Successive Waves of the COVID-19 Pandemic Had an Increasing Impact on Chronic Cardiovascular Patients in a Western Region of Romania
Abstract
1. Introduction
2. Patients and Methods
2.1. Study Design and Participants
2.2. Study Variables and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Patients’ Characteristics and Study Outcomes
3.2. Relationship between Primary and Secondary Outcomes
3.3. Relationship between Patients’ Characteristics and the Primary Outcome
3.4. Results of Paraclinical Investigations in Relation to the Primary Outcome
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walls, A.C.; Park, Y.-J.; Tortorici, M.A.; Wall, A.; McGuire, A.T.; Veesler, D. Structure, Function, and Antigenicity of the SARS-CoV-2 Spike Glycoprotein. Cell 2020, 181, 281–292.e6. [Google Scholar] [CrossRef]
- Hamming, I.; Timens, W.; Bulthuis, M.; Lely, A.; Navis, G.; van Goor, H. Tissue Distribution of ACE2 Protein, the Functional Receptor for SARS Coronavirus. A First Step in Understanding SARS Pathogenesis. J. Pathol. 2004, 203, 631–637. [Google Scholar] [CrossRef]
- Xiong, T.-Y.; Redwood, S.; Prendergast, B.; Chen, M. Coronaviruses and the Cardiovascular System: Acute and Long-Term Implications. Eur. Heart J. 2020, 41, 1798–1800. [Google Scholar] [CrossRef]
- Richardson, S.; Hirsch, J.S.; Narasimhan, M.; Crawford, J.M.; McGinn, T.; Davidson, K.W.; Barnaby, D.P.; Becker, L.B.; Chelico, J.D.; Cohen, S.L.; et al. Presenting Characteristics, Comorbidities, and Outcomes among 5700 Patients Hospitalized with COVID-19 in the New York City Area. JAMA 2020, 323, 2052. [Google Scholar] [CrossRef]
- Driggin, E.; Madhavan, M.v.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems during the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef]
- Zheng, Y.-Y.; Ma, Y.-T.; Zhang, J.-Y.; Xie, X. COVID-19 and the Cardiovascular System. Nat. Rev. Cardiol. 2020, 17, 259–260. [Google Scholar] [CrossRef]
- Guo, T.; Fan, Y.; Chen, M.; Wu, X.; Zhang, L.; He, T.; Wang, H.; Wan, J.; Wang, X.; Lu, Z. Cardiovascular Implications of Fatal Outcomes of Patients with Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 811. [Google Scholar] [CrossRef]
- Phelps, M.; Christensen, D.M.; Gerds, T.; Fosbøl, E.; Torp-Pedersen, C.; Schou, M.; Køber, L.; Kragholm, K.; Andersson, C.; Biering-Sørensen, T.; et al. Cardiovascular Comorbidities as Predictors for Severe COVID-19 Infection or Death. Eur. Heart J. Qual. Care Clin. Outcomes 2021, 7, 172–180. [Google Scholar] [CrossRef]
- Chatzilena, A.; Hyams, C.; Challen, R.; Marlow, R.; King, J.; Adegbite, D.; Kinney, J.; Clout, M.; Maskell, N.; Oliver, J.; et al. Effectiveness of BNT162b2 COVID-19 Vaccination in Prevention of Hospitalisations and Severe Disease in Adults with SARS-CoV-2 Delta (B.1.617.2) and Omicron (B.1.1.529) Variant between June 2021 and July 2022: A Prospective Test Negative Case–Control Study. Lancet Reg. Health Eur. 2023, 25, 100552. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Kuan, C.-C. Vaccination to Reduce Severe COVID-19 and Mortality in COVID-19 Patients: A Systematic Review and Meta-Analysis. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 1770–1776. [Google Scholar] [CrossRef]
- Xie, Y.; Xu, E.; Bowe, B.; Al-Aly, Z. Long-Term Cardiovascular Outcomes of COVID-19. Nat. Med. 2022, 28, 583–590. [Google Scholar] [CrossRef]
- Coronavirus Country Profiles. Available online: https://ourworldindata.org/coronavirus#coronavirus-country-profiles (accessed on 25 February 2023).
- Fisman, D.N.; Tuite, A.R. Evaluation of the Relative Virulence of Novel SARS-CoV-2 Variants: A Retrospective Cohort Study in Ontario, Canada. Can. Med. Assoc. J. 2021, 193, E1619–E1625. [Google Scholar] [CrossRef]
- Zali, A.; Khodadoost, M.; Gholamzadeh, S.; Janbazi, S.; Piri, H.; Taraghikhah, N.; Hannani, K.; Looha, M.A.; Mohammadi, G. Mortality among Hospitalized COVID-19 Patients during Surges of SARS-CoV-2 Alpha (B.1.1.7) and Delta (B.1.617.2) Variants. Sci. Rep. 2022, 12, 18918. [Google Scholar] [CrossRef]
- Florensa, D.; Mateo, J.; Spaimoc, R.; Miret, C.; Godoy, S.; Solsona, F.; Godoy, P. Severity of COVID-19 Cases in the Months of Predominance of the Alpha and Delta Variants. Sci. Rep. 2022, 12, 15456. [Google Scholar] [CrossRef]
- Yao, L.; Graff, J.C.; Aleya, L.; Ma, J.; Cao, Y.; Wei, W.; Sun, S.; Wang, C.; Jiao, Y.; Gu, W.; et al. Mortality in Four Waves of COVID-19 Is Differently Associated with Healthcare Capacities Affected by Economic Disparities. Trop. Med. Infect. Dis. 2022, 7, 241. [Google Scholar] [CrossRef]
- World Bank National Account Data. Available online: https://data.worldbank.org/indicator/NY.GDP.MKTP.CD?locations=RO (accessed on 25 February 2023).
- Vana, D. Graphs.r.o. Available online: https://www.graphs.ro/ (accessed on 21 February 2023).
- Hale, T.; Angrist, N.; Goldszmidt, R.; Kira, B.; Petherick, A.; Phillips, T.; Webster, S.; Cameron-Blake, E.; Hallas, L.; Majumdar, S.; et al. A Global Panel Database of Pandemic Policies (Oxford COVID-19 Government Response Tracker). Nat. Hum. Behav. 2021, 5, 529–538. [Google Scholar] [CrossRef]
- Blavatnik School of Government. Variation in Government Responses to COVID-19. Available online: https://www.bsg.ox.ac.uk/research/publications/variation-government-responses-covid-19 (accessed on 21 February 2023).
- Liang, C.; Zhang, W.; Li, S.; Qin, G. Coronary Heart Disease and COVID-19: A Meta-Analysis. Med. Clin. 2021, 156, 547–554. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Wang, X.; Jia, X.; Li, J.; Lei, H.; Liu, Z.; Liao, F.; Ji, M.; Lv, X.; et al. Do Underlying Cardiovascular Diseases Have Any Impact on Hospitalised Patients with COVID-19? Heart 2020, 106, 1148–1153. [Google Scholar] [CrossRef]
- Cummings, M.J.; Baldwin, M.R.; Abrams, D.; Jacobson, S.D.; Meyer, B.J.; Balough, E.M.; Aaron, J.G.; Claassen, J.; Rabbani, L.E.; Hastie, J.; et al. Epidemiology, Clinical Course, and Outcomes of Critically Ill Adults with COVID-19 in New York City: A Prospective Cohort Study. Lancet 2020, 395, 1763–1770. [Google Scholar] [CrossRef]
- Petrilli, C.M.; Jones, S.A.; Yang, J.; Rajagopalan, H.; O’Donnell, L.; Chernyak, Y.; Tobin, K.A.; Cerfolio, R.J.; Francois, F.; Horwitz, L.I. Factors Associated with Hospital Admission and Critical Illness among 5279 People with Coronavirus Disease 2019 in New York City: Prospective Cohort Study. BMJ 2020, 369, m1966. [Google Scholar] [CrossRef]
- Singh, R.; Rathore, S.S.; Khan, H.; Karale, S.; Chawla, Y.; Iqbal, K.; Bhurwal, A.; Tekin, A.; Jain, N.; Mehra, I.; et al. Association of Obesity with COVID-19 Severity and Mortality: An Updated Systemic Review, Meta-Analysis, and Meta-Regression. Front. Endocrinol. 2022, 13, 52. [Google Scholar] [CrossRef] [PubMed]
- Hentsch, L.; Cocetta, S.; Allali, G.; Santana, I.; Eason, R.; Adam, E.; Janssens, J.-P. Breathlessness and COVID-19: A Call for Research. Respiration 2021, 100, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xue, S.; Xu, J.; Ge, H.; Mao, Q.; Xu, X.; Jiang, H. Clinical Characteristics and Related Risk Factors of Disease Severity in 101 COVID-19 Patients Hospitalized in Wuhan, China. Acta Pharmacol. Sin. 2022, 43, 64–75. [Google Scholar] [CrossRef] [PubMed]
- Baigent, C.; Windecker, S.; Andreini, D.; Arbelo, E.; Barbato, E.; Bartorelli, A.L.; Baumbach, A.; Behr, E.R.; Berti, S.; Bueno, H.; et al. European Society of Cardiology Guidance for the Diagnosis and Management of Cardiovascular Disease during the COVID-19 Pandemic: Part 1—Epidemiology, Pathophysiology, and Diagnosis. Eur. Heart J. 2022, 43, 1033–1058. [Google Scholar] [CrossRef]
- The Task Force for the Management of COVID-19 of the European Society of Cardiology. Corrigendum to: European Society of Cardiology Guidance for the Diagnosis and Management of Cardiovascular Disease during the COVID-19 Pandemic: Part 1—Epidemiology, Pathophysiology, and Diagnosis; and ESC Guidance for the Diagnosis and Management of Cardiovascular Disease during the COVID-19 Pandemic: Part 2—Care Pathways, Treatment, and Follow-Up. Eur. Heart J. 2022, 43, 1033–1058. [Google Scholar] [CrossRef]
- Frencken, J.F.; Donker, D.W.; Spitoni, C.; Koster-Brouwer, M.E.; Soliman, I.W.; Ong, D.S.Y.; Horn, J.; van der Poll, T.; van Klei, W.A.; Bonten, M.J.M.; et al. Myocardial Injury in Patients with Sepsis and Its Association with Long-Term Outcome. Circ. Cardiovasc. Qual. Outcomes 2018, 11, e004040. [Google Scholar] [CrossRef]
- Mahat, R.K.; Panda, S.; Rathore, V.; Swain, S.; Yadav, L.; Sah, S.P. The Dynamics of Inflammatory Markers in Coronavirus Disease-2019 (COVID-19) Patients: A Systematic Review and Meta-Analysis. Clin. Epidemiol. Glob. Health 2021, 11, 100727. [Google Scholar] [CrossRef]
- Lalani, K.; Seshadri, S.; Samanth, J.; Thomas, J.J.; Rao, M.S.; Kotian, N.; Satheesh, J.; Nayak, K. Cardiovascular Complications and Predictors of Mortality in Hospitalized Patients with COVID-19: A Cross-Sectional Study from the Indian Subcontinent. Trop. Med. Health 2022, 50, 55. [Google Scholar] [CrossRef]
- Verduri, A.; Short, R.; Carter, B.; Braude, P.; Vilches-Moraga, A.; Quinn, T.J.; Collins, J.; Lumsden, J.; McCarthy, K.; Evans, L.; et al. Comparison between First and Second Wave of COVID-19 Outbreak in Older People: The COPE Multicentre European Observational Cohort Study. Eur. J. Public Health 2022, 32, 807–812. [Google Scholar] [CrossRef]
- Wibowo, A.; Pranata, R.; Akbar, M.R.; Purnomowati, A.; Martha, J.W. Prognostic Performance of Troponin in COVID-19: A Diagnostic Meta-Analysis and Meta-Regression. Int. J. Infect. Dis. 2021, 105, 312–318. [Google Scholar] [CrossRef]
- Kumar, A.; Karn, E.; Trivedi, K.; Kumar, P.; Chauhan, G.; Kumari, A.; Pant, P.; Munisamy, M.; Prakash, J.; Sarkar, P.G.; et al. Procalcitonin as a Predictive Marker in COVID-19: A Systematic Review and Meta-Analysis. PLoS ONE 2022, 17, e0272840. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.S.; Wong, T.Y.; Russ, B.P.; Horspool, A.M.; Miller, O.A.; Rader, N.A.; Givi, J.P.; Winters, M.T.; Wong, Z.Y.A.; Cyphert, H.A.; et al. SARS-CoV-2 Delta Variant Induces Enhanced Pathology and Inflammatory Responses in K18-HACE2 Mice. PLoS ONE 2022, 17, e0273430. [Google Scholar] [CrossRef] [PubMed]
- Wan, E.Y.F.; Mathur, S.; Zhang, R.; Yan, V.K.C.; Lai, F.T.T.; Chui, C.S.L.; Li, X.; Wong, C.K.H.; Chan, E.W.Y.; Yiu, K.H.; et al. Association of COVID-19 with Short- and Long-Term Risk of Cardiovascular Disease and Mortality: A Prospective Cohort in UK Biobank. Cardiovasc. Res. 2023. [Google Scholar] [CrossRef] [PubMed]
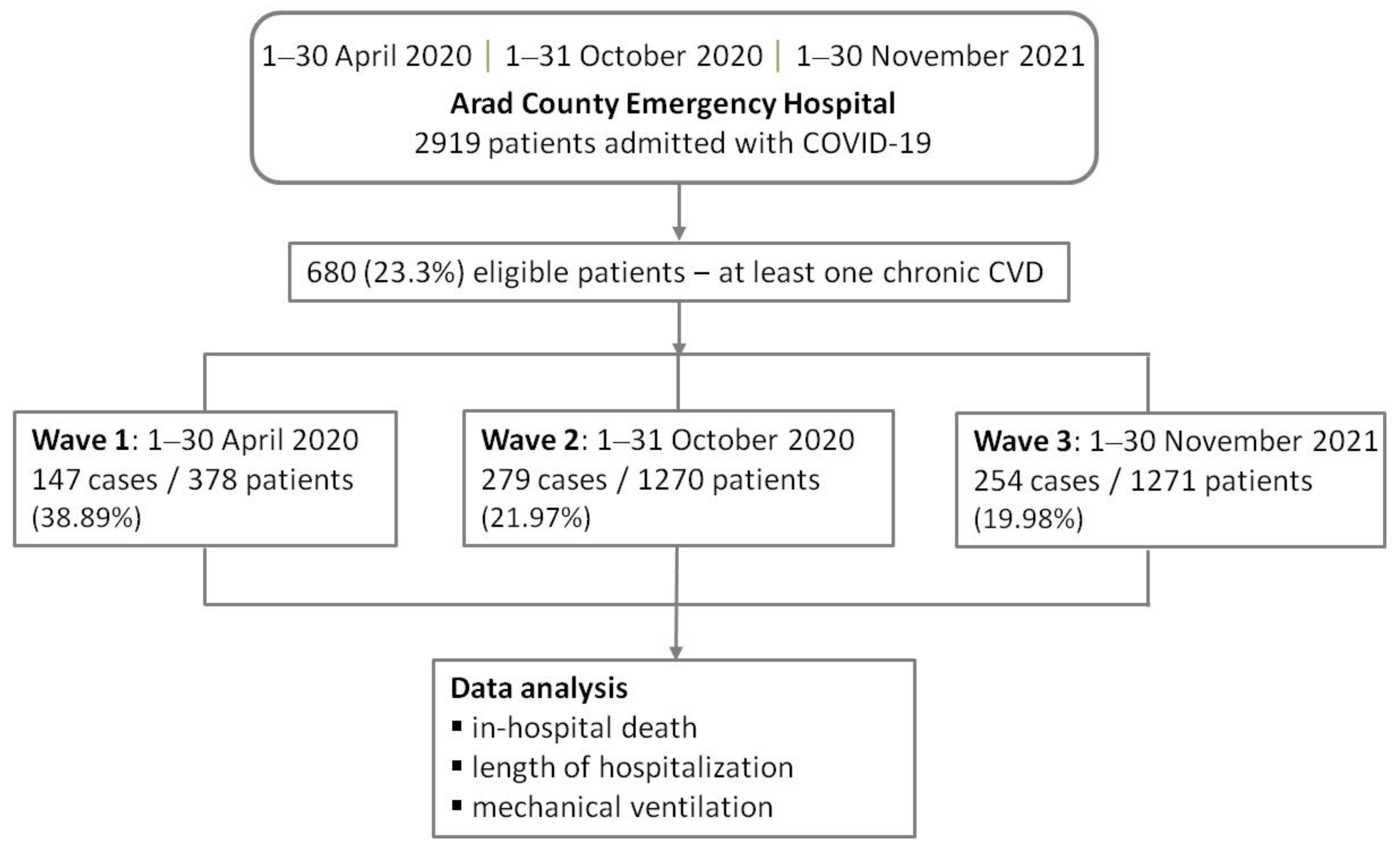
| Characteristic/ Variable | All Patients (Total 680) | Wave 1 April 2020 (Total 147) | Wave 2 Nov 2020 (Total 279) | Wave 3 Oct 2021 (Total 254) | p-Value (a), (b) |
|---|---|---|---|---|---|
| Age in years (a) | 66.22 ± 11.44 | 63.18 ± 9.77 | 65.56 ± 12.72 | 68.71 ± 10.31 | <0.001 ** |
| 67 (59–74) | 64 (56.5–70) | 67 (57–74) | 70 (63–76) | ||
| Sex, M (b) | 328 (48.2%) | 85 (57.8%) | 143 (51.3%) | 100 (39.4%) | 0.001 ** |
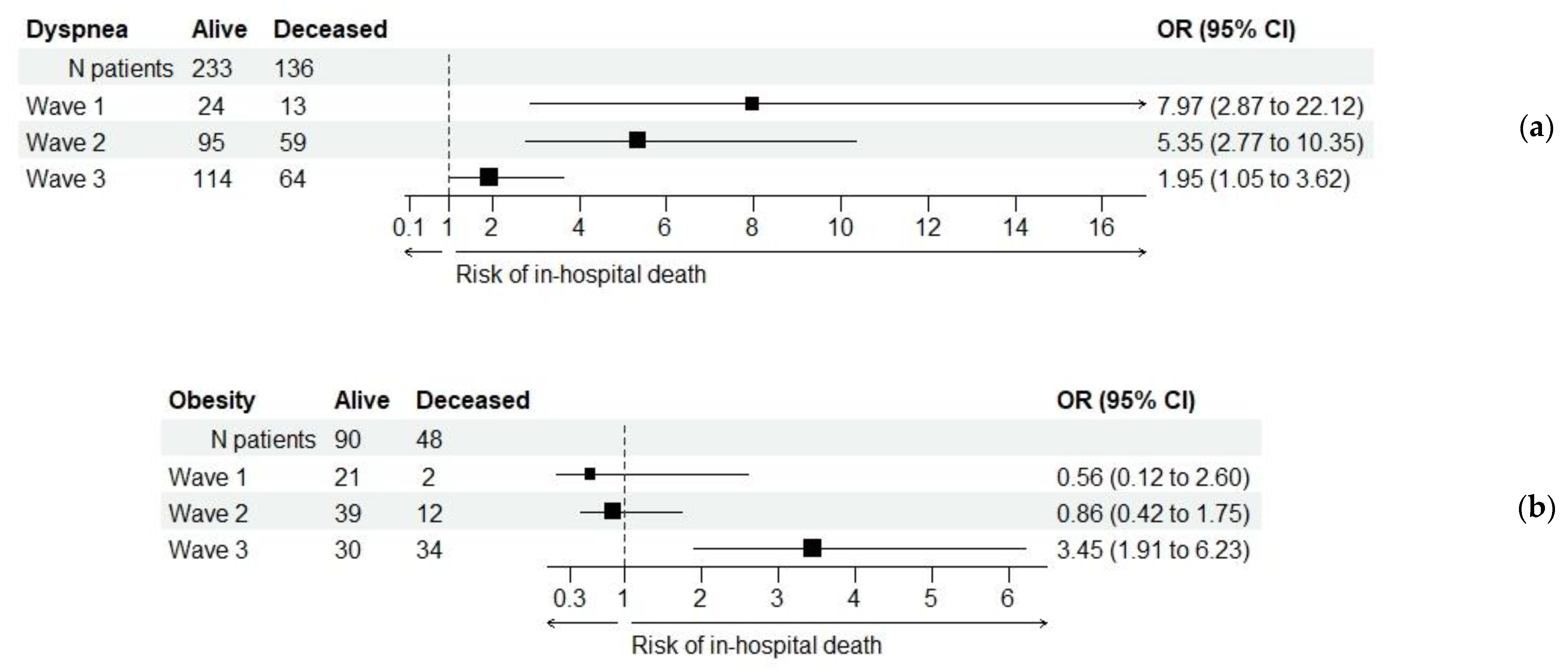
| Dyspnea (b) | 369 (54.3%) | 37 (25.2%) | 154 (55.2%) | 178 (70.1%) | <0.001 ** |
| Hypertension (b) | 643 (94.5%) | 144 (98%) | 279 (100%) | 220 (86.6%) | <0.001 ** |
| IHD (b) | 89 (13.1%) | 12 (8.2%) | 29 (10.4%) | 48 (18.9%) | 0.002 * |
| VHD (b) | 16 (2.4%) | 2 (1.4%) | 2 (0.7%) | 12 (4.7%) | 0.006 ** |
| Pulmonary embolism (b) | 3 (0.4%) | 1 (0.7%) | 0 | 2 (0.8%) | 0.333 |
| CKD (b) | 61 (9%) | 10 (6.8%) | 23 (8.2%) | 28 (11%) | 0.311 |
| Stroke (b) | 46 (6.8%) | 9 (6.1%) | 13 (4.7%) | 24 (9.4%) | 0.084 |
| COPD (b) | 44 (6.5%) | 18 (12.2%) | 26 (9.3%) | 0 | <0.001 ** |
| DM (b) | 245 (36%) | 51 (34.7%) | 100 (35.8%) | 94 (37%) | 0.894 |
| Obesity (b) | 138 (20.3%) | 23 (15.6%) | 51 (18.3%) | 64 (25.2%) | 0.040 * |
| Score XR (a) | 2.58 ± 2.56 | 2.22 ± 2.13 | 3.21 ± 2.58 | 2.11 ± 2.62 | <0.001 ** |
| 2 (0–4) | 2 (0–4) | 3 (0–6) | 0 (0–4) | ||
| Vaccinated (b) | 37 (5.4%) | – | – | 37 (14.6%) | – |
| O2 simple mask (b) | 277 (40.7%) | 51 (34.7%) | 90 (32.3%) | 136 (53.5%) | <0.001 ** |
| NIV (b) | 185 (27.2%) | 51 (34.7%) | 130 (46.6%) | 4 (1.6%) | <0.001 ** |
| OTI (b) | 94 (13.8%) | 12 (8.2%) | 25 (9%) | 57 (22.4%) | <0.001 ** |
| Characteristic/ Variable | All Patients (Total 680) | Wave 1 April 2020 (Total 147) | Wave 2 Nov 2020 (Total 279) | Wave 3 Oct 2021 (Total 254) | p-Value (a), (b) |
|---|---|---|---|---|---|
| Death (a) | 173 (25.4%) | 20 (13.6%) | 72 (25.8%) | 81 (31.9%) | <0.001 ** |
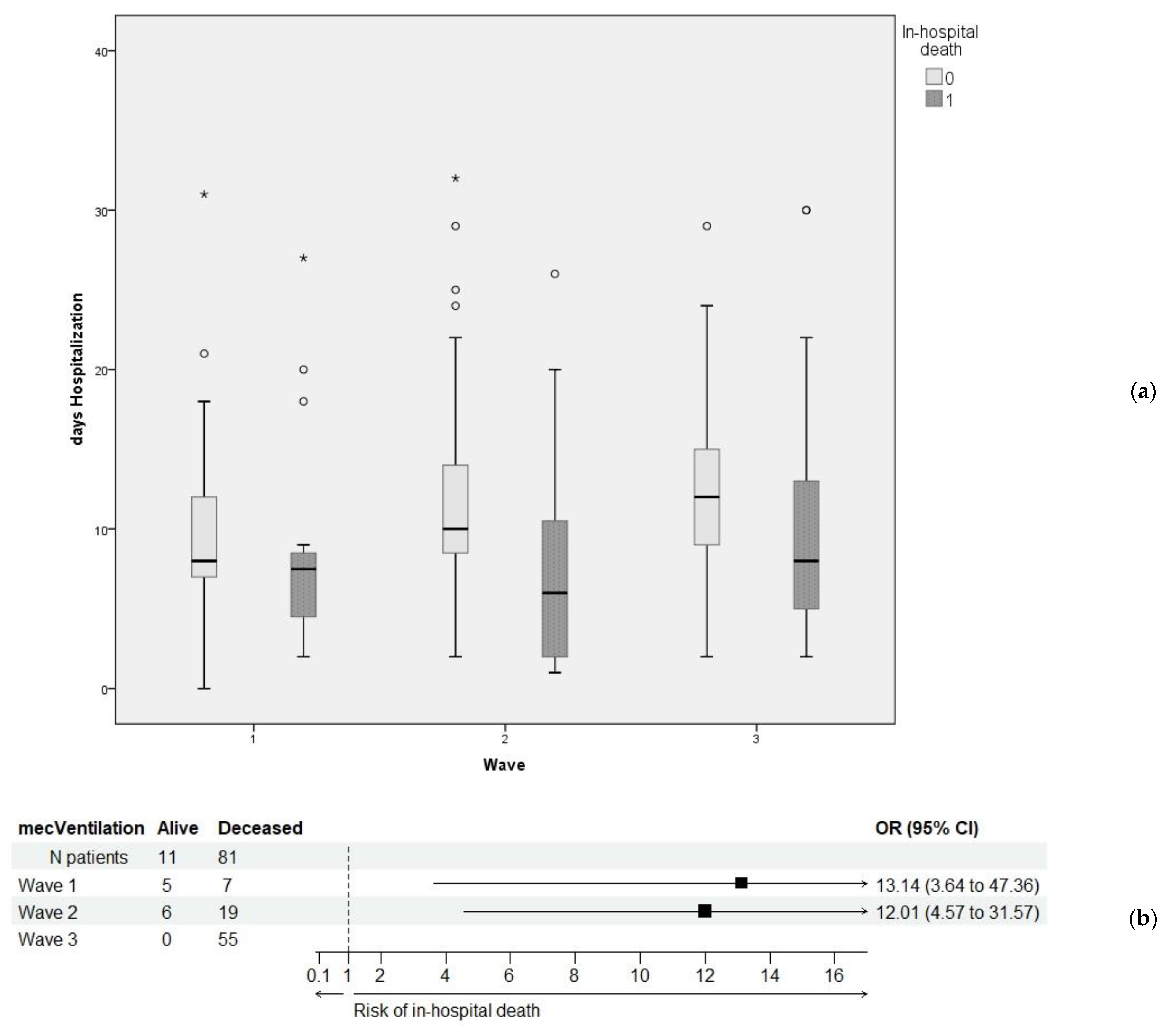
| Days hosp (b) | 10.44 ± 5.19 | 8.94 ± 4.74 | 10.19 ± 5.06 | 11.58 ± 5.34 | <0.001 ** |
| 10 (8–13) | 8 (7–11) | 10 (8–13) | 11 (8–15) | ||
| Mechanical ventilation (a) | 92 (13.5%) | 12 (8.2%) | 25 (9%) | 55 (21.7%) | <0.001 ** |
| Characteristic/ Variable | All Patients (Total 680) | Discharged Alive (Total 507) | In-Hospital Death (Total 173) | p-Value (a), (b) |
|---|---|---|---|---|
| Days hosp (a) | 10.44 ± 5.19 | 11.16 ± 4.64 | 8.32 ± 6.08 | <0.001 ** |
| 10 (8–13) | 10 (8–14) | 7 (3–11) | ||
| Mechanical ventilation (b), (c) | 92 (13.5%) | 11 (12%) | 81 (88%) | <0.001 ** |
| Characteristic/ Variable | All Patients (Total 680) | Discharged Alive (Total 507) | In-Hospital Death (Total 173) | p-Value (a), (b) |
|---|---|---|---|---|
| Age in years (a) | 66.22 ± 11.44 | 64.73 ± 11.45 | 70.59 ± 10.26 | <0.001 ** |
| 67 (59–74) | 66 (57–73) | 71 (64–77) | ||
| Sex M (b), (c) | 328 (48.2%) | 238 (72.6%) | 90 (27.4%) | 0.036 * |
| Sex F (b), (c) | 352 (51.8%) | 269 (76.4%) | 83 (23.6%) | |
| Dyspnea (b), (c) | 369 (54.3%) | 233 (63.1%) | 136 (36.9%) | <0.001 ** |
| Hypertension (b), (c) | 643 (94.6%) | 486 (95.9%) | 157 (90.8%) | 0.011 * |
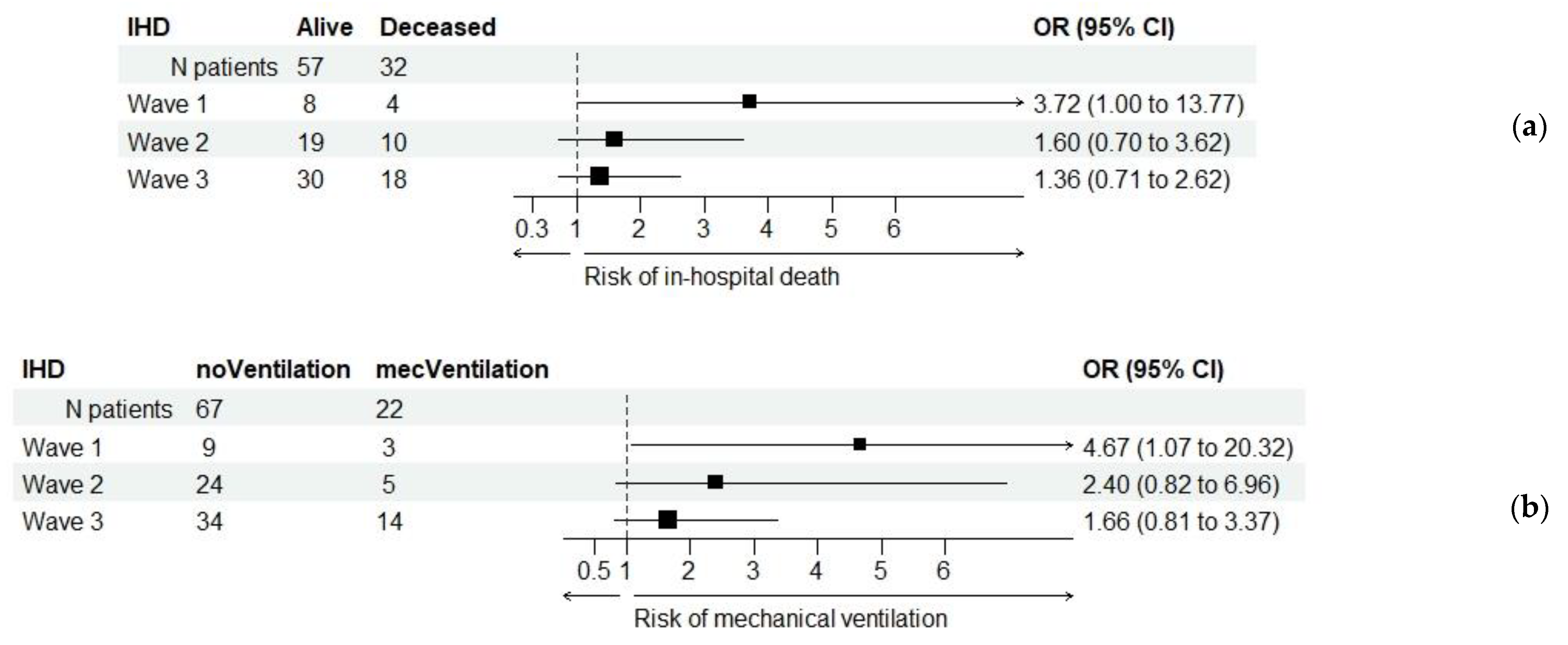
| IHD (b), (c) | 89 (13.1%) | 57 (64%) | 32 (36%) | 0.015 * |
| VHD (b), (c) | 16 (2.4%) | 9 (56.2%) | 7 (43.8%) | 0.141 |
| Pulmonary embolism (b), (c) | 3 (0.4%) | 1 (33.3%) | 3 (66.7%) | 0.161 |
| CKD (b), (c) | 61 (9%) | 40 (65.6%) | 21 (34.4%) | 0.091 # |
| Stroke(b), (c) | 46 (6.8%) | 28 (60.9%) | 18 (39.1%) | 0.027 * |
| COPD (b), (c) | 44 (6.5%) | 36 (81.8%) | 8 (18.2%) | 0.253 |
| DM (b), (c) | 245 (36%) | 175 (71.4%) | 70 (28.6%) | 0.160 |
| Obesity (b), (c) | 138 (20.3%) | 90 (65.2%) | 48 (34.8%) | 0.005 ** |
| Score XR (a) | 2.58 ± 2.56 | 2.44 ± 2.38 | 3.01 ± 2.98 | <0.106 |
| 2 (0–4) | 2 (0–4) | 2 (0–6) | ||
| Vaccinated (b) | 37 (5.4%) | 26 (70.3%) | 11 (29.7%) | 0.76 |
| O2 simple mask (b) | 277 (40.7%) | 231 (83.4%) | 46 (16.6%) | <0.001 ** |
| NIV (b) | 185 (27.2%) | 166 (89.7%) | 19 (10.3%) | <0.001 ** |
| OTI (b) | 94 (13.8%) | 39 (41.5%) | 55 (58.5%) | <0.001 ** |
| Ischemic Heart Disease | In-Hospital Death | p-Value | OR (95% CI) | |||
|---|---|---|---|---|---|---|
| – | + | Total | ||||
| All patients | – | 450 | 141 | 591 | 0.015 * | 1.792 (1.117; 2.874) |
| + | 57 | 32 | 89 | |||
| Wave 1 | – | 119 | 16 | 135 | 0.060 | 3.719 (1.005; 13.766) |
| + | 8 | 4 | 12 | |||
| Wave 2 | – | 188 | 62 | 250 | 0.259 | 1.596 (0.704; 3.615) |
| + | 19 | 10 | 29 | |||
| Wave 3 | – | 143 | 63 | 206 | 0.354 | 1.362 (0.707; 2.622) |
| + | 30 | 18 | 48 | |||
| Breslow–Day test: chi-square = 1.896 (2 df), p = 0.387 | NOTE for separate strata analysis: statistically non-significant difference between the strata and a marginally significant overall OR value | |||||
| Mantel–Haenszel test: chi-square = 3.387 (1 df), p = 0.066 | ||||||
| Mantel–Haenszel common estimate for risk OR = 1.604, 95% CI (0.996; 2.586) | ||||||
| Variable | Wave | All Patients | Discharged Alive | In-Hospital Deaths | p-Value (a) |
|---|---|---|---|---|---|
| N/L ratio (a) | 1 | n = 146 | n = 127 | n = 19 | |
| 3.09 (1.87–6.49) | 2.79 (1.71–4.59) | 13.42 (7.37–18.68) | <0.001 ** | ||
| 2 | n = 270 | n = 198 | n = 72 | ||
| 5.845 (3.00–11.53) | 4.90 (2.83–8.43) | 12.03 (5.19–19.29) | <0.001 ** | ||
| 3 | n = 254 | n = 173 | n = 81 | ||
| 5.92 (3.86–9.85) | 5.1 (3.33–7.65) | 7.92 (5.41–15.69) | <0.001 ** | ||
| T/L ratio (a) | 1 | n = 146 | n = 127 | n = 19 | |
| 9.175 (6.07–18.11) | 8.14 (5.75–14.33) | 29.30 (17.03–59.64) | <0.001 ** | ||
| 2 | n = 270 | n = 198 | n = 72 | ||
| 15.26 (7.91–31.56) | 13.41 (7.48–26.04) | 25.14 (9.69–52.09) | < 0.001** | ||
| 3 | n = 254 | n = 173 | n = 81 | ||
| 17.22 (9.78–31.12) | 14.21 (8.76–25.67) | 22.53 (13.65–44.62) | <0.001 ** | ||
| Fibrinogen (a) | 1 | n = 147 | n = 127 | n = 20 | |
| 501 (387.5–625) | 500 (380.5–597) | 599.5 (434–718.5) | 0.045 * | ||
| 2 | n = 182 | n = 146 | n = 36 | ||
| 585 (452–704) | 564.5 (445–699) | 657 (502.5–744.5) | 0.036 * | ||
| 3 | n = 247 | n = 166 | n = 81 | ||
| 540 (439–651) | 540 (436–646) | 534 (451–670) | 0.827 | ||
| CRP (a) | 1 | n = 136 | n = 119 | n = 17 | |
| 24.71 (5.395–76.66) | 18.35 (4.955–48.495) | 104.82 (83.07–155.64) | <0.001 ** | ||
| 2 | n = 165 | n = 130 | n = 35 | ||
| 39.9 (8.84–95.06) | 27.885 (7.93–71.88) | 85.27 (37.29–188.815) | <0.001 ** | ||
| 3 | n = 240 | n = 164 | n = 76 | ||
| 86.84 (40.735–139.44) | 77.37 (33.73–130.955) | 105.515 (57.06–163.2) | 0.002 ** | ||
| Procalcitonine (a) | 1 | n = 146 | n = 126 | n = 20 | |
| 0.0645 (0.048–0.168) | 0.06 (0.047–0.109) | 0.526 (0.26–1.875) | <0.001 ** | ||
| 2 | n = 111 | n = 93 | n = 18 | ||
| 0.10 (0.06–0.22) | 0.09 (0.05–0.17) | 0.32 (0.15–1.83) | <0.001 ** | ||
| 3 | n = 209 | n = 146 | n = 63 | ||
| 0.12 (0.06–0.25) | 0.09 (0.06–0.17) | 0.24 (0.13–1.18) | <0.001 ** | ||
| Troponine (a) | 1 | n = 52 | n = 39 | n = 13 | |
| 11.2 (7.38–27.735) | 9.64 (6.74–12.93) | 38 (29–107) | <0.001 ** | ||
| 2 | n = 29 | n = 22 | n = 7 | ||
| 8.6 (5.04–21.9) | 6.935 (4.7–12.05) | 41.75 (28.405–73.51) | 0.001 ** | ||
| 3 | n = 66 | n = 32 | n = 34 | ||
| 16.75 (10.9–36.7) | 12.8 (9.15–19.3) | 25.25 (13.7–102.7) | <0.001 ** | ||
| IL-6 (a) | 1 | n = 6 | n = 6 | – | |
| 6.45 (2.44–7.07) | 6.45 (2.44–7.07) | – | – | ||
| 2 | – | – | – | – | |
| 3 | n = 207 | n = 138 | n = 69 | ||
| 50.05 (18.4–101.15) | 36.96 (11.99–74.35) | 81.9 (32.38–173) | <0.001 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tudora, A.; Lungeanu, D.; Pop-Moldovan, A.; Puschita, M.; Lala, R.I. Successive Waves of the COVID-19 Pandemic Had an Increasing Impact on Chronic Cardiovascular Patients in a Western Region of Romania. Healthcare 2023, 11, 1183. https://doi.org/10.3390/healthcare11081183
Tudora A, Lungeanu D, Pop-Moldovan A, Puschita M, Lala RI. Successive Waves of the COVID-19 Pandemic Had an Increasing Impact on Chronic Cardiovascular Patients in a Western Region of Romania. Healthcare. 2023; 11(8):1183. https://doi.org/10.3390/healthcare11081183
Chicago/Turabian StyleTudora, Adelina, Diana Lungeanu, Adina Pop-Moldovan, Maria Puschita, and Radu I. Lala. 2023. "Successive Waves of the COVID-19 Pandemic Had an Increasing Impact on Chronic Cardiovascular Patients in a Western Region of Romania" Healthcare 11, no. 8: 1183. https://doi.org/10.3390/healthcare11081183
APA StyleTudora, A., Lungeanu, D., Pop-Moldovan, A., Puschita, M., & Lala, R. I. (2023). Successive Waves of the COVID-19 Pandemic Had an Increasing Impact on Chronic Cardiovascular Patients in a Western Region of Romania. Healthcare, 11(8), 1183. https://doi.org/10.3390/healthcare11081183







