The Feasibility and Efficacy of Remote App-Guided Home Exercises for Frozen Shoulder: A Pilot Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Study Flow
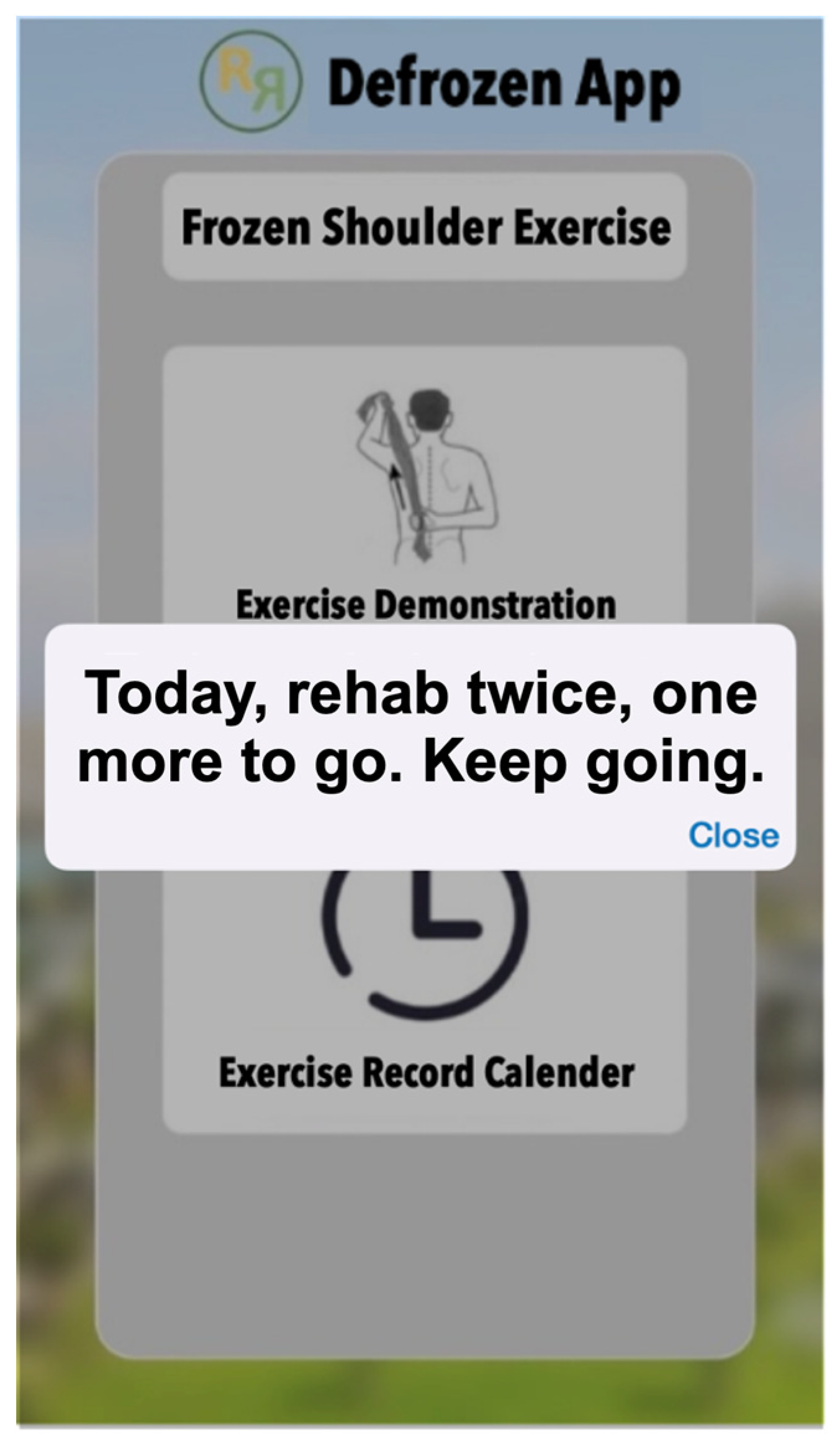
2.3. Defrozen-app for Demonstrating Home Exercises for Frozen Shoulder
2.4. Outcome Measures
2.5. The Feasibility Scaling Tools
2.6. Clinical Outcome Measures
3. Results
3.1. Participants
3.2. Feasibility Scoring
3.2.1. Adherence to Therapy
3.2.2. Technology Acceptance Model (TAM2)
3.2.3. System Usability Scale (SUS)
3.2.4. User Satisfaction and Engagement (USE) Questionnaire
3.3. Clinical Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karbowiak, M.; Holme, T.; Mirza, M.; Siddiqui, N. Frozen shoulder. BMJ 2022, 377, e068547. [Google Scholar] [CrossRef] [PubMed]
- Razmjou, H.; Christakis, M. Clinical and Radiological Examination of the Shoulder Joint: A Guide for Advanced Practice Physiotherapists; Springer Nature: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Walker-Bone, K.; Palmer, K.T.; Reading, I.; Coggon, D.; Cooper, C. Prevalence and impact of musculoskeletal disorders of the upper limb in the general population. Arthritis Rheum. 2004, 51, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Cohen, C.; Ejnisman, B. Epidemiology of Frozen Shoulder. In Shoulder Stiffness: Current Concepts and Concerns; Itoi, E., Arce, G., Bain, G.I., Diercks, R.L., Guttmann, D., Imhoff, A.B., Mazzocca, A.D., Sugaya, H., Yoo, Y.-S., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 21–30. [Google Scholar]
- Millar, N.L.; Meakins, A.; Struyf, F.; Willmore, E.; Campbell, A.L.; Kirwan, P.D.; Akbar, M.; Moore, L.; Ronquillo, J.C.; Murrell, G.A.C.; et al. Frozen shoulder. Nat. Rev. Dis. Primers 2022, 8, 59. [Google Scholar] [CrossRef] [PubMed]
- Mertens, M.G.; Meert, L.; Struyf, F.; Schwank, A.; Meeus, M. Exercise Therapy Is Effective for Improvement in Range of Motion, Function, and Pain in Patients with Frozen Shoulder: A Systematic Review and Meta-analysis. Arch. Phys. Med. Rehabil. 2022, 103, 998–1012.e14. [Google Scholar] [CrossRef] [PubMed]
- Russell, S.; Jariwala, A.; Conlon, R.; Selfe, J.; Richards, J.; Walton, M. A blinded, randomized, controlled trial assessing conservative management strategies for frozen shoulder. J. Shoulder Elb. Surg. 2014, 23, 500–507. [Google Scholar] [CrossRef] [PubMed]
- Robinson, P.M.; Norris, J.; Roberts, C.P. Randomized controlled trial of supervised physiotherapy versus a home exercise pro-gram after hydrodilatation for the management of primary frozen shoulder. J. Shoulder Elb. Surg. 2017, 26, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Challoumas, D.; Biddle, M.; McLean, M.; Millar, N.L. Comparison of Treatments for Frozen Shoulder: A Systematic Review and Meta-analysis. JAMA Netw. Open 2020, 3, e2029581. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Kim, D.H.; Bae, K.C.; Lee, D.; Kim, K. Proper site of corticosteroid injection for the treatment of idiopathic frozen shoulder: Results from a randomized trial. Jt. Bone Spine 2016, 83, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Pandey, V.; Madi, S. Clinical Guidelines in the Management of Frozen Shoulder: An Update! Indian J. Orthop. 2021, 55, 299–309. [Google Scholar] [CrossRef]
- Gleyze, P.; Clavert, P.; Flurin, P.H.; Laprelle, E.; Katz, D.; Toussaint, B.; Benkalfate, T.; Charousset, C.; Joudet, T.; Georges, T.; et al. Management of the stiff shoulder. A prospective multicenter comparative study of the six main techniques in use: 235 cases. Orthop. Traumatol. Surg. Res. 2011, 97 (Suppl. S8), S167–S181. [Google Scholar] [CrossRef]
- Hardage, J.; Peel, C.; Morris, D.; Graham, C.; Brown, C.J.; Foushee, R.H.; Braswell, J. Adherence to Exercise Scale for Older Patients (AESOP): A Measure for Predicting Exercise Adherence in Older Adults after Discharge from Home Health Physical Therapy. J. Geriatr. Phys. Ther. 2007, 30, 69. [Google Scholar] [CrossRef]
- Chen, H.C.; Chuang, T.Y.; Lin, P.C.; Lin, Y.K.; Chuang, Y.H. Effects of Messages Delivered by Mobile Phone on Increasing Compliance with Shoulder Exercises among Patients with a Frozen Shoulder. J. Nurs. Scholarsh. 2017, 49, 429–437. [Google Scholar] [CrossRef]
- Liao, C.H.; Wu, Y.T.; Cheng, C.T.; Ooyang, C.H.; Kang, S.C.; Fu, C.Y.; Hsu, Y.P.; Hsieh, C.H.; Chen, C.C. An Image-Based Mobile Health App for Postdrainage Monitoring: Usability Study. J. Med. Internet Res. 2020, 22, e17686. [Google Scholar] [CrossRef]
- World Health Organization. MHealth: New Horizons for Health Through Mobile Technologies; World Health Organization: Geneva, Switzerland, 2011.
- Sharma, S.P.; Bærheim, A.; Kvåle, A. Passive range of motion in patients with adhesive shoulder capsulitis, an intertester re-liability study over eight weeks. BMC Musculoskelet. Disord. 2015, 16, 37. [Google Scholar] [CrossRef]
- Julious, S.A. Sample size of 12 per group rule of thumb for a pilot study. Pharm. Stat. 2005, 4, 287–291. [Google Scholar] [CrossRef]
- Venkatesh, V.; Davis, F.D. A Theoretical Extension of the Technology Acceptance Model: Four Longitudinal Field Studies. Manag. Sci. 2000, 46, 186–204. [Google Scholar] [CrossRef]
- Jordan, P.W.; Thomas, B.; McClelland, I.L.; Weerdmeester, B. Usability Evaluation in Industry; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Dawson, J.; Fitzpatrick, R.; Carr, A. Questionnaire on the perceptions of patients about shoulder surgery. J. Bone Jt. Surg. Br. 1996, 78, 593–600. [Google Scholar] [CrossRef]
- Deng, Z.; Li, Z.; Li, X.; Chen, Z.; Shen, C.; Sun, X.; Shu, H.; Wu, J.; Tang, K. Comparison of Outcomes of Two Different Corticosteroid Injection Approaches for Primary Frozen Shoulder: A Randomized Controlled Study. J. Rehabil. Med. 2023, 55, jrm00361. [Google Scholar] [CrossRef] [PubMed]
- Dawson, J.; Rogers, K.; Fitzpatrick, R.; Carr, A. The Oxford shoulder score revisited. Arch. Orthop. Trauma. Surg. 2009, 129, 119–123. [Google Scholar] [CrossRef]
- Xu, X.; Wang, F.; Wang, X.; Wei, X.; Wang, Z. Chinese cross-cultural adaptation and validation of the Oxford shoulder score. Health Qual. Life Outcomes 2015, 13, 193. [Google Scholar] [CrossRef]
- Pfeifer, A.C.; Uddin, R.; Schröder-Pfeifer, P.; Holl, F.; Swoboda, W.; Schiltenwolf, M. Mobile Application-Based Interventions for Chronic Pain Patients: A Systematic Review and Meta-Analysis of Effectiveness. J. Clin. Med. Res. 2020, 9, 3557. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.H.; Bae, K.C.; Kim, D.H. Treatment Strategy for Frozen Shoulder. Clin. Orthop. Surg. 2019, 11, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Szajna, B. Empirical Evaluation of the Revised Technology Acceptance Model. Manag. Sci. 1996, 42, 85–92. [Google Scholar] [CrossRef]
- Stütz, T.; Emsenhuber, G.; Huber, D.; Domhardt, M.; Tiefengrabner, M.; Oostingh, G.J.; Fötschl, U.; Matis, N.; Ginzinger, S. Mobile Phone-Supported Physiotherapy for Frozen Shoulder: Feasibility Assessment Based on a Usability Study. JMIR Rehabil. Assist. Technol. 2017, 4, e7085. [Google Scholar] [CrossRef] [PubMed]
- Lund, A. Measuring usability with the use questionnaire. Usability and user experience newsletter of the STC usability SIG. Usability Interface 2001, 8, 3–6. [Google Scholar]
- Bangor, A.; Kortum, P.T.; Miller, J.T. An Empirical Evaluation of the System Usability Scale. Int. J. Hum. Comput. Interact. 2008, 24, 574–594. [Google Scholar] [CrossRef]
- Mintken, P.E.; Glynn, P.; Cleland, J.A. Psychometric properties of the shortened disabilities of the Arm, Shoulder, and Hand Questionnaire (QuickDASH) and Numeric Pain Rating Scale in patients with shoulder pain. J. Shoulder Elb. Surg. 2009, 18, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Rangan, A.; Brealey, S.D.; Keding, A.; Corbacho, B.; Northgraves, M.; Kottam, L.; Goodchild, L.; Srikesavan, C.; Rex, S.; Charalambous, C.P.; et al. Management of adults with primary frozen shoulder in secondary care (UK FROST): A multicentre, pragmatic, three-arm, superiority randomised clinical trial. Lancet 2020, 396, 977–989. [Google Scholar] [CrossRef]
- Beaton, D.E.; Wright, J.G.; Katz, J.N.; Upper Extremity Collaborative Group. Development of the QuickDASH: Comparison of three item-reduction approaches. J. Bone Jt. Surg. Am. 2005, 87, 1038–1046. [Google Scholar]
- Gummesson, C.; Ward, M.M.; Atroshi, I. The shortened disabilities of the arm, shoulder and hand questionnaire (QuickDASH): Validity and reliability based on responses within the full-length DASH. BMC Musculoskelet. Disord. 2006, 7, 44. [Google Scholar] [CrossRef]
- Liang, H.W.; Wang, H.K.; Yao, G.; Horng, Y.S.; Hou, S.M. Psychometric evaluation of the Taiwan version of the Disability of the Arm, Shoulder, and Hand (DASH) questionnaire. J. Formos. Med. Assoc. 2004, 103, 773–779. [Google Scholar] [PubMed]
- Namdari, S.; Yagnik, G.; Ebaugh, D.D.; Nagda, S.; Ramsey, M.L.; Williams, G.R.; Mehta, S. Defining functional shoulder range of motion for activities of daily living. J. Shoulder Elb. Surg. 2012, 21, 1177–1183. [Google Scholar] [CrossRef]
- Whitehead, A.L.; Sully, B.G.O.; Campbell, M.J. Pilot and feasibility studies: Is there a difference from each other and from a randomised controlled trial? Contemp. Clin. Trials 2014, 38, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.E.; Harvey, L.A.; Avdalis, C.; Chen, L.W.; Jeyalingam, S.; Pratt, C.A.; Tatum, H.J.; Bowden, J.L.; Lucas, B.R. An app with remote support achieves better adherence to home exercise programs than paper handouts in people with musculoskeletal conditions: A randomised trial. J. Physiother. 2017, 63, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Willoughby, J.F. Do Fitness Apps Need Text Reminders? An Experiment Testing Goal-Setting Text Message Reminders to Promote Self-Monitoring. J. Health Commun. 2018, 23, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Bullard, T.; Ji, M.; An, R.; Trinh, L.; Mackenzie, M.; Mullen, S.P. A systematic review and meta-analysis of adherence to physical activity interventions among three chronic conditions: Cancer, cardiovascular disease, and diabetes. BMC Public Health 2019, 19, 636. [Google Scholar] [CrossRef] [PubMed]
- Nicolson, P.J.A.; Hinman, R.S.; Kasza, J.; Bennell, K.L. Trajectories of adherence to home-based exercise programs among people with knee osteoarthritis. Osteoarthr. Cartil. 2018, 26, 513–521. [Google Scholar] [CrossRef] [PubMed]
- Arensman, R.; Kloek, C.; Pisters, M.; Koppenaal, T.; Ostelo, R.; Veenhof, C. Patient Perspectives on Using a Smartphone App to Support Home-Based Exercise During Physical Therapy Treatment: Qualitative Study. JMIR Hum. Factors 2022, 9, e35316. [Google Scholar] [CrossRef] [PubMed]
- Major, D.H.; Grotle, M.; Littlewood, C.; Brox, J.I.; Matre, D.; Gallet, H.; Roe, Y. Adherence to self-managed exercises for patients with persistent subacromial pain: The Ad-Shoulder feasibility study. Pilot. Feasibility Stud. 2021, 7, 31. [Google Scholar] [CrossRef]
- Steiner, B.; Richter, I.; Elgert, L.; Haux, R.; Wolf, K.H. Mobile Health Applications for Rehabilitation of Musculoskeletal Diseases of the Shoulder: A Systematic Analysis. Stud. Health Technol. Inform. 2021, 278, 195–202. [Google Scholar]
- Ongvisatepaiboon, K.; Vanijja, V.; Chignell, M.; Mekhora, K.; Chan, J.H. Smartphone-Based Audio-Biofeedback System for Shoulder Joint Tele-Rehabilitation. J. Med. Imaging Health Inform. 2016, 6, 1127–1134. [Google Scholar] [CrossRef]
- Carbonaro, N.; Lucchesi, I.; Lorusssi, F.; Tognetti, A. Tele-monitoring, and tele-rehabilitation of the shoulder muscular-skeletal diseases through wearable systems. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2018, 2018, 4410–4413. [Google Scholar]
- Chiensriwimol, N.; Mongkolnam, P.; Chan, J.H. Frozen Shoulder Rehabilitation: Exercise Simulation and Usability Study. In Proceedings of the Ninth International Symposium on Information and Communication Technology. SoICT 2018, Danang City, Vietnam, 6–7 December 2018; Association for Computing Machinery: New York, NY, USA, 2018; pp. 257–264. [Google Scholar]
- Chung, C.E.; Chen, C.H. The App Game Interface Design for Frozen Shoulder Rehabilitation. In Advances in Ergonomics Modeling, Usability & Special Populations; Springer International Publishing: Berlin/Heidelberg, Germany, 2017; pp. 507–516. [Google Scholar]
- Rahman, M.; Kankanhalli, A.; Wadhwa, B.; Hua, Y.C.; Kei, C.K.; Hoon, L.J.; Jayakkumar, S.; Lin, C.C. GEAR: A Mobile Game-Assisted Rehabilitation System. In Proceedings of the 2016 IEEE International Conference on Healthcare Informatics (ICHI), Chicago, IL, USA, 4–7 October 2016; pp. 511–516. [Google Scholar]
- Choi, Y.; Nam, J.; Yang, D.; Jung, W.; Lee, H.R.; Kim, S.H. Effect of smartphone application-supported self-rehabilitation for frozen shoulder: A prospective randomized control study. Clin. Rehabil. 2019, 33, 653–660. [Google Scholar] [CrossRef]




| Demographics | Defrozen-App Participants (n = 13) |
|---|---|
| Age (years), median (IQR) | 54 (IQR: 49–60) |
| Gender (male/female) | 6:7 |
| Dominant side involvement | 6 (46%) |
| Duration of symptoms (months), median (IQR) | 3 (IQR: 2–3) |
| Employment status summary | |
| In paid work | 6 (46%) |
| Not in paid work | 7 (54%) |
| Previous physiotherapy for affected shoulder | |
| Yes | 2 (15%) |
| No | 11 (85%) |
| Questionnaires | Score |
|---|---|
| TAM-2: Intention to use | 4.4 ± 1.1 |
| TAM-2: Perceived usefulness | 4.5 ± 0.9 |
| TAM-2: Perceived ease of use | 4.8 ± 0.5 |
| SUS | 81.7 ± 15.7 |
| USE: Ease of learning | 4.9 ± 0.4 |
| USE: Satisfaction | 4.3 ± 0.6 |
| ID | Approach Time (Times/Week) | Average Approach Times/Day | Adherence Rate (Suggest 3 Times per Day), % | |||
|---|---|---|---|---|---|---|
| Week 1 | Week 2 | Week 3 | Week 4 | |||
| 1 | 14 | 14 | 14 | 14 | 2.0 | 67 |
| 2 | 12 | 17 | 5 | 0 | 1.2 | 40 |
| 3 | 27 | 24 | 28 | 30 | 3.9 | 100 |
| 4 | 28 | 28 | 28 | 28 | 4.0 | 100 |
| 5 | 29 | 25 | 16 | 0 | 2.5 | 83 |
| 6 | 25 | 16 | 0 | 0 | 1.5 | 50 |
| 7 | 20 | 21 | 28 | 25 | 3.4 | 100 |
| 8 | 4 | 0 | 0 | 0 | 0.1 | 3 |
| 9 | 25 | 26 | 28 | 28 | 3.8 | 100 |
| 10 | 11 | 7 | 0 | 0 | 0.6 | 20 |
| 11 | 24 | 25 | 24 | 23 | 3.4 | 100 |
| 12 | 27 | 26 | 8 | 0 | 2.2 | 70 |
| 13 | 26 | 20 | 27 | 27 | 3.6 | 100 |
| Outcome Variables | Baseline | 4-Week | p Value | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Pain NRS | 6 | 5–7 | 2 | 1–2 | 0.015 |
| OSS | 29 | 26–34 | 41 | 39–43 | 0.013 |
| QuickDASH score | 34.1 | 25–47.2 | 13.6 | 9–18 | 0.028 |
| Range of motion of shoulder | |||||
| Flexion | 130 | 110–132 | 150 | 135–160 | 0.008 |
| Extension | 40 | 35–50 | 58.3 | 50–62 | 0.012 |
| Abduction | 90 | 50–100 | 143 | 130–180 | 0.008 |
| Internal rotation | 45 | 10–60 | 65 | 46–67.3 | 0.024 |
| External rotation | 40 | 35–80 | 69 | 62.7–79 | 0.086 |
| Horizontal adduction | 100 | 80–102 | 120 | 116–125 | 0.009 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-J.; Chung, C.-Y.; Chen, C.P.C.; Hsieh, Y.-W.; Wang, C.-F.; Chen, C.-C. The Feasibility and Efficacy of Remote App-Guided Home Exercises for Frozen Shoulder: A Pilot Study. Healthcare 2024, 12, 1095. https://doi.org/10.3390/healthcare12111095
Lin Y-J, Chung C-Y, Chen CPC, Hsieh Y-W, Wang C-F, Chen C-C. The Feasibility and Efficacy of Remote App-Guided Home Exercises for Frozen Shoulder: A Pilot Study. Healthcare. 2024; 12(11):1095. https://doi.org/10.3390/healthcare12111095
Chicago/Turabian StyleLin, Yi-Jun, Chia-Ying Chung, Carl P. C. Chen, Yu-Wei Hsieh, Ching-Fu Wang, and Chih-Chi Chen. 2024. "The Feasibility and Efficacy of Remote App-Guided Home Exercises for Frozen Shoulder: A Pilot Study" Healthcare 12, no. 11: 1095. https://doi.org/10.3390/healthcare12111095
APA StyleLin, Y.-J., Chung, C.-Y., Chen, C. P. C., Hsieh, Y.-W., Wang, C.-F., & Chen, C.-C. (2024). The Feasibility and Efficacy of Remote App-Guided Home Exercises for Frozen Shoulder: A Pilot Study. Healthcare, 12(11), 1095. https://doi.org/10.3390/healthcare12111095







