 326. [Google Scholar]
- Satake, S.; Senda, K.; Hong, Y.J.; Miura, H.; Endo, H.; Sakurai, T.; Kondo, I.; Toba, K. Validity of the Kihon Checklist for assessing frailty status. Geriatr. Gerontol. Int. 2016, 16, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Sewo Sampaio, P.Y.; Sampaio, R.A.C.; Yamada, M.; Arai, H. Systematic review of the Kihon Checklist: Is it a reliable assessment of frailty? Geriatr. Gerontol. Int. 2016, 16, 893–902. [Google Scholar] [CrossRef] [PubMed]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Sugawara, Y.; Hozawa, A.; Tsuji, I. Predictive ability of a simple subjective memory complaints scale for incident dementia: Evaluation of Japan’s national checklist, the “Kihon Checklist”. Geriatr. Gerontol. Int. 2017, 17, 1300–1305. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Ogita, M.; Arai, H. Self-reported cognitive frailty predicts adverse health outcomes for community-dwelling older adults based on an analysis of sex and age. J. Nutr. Health Aging 2019, 23, 654–664. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, Y.; Sato, S.; Takahashi, M.; Takeda, N.; Matsushita, M.; Kitabatake, Y.; Maruo, K.; Arao, T. The association of single and combined factors of sedentary behavior and physical activity with subjective cognitive complaints among community-dwelling older adults: Cross-sectional study. PLoS ONE 2018, 13, e0195384. [Google Scholar] [CrossRef]
- Okajima, I.; Nakajima, S.; Kobayashi, M.; Inoue, Y. Development and validation of the Japanese version of the Athens Insomnia Scale. Psychiatry Clin. Neurosci. 2013, 67, 420–425. [Google Scholar] [CrossRef]
- Enomoto, K.; Adachi, T.; Yamada, K.; Inoue, D.; Nakanishi, M.; Nishigami, T.; Shibata, M. Reliability and validity of the Athens Insomnia Scale in chronic pain patients. J. Pain Res. 2018, 11, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Hoyl, M.T.; Alessi, C.A.; Harker, J.O.; Josephson, K.R.; Pietruszka, F.M.; Koelfgen, M.; Mervis, J.R.; Fitten, L.J.; Rubenstein, L.Z. Development and testing of a five-item version of the Geriatric Depression Scale. J. Am. Geriatr. Soc. 1999, 47, 873–878. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Murata, C.; Hirai, H.; Kondo, N.; Kondo, K.; Ueda, K.; Ichida, N. Predictive validity of GDS5 using AGES project data. Kousei Shihyou 2014, 61, 7–12. (In Japanese) [Google Scholar]
- Niu, H.; Álvarez-Álvarez, I.; Guillén-Grima, F.; Aguinaga-Ontoso, I. Prevalence and incidence of Alzheimer’s disease in Europe: A meta-analysis. Neurologia 2017, 32, 523–532. [Google Scholar] [CrossRef] [PubMed]
- Daviglus, M.L.; Bell, C.C.; Berrettini, W.; Bowen, P.E.; Connolly, E.S., Jr.; Cox, N.J.; Dunbar-Jacob, J.M.; Granieri, E.C.; Hunt, G.; McGarry, K.; et al. National Institutes of Health State-of-the-Science Conference statement: Preventing alzheimer disease and cognitive decline. Ann. Intern. Med. 2010, 153, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Van Patten, R.; Tremont, G. Public knowledge of late-life cognitive decline and dementia in an international sample. Dementia 2020, 19, 1758–1776. [Google Scholar] [CrossRef] [PubMed]
- Sierra, C. Hypertension and the risk of dementia. Front. Cardiovasc. Med. 2020, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Cholerton, B.; Baker, L.D.; Montine, T.J.; Craft, S. Type 2 diabetes, cognition, and dementia in older adults: Toward a precision health approach. Diabetes Spectr. 2016, 29, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Liu, X.; Zhu, R.; Zhao, J.; Wang, Q. Lipid levels and the risk of dementia: A dose-response meta-analysis of prospective cohort studies. Ann. Clin. Transl. Neurol. 2022, 9, 296–311. [Google Scholar] [CrossRef]
- Jia, R.; Wang, Q.; Huang, H.; Yang, Y.; Chung, Y.F.; Liang, T. Cardiovascular disease risk models and dementia or cognitive decline: A systematic review. Front. Aging Neurosci. 2023, 15, 1257367. [Google Scholar] [CrossRef]
- Myrstad, C.; Engdahl, B.L.; Costafreda, S.G.; Krokstad, S.; Lin, F.; Livingston, G.; Strand, B.H.; Øhre, B.; Selbæk, G. Hearing impairment and risk of dementia in the HUNT Study (HUNT4 70+): A Norwegian cohort study. EClinicalMedicine 2023, 66, 102319. [Google Scholar] [CrossRef] [PubMed]
- Gu, D.; Ou, S.; Liu, G. Traumatic brain injury and risk of dementia and Alzheimer’s disease: A systematic review and meta-analysis. Neuroepidemiology 2022, 56, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Wiegmann, C.; Mick, I.; Brandl, E.J.; Heinz, A.; Gutwinski, S. Alcohol and dementia—What is the link? A systematic review. Neuropsychiatr. Dis. Treat. 2020, 16, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Ohara, T.; Ninomiya, T.; Hata, J.; Ozawa, M.; Yoshida, D.; Mukai, N.; Nagata, M.; Iwaki, T.; Kitazono, T.; Kanba, S.; et al. Midlife and late-life smoking and risk of dementia in the community: The Hisayama study. J. Am. Geriatr. Soc. 2015, 63, 2332–2339. [Google Scholar] [CrossRef] [PubMed]
- Guarnera, J.; Yuen, E.; Macpherson, H. The impact of loneliness and social isolation on cognitive aging: A narrative review. J. Alzheimer’s Dis. Rep. 2023, 7, 699–714. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liu, H.Y.; Cheng, Y.C.; Su, C.H. Exercise dosage in reducing the risk of dementia development: Mode, duration, and intensity-A narrative review. Int. J. Environ. Res. Public Health 2021, 18, 13331. [Google Scholar] [CrossRef] [PubMed]
- Okura, M.; Ogita, M.; Yamamoto, M.; Nakai, T.; Numata, T.; Arai, H. The relationship of community activities with cognitive impairment and depressive mood independent of mobility disorder in Japanese older adults. Arch. Gerontol. Geriatr. 2017, 70, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Goda, A.; Murata, S.; Nakano, H.; Shiraiwa, K.; Abiko, T.; Nonaka, K.; Iwase, H.; Anami, K.; Horie, J. Subjective and objective mental and physical functions affect subjective cognitive decline in community-dwelling elderly Japanese people. Healthcare 2020, 8, 347. [Google Scholar] [CrossRef] [PubMed]
- Tomata, Y.; Sugiyama, K.; Kaiho, Y.; Honkura, K.; Watanabe, T.; Zhang, S.; Sugawara, Y.; Tsuji, I. Green tea consumption and the risk of incident dementia in elderly Japanese: The Ohsaki cohort 2006 study. Am. J. Geriatr. Psychiatry 2016, 24, 881–889. [Google Scholar] [CrossRef]
- Wen, C.; Hu, H.; Ou, Y.N.; Bi, Y.L.; Ma, Y.H.; Tan, L.; Yu, J.T. Risk factors for subjective cognitive decline: The CABLE study. Transl. Psychiatry 2021, 11, 576. [Google Scholar] [CrossRef]
- Wei, Y.C.; Huang, L.Y.; Chen, C.K.; Lin, C.; Shyu, Y.C.; Chen, Y.L.; Huang, W.Y.; Lin, C.P. Subjective cognitive decline in the community is affected at multiple aspects of mental health and life quality: A cross-sectional study of the community medicine of Keelung Chang Gung Memorial Hospital. Dement. Geriatr. Cogn. Dis. Extra 2019, 9, 152–162. [Google Scholar] [CrossRef] [PubMed]
- Peterson, R.L.; Carvajal, S.C.; McGuire, L.C.; Fain, M.J.; Bell, M.L. State inequality, socioeconomic position and subjective cognitive decline in the United States. SSM Popul. Health 2019, 7, 100357. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.G.; Geerligs, L.; Claassen, J.A.H.R.; Overdorp, E.J.; Brazil, I.A.; Kessels, R.P.C.; Oosterman, J.M. Positive effects of education on cognitive functioning depend on clinical status and neuropathological severity. Front. Hum. Neurosci. 2021, 15, 723728. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.Q.; Lin, L.H.; Ding, K.R.; Ke, Y.F.; Huang, J.H.; Hou, C.L.; Jia, F.J.; Wang, S.B. The role of depression and anxiety in the relationship between poor sleep quality and subjective cognitive decline in Chinese elderly: Exploring parallel, serial, and moderated mediation. J. Affect. Disord. 2021, 294, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Zullo, L.; Clark, C.; Gholam, M.; Castelao, E.; von Gunten, A.; Preisig, M.; Popp, J. Factors associated with subjective cognitive decline in dementia-free older adults-A population-based study. Int. J. Geriatr. Psychiatry 2021, 36, 1188–1196. [Google Scholar] [CrossRef] [PubMed]
- Rajczyk, J.I.; Ferketich, A.; Wing, J.J. Relation between smoking status and subjective cognitive decline in middle age and older adults: A cross-sectional analysis of 2019 behavioral risk factor surveillance system data. J. Alzheimer’s Dis. 2023, 91, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Joo, H.J.; Joo, J.H.; Kwon, J.; Jang, B.N.; Park, E.C. Association between quality and duration of sleep and subjective cognitive decline: A cross-sectional study in South Korea. Sci. Rep. 2021, 11, 16989. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Chang, I.B.; Kim, Y.H.; Min, C.Y.; Yoo, D.M.; Choi, H.G. Association between various types or statuses of smoking and subjective cognitive decline based on a community health survey of Korean adults. Front. Neurol. 2022, 13, 810830. [Google Scholar] [CrossRef] [PubMed]
- Exalto, L.G.; Hendriksen, H.M.A.; Barkhof, F.; van den Bosch, K.A.; Ebenau, J.L.; van Leeuwenstijn-Koopman, M.; Prins, N.D.; Teunissen, C.E.; Visser, L.N.C.; Scheltens, P.; et al. Subjective cognitive decline and self-reported sleep problems: The SCIENCe project. Alzheimer’s Dement. 2022, 14, e12287. [Google Scholar] [CrossRef]
- Kim, J.H.; Ahn, J.H.; Min, C.Y.; Yoo, D.M.; Choi, H.G. Association between sleep quality and subjective cognitive decline: Evidence from a community health survey. Sleep Med. 2021, 83, 123–131. [Google Scholar] [CrossRef]
- Kim, M.; Lim, K.C.; Ko, H. Factors influencing subjective cognitive function among community-dwelling older adults. Geriatr. Nurs. 2021, 42, 1070–1076. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ju, Y.J.; Park, E.C.; Lee, S.Y. Effect of poor sleep quality on subjective cognitive decline (SCD) or SCD-related functional difficulties: Results from 220,000 nationwide general populations without dementia. J. Affect. Disord. 2020, 260, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Leng, M.; Yin, H.; Zhang, P.; Jia, Y.; Hu, M.; Li, G.; Wang, C.; Chen, L. Sleep quality and health-related quality of life in older people with subjective cognitive decline, mild cognitive impairment, and Alzheimer disease. J. Nerv. Ment. Dis. 2020, 208, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.H.; Wang, S.B.; Xu, W.Q.; Hu, Q.; Zhang, P.; Ke, Y.F.; Huang, J.H.; Ding, K.R.; Li, X.L.; Hou, C.L.; et al. Subjective cognitive decline symptoms and its association with socio-demographic characteristics and common chronic diseases in the southern Chinese older adults. BMC Public Health 2022, 22, 127. [Google Scholar] [CrossRef] [PubMed]
- Wardle-Pinkston, S.; Slavish, D.C.; Taylor, D.J. Insomnia and cognitive performance: A systematic review and meta-analysis. Sleep Med. Rev. 2019, 48, 101205. [Google Scholar] [CrossRef] [PubMed]
- Winer, J.R.; Deters, K.D.; Kennedy, G.; Jin, M.; Goldstein-Piekarski, A.; Poston, K.L.; Mormino, E.C. Association of short and long sleep duration with amyloid-β burden and cognition in aging. JAMA Neurol. 2021, 78, 1187–1196. [Google Scholar] [CrossRef] [PubMed]
- Fasiello, E.; Gorgoni, M.; Scarpelli, S.; Alfonsi, V.; Ferini Strambi, L.; De Gennaro, L. Functional connectivity changes in insomnia disorder: A systematic review. Sleep Med. Rev. 2022, 61, 101569. [Google Scholar] [CrossRef]
- Sharma, N.; Murari, G.; Vandermorris, S.; Verhoeff, N.P.L.G.; Herrmann, N.; Chen, J.J.; Mah, L. Functional connectivity between the posterior default mode network and parahippocampal gyrus is disrupted in older adults with subjective cognitive decline and correlates with subjective memory ability. J. Alzheimer’s Dis. 2021, 82, 435–445. [Google Scholar] [CrossRef]
- Viviano, R.P.; Damoiseaux, J.S. Longitudinal change in hippocampal and dorsal anterior insulae functional connectivity in subjective cognitive decline. Alzheimer’s Res. Ther. 2021, 13, 108. [Google Scholar] [CrossRef]
- Wang, S.; Sun, H.; Hu, G.; Xue, C.; Qi, W.; Rao, J.; Zhang, F.; Zhang, X.; Chen, J. Altered insular subregional connectivity associated with cognitions for distinguishing the spectrum of pre-clinical Alzheimer’s disease. Front. Aging Neurosci. 2021, 13, 597455. [Google Scholar] [CrossRef]
- Winkelman, J.W.; Lecea, L. Sleep and neuropsychiatric illness. Neuropsychopharmacology 2020, 45, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.E.; Kamerow, D.B. Epidemiologic study of sleep disturbances and psychiatric disorders: An opportunity for prevention? JAMA 1989, 262, 1479–1484. [Google Scholar] [CrossRef] [PubMed]
- Sannemann, L.; Schild, A.K.; Altenstein, S.; Bartels, C.; Brosseron, F.; Buerger, K.; Cosma, N.C.; Fliessbach, K.; Freiesleben, S.D.; Glanz, W.; et al. Neuropsychiatric symptoms in at-risk groups for AD dementia and their association with worry and AD biomarkers-results from the DELCODE study. Alzheimer’s Res. Ther. 2020, 12, 131. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Ju, Y.J.; Chun, K.H.; Lee, S.Y. The frequency of sleep medication use and the risk of subjective cognitive decline (SCD) or SCD with functional difficulties in elderly individuals without dementia. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 1693–1698. [Google Scholar] [CrossRef] [PubMed]
- Mishima, K.; DiBonaventura, M.D.; Gross, H. The burden of insomnia in Japan. Nat. Sci. Sleep. 2015, 7, 1–11. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cervilla, J.A.; Prince, M.; Mann, A. Smoking, drinking, and incident cognitive impairment: A cohort community based study included in the Gospel Oak project. J. Neurol. Neurosurg. Psychiatry 2000, 68, 622–626. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ju, Y.J.; Lee, J.E.; Kim, Y.T.; Hong, S.C.; Choi, Y.J.; Song, M.K.; Kim, H.Y. Factors associated with poor sleep quality in the Korean general population: Providing information from the Korean version of the Pittsburgh Sleep Quality Index. J. Affect. Disord. 2020, 271, 49–58. [Google Scholar] [CrossRef]
- Innes, K.E.; Selfe, T.K.; Khalsa, D.S.; Kandati, S. Effects of Meditation versus Music Listening on Perceived Stress, Mood, Sleep, and Quality of Life in Adults with Early Memory Loss: A Pilot randomized controlled trial. J. Alzheimer’s Dis. 2016, 52, 1277–1298. [Google Scholar] [CrossRef]
| Variable | SCC | Non-SCC | |
|---|---|---|---|
| AIS-J | No insomnia | 49 | 110 |
| Nocturnal insomnia | 27 | 21 | |
| Pathological insomnia | 20 | 14 | |
| Variable | SCC | Non-SCC | p-Value | |
|---|---|---|---|---|
| (n = 96) | (n = 145) | |||
| Attribute | Age (yr) ☨ | 78.25 ± 5.95 | 76.70 ± 5.80 | 0.05 |
| Sex: Male/Female (n) | 19/77 | 20/125 | 0.22 | |
| BMI (kg/m2) * | 22.42 ± 3.43 | 22.48 ± 3.29 | 0.72 | |
| Educational history (yr) * | 11.178 ± 2.23 | 11.91 ± 2.41 | 0.02 | |
| Mental and physical indicator | AIS-J (score) * | 3.70 ± 3.09 | 2.41 ± 2.20 | 0.001 |
| GDS-5 (score) * | 0.95 ± 1.20 | 0.52 ± 0.84 | 0.004 | |
| MMSE (score) * | 27.88 ± 1.95 | 28.39 ± 1.85 | 0.02 | |
| Medical visits due to disease | Hypertension: yes/no (n) | 45/51 | 52/93 | 0.09 |
| Diabetes: yes/no (n) | 10/86 | 16/129 | 0.88 | |
| Hyperlipidemia: yes/no (n) | 13/83 | 34/111 | 0.06 | |
| Cerebrovascular disease: yes/no (n) | 5/91 | 5/140 | 0.50 | |
| Presence of dementia risk | Hearing loss: yes/no (n) | 28/68 | 32/113 | 0.21 |
| History of head injury: yes/no (n) | 8/88 | 6/139 | 0.17 | |
| Past or current drinking habits: yes/no (n) | 27/69 | 39/106 | 0.83 | |
| Past or current smoking habits: yes/no (n) | 23/73 | 13/132 | 0.001 | |
| Social isolation: yes/no (n) | 9/87 | 20/125 | 0.30 | |
| Physical inactivity: yes/no (n) | 28/67 | 68/114 | 0.17 | |
| Variable | Univariate Analysis | Multivariate Analysis | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 95% CI for OR | 95% CI for OR | |||||||||
| OR | Lower | Upper | p | OR | Lower | Upper | p | |||
| Attribute | Age (yr) | 1.05 | 1.00 | 1.09 | 0.05 | 1.03 | 0.97 | 1.09 | 0.37 | |
| Sex | Female | 0.65 | 0.33 | 1.29 | 0.22 | 0.68 | 0.21 | 2.23 | 0.53 | |
| Male | 1.00 | 1.00 | ||||||||
| BMI (kg/m2) | 0.99 | 0.92 | 1.07 | 0.89 | 0.97 | 0.88 | 1.06 | 0.46 | ||
| Educational history (yr) | 0.87 | 0.78 | 0.98 | 0.02 | 0.87 | 0.75 | 1.00 | 0.06 | ||
| Mental and physical indicator | AIS-J (score) | 1.21 | 1.09 | 1.34 | 0.001 | 1.16 | 1.03 | 1.31 | 0.02 | |
| GDS-5 (score) | 1.52 | 1.17 | 1.98 | 0.004 | 1.33 | 0.98 | 1.82 | 0.07 | ||
| MMSE (score) | 0.87 | 0.76 | 0.99 | 0.04 | 0.91 | 0.77 | 1.07 | 0.25 | ||
| Medical visits due to disease | Hypertension | Yes | 1.58 | 0.93 | 2.67 | 0.09 | 1.68 | 0.90 | 3.16 | 0.11 |
| No | 1.00 | 1.00 | ||||||||
| Diabetes | Yes | 0.94 | 0.41 | 2.16 | 0.88 | 0.88 | 0.33 | 2.35 | 0.80 | |
| No | 1.00 | 1.00 | ||||||||
| Hyperlipidemia | Yes | 0.51 | 0.25 | 1.03 | 0.06 | 0.59 | 0.27 | 1.28 | 0.18 | |
| No | 1.00 | 1.00 | ||||||||
| Cerebrovascular disease | Yes | 1.54 | 0.43 | 5.46 | 0.51 | 1.26 | 0.28 | 5.66 | 0.77 | |
| No | 1.00 | 1.00 | ||||||||
| Presence of dementia risk | Hearing loss | Yes | 1.45 | 0.81 | 2.62 | 0.21 | 0.99 | 0.48 | 2.03 | 0.98 |
| No | 1.00 | 1.00 | ||||||||
| History of head injury | Yes | 2.11 | 0.71 | 6.27 | 0.18 | 2.42 | 0.65 | 8.99 | 0.19 | |
| No | 1.00 | 1.00 | ||||||||
| Past or current drinking habits | Yes | 1.06 | 0.60 | 1.89 | 0.83 | 0.80 | 0.37 | 1.75 | 0.58 | |
| No | 1.00 | 1.00 | ||||||||
| Past or current smoking habits | Yes | 3.20 | 1.53 | 6.69 | 0.001 | 3.48 | 1.24 | 9.70 | 0.02 | |
| No | 1.00 | 1.00 | ||||||||
| Social isolation | Yes | 0.65 | 0.28 | 1.49 | 0.30 | 0.48 | 0.18 | 1.23 | 0.12 | |
| No | 1.00 | 1.00 | ||||||||
| Physical inactivity | Yes | 1.51 | 0.84 | 2.74 | 0.17 | 1.55 | 0.76 | 3.18 | 0.23 | |
| No | 1.00 | 1.00 | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goda, A.; Nakano, H.; Kikuchi, Y.; Mori, K.; Mitsumaru, N.; Murata, S. Association between Subjective Cognitive Complaints and Sleep Disturbance among Community-Dwelling Elderly Individuals in Japan. Healthcare 2024, 12, 1245. https://doi.org/10.3390/healthcare12131245
Goda A, Nakano H, Kikuchi Y, Mori K, Mitsumaru N, Murata S. Association between Subjective Cognitive Complaints and Sleep Disturbance among Community-Dwelling Elderly Individuals in Japan. Healthcare. 2024; 12(13):1245. https://doi.org/10.3390/healthcare12131245
Chicago/Turabian StyleGoda, Akio, Hideki Nakano, Yuki Kikuchi, Kohei Mori, Nozomi Mitsumaru, and Shin Murata. 2024. "Association between Subjective Cognitive Complaints and Sleep Disturbance among Community-Dwelling Elderly Individuals in Japan" Healthcare 12, no. 13: 1245. https://doi.org/10.3390/healthcare12131245
APA StyleGoda, A., Nakano, H., Kikuchi, Y., Mori, K., Mitsumaru, N., & Murata, S. (2024). Association between Subjective Cognitive Complaints and Sleep Disturbance among Community-Dwelling Elderly Individuals in Japan. Healthcare, 12(13), 1245. https://doi.org/10.3390/healthcare12131245






