CBCT Evaluation of Periapical Pathologies in Maxillary Posterior Teeth and Their Relationship with Maxillary Sinus Mucosal Thickening
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maillet, M.; Bowles, W.R.; McClanahan, S.; John, M.T.; Ahmad, M. Cone-beam computed tomography evaluation of maxillary sinusitis. J. Endod. 2011, 37, 753–757. [Google Scholar] [CrossRef] [PubMed]
- Vijayan, A.; Sreejith, V.; Surendran, R.; Ahamed, G. Orbital abscess arising from an odontogenic infection. J. Contemp. Dent. Pract. 2012, 13, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Mehra, P.; Murad, H. Maxillary sinus disease of odontogenic origin. Otolaryngol. Clin. N. Am. 2004, 37, 347–364. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, D.; Eroglu, M.; Salihoglu, M.; Yildirim, A.O.; Karagoz, H.; Erkan, M. The relationship between dental indentation and maxillary sinusitis. Open J. Med. Imaging 2013, 3, 65–68. [Google Scholar] [CrossRef]
- Little, R.E.; Long, C.M.; Loehrl, T.A.; Poetker, D.M. Odontogenic sinusitis: A review of the current literature. Laryngoscope Investig. Otolaryngol. 2018, 3, 110–114. [Google Scholar] [CrossRef] [PubMed]
- Kuan, E.C.; Suh, J.D. Systemic and odontogenic etiologies in chronic rhinosinusitis. Otolaryngol. Clin. N. Am. 2017, 50, 95–111. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Z.; Zhang, L.; Zhou, X.; Zheng, Q.; Duan, X.; Zheng, G.; Wang, H.; Huang, D. Associations between maxillary sinus mucosal thickening and apical periodontitis using cone-beam computed tomography scanning: A retrospective study. J. Endod. 2012, 38, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Nash, D.; Wald, E. Sinusitis. Pediatr. Rev. 2001, 22, 111–117. [Google Scholar] [CrossRef]
- Goller-Bulut, D.; Sekerci, A.E.; Köse, E.; Sisman, Y. Cone beam computed tomographic analysis of maxillary premolars and molars to detect the relationship between periapical and marginal bone loss and mucosal thickness of maxillary sinus. Med. Oral Patol. Oral Cir. Bucal 2015, 20, e572–e579. [Google Scholar] [CrossRef]
- Tassoker, M. What are the risk factors for maxillary sinus pathologies? A CBCT study. Oral Radiol. 2020, 36, 80–84. [Google Scholar] [CrossRef]
- Vallo, J.; Suominen-Taipale, L.; Huumonen, S.; Soikkonen, K.; Norblad, A. Prevalence of mucosal abnormalities of the maxillary sinus and their relationship to dental disease in panoramic radiography: Results from the Health 2000 Health Examination Survey. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, e80–e87. [Google Scholar] [CrossRef] [PubMed]
- Brüllmann, D.D.; Schmidtmann, I.; Hornstein, S.; Schulze, R.K. Correlation of cone beam computed tomography (CBCT) findings in the maxillary sinus with dental diagnoses: A retrospective cross-sectional study. Clin. Oral Investig. 2012, 16, 1023–1029. [Google Scholar] [CrossRef]
- Marin, S.; Kirnbauer, B.; Rugani, P.; Payer, M.; Jakse, N. Potential risk factors for maxillary sinus membrane perforation and treatment outcome analysis. Clin. Implant Dent. Relat. Res. 2019, 21, 66–72. [Google Scholar] [CrossRef]
- Cayo-Rojas, C.F.; Begazo-Jiménez, L.A.; Romero-Solórzano, L.B.; Nicho-Valladares, M.K.; Gaviria-Martínez, A.; Cervantes-Ganoza, L.A. Periapical lesions and their relationship to Schneider’s membrane in cone-beam computed tomography. Int. J. Dent. 2020, 2020, 8450315. [Google Scholar] [CrossRef]
- Maska, B.; Lin, G.H.; Othman, A.; Behdin, S.; Travan, S.; Benavides, E.; Kapila, Y. Dental implants and grafting success remain high despite large variations in maxillary sinus mucosal thickening. Int. J. Implant Dent. 2017, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Cagici, C.A.; Yilmazer, C.; Hurcan, C.; Ozer, C.; Ozer, F. Appropriate interslice gap for screening coronal paranasal sinus tomography for mucosal thickening. Eur. Arch. Otorhinolaryngol. 2009, 266, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Janner, S.F.M.; Caversaccio, M.D.; Dubach, P.; Sendi, P.; Buser, D.; Bornstein, M.M. Characteristics and dimensions of the Schneiderian membrane: A radiographic analysis using cone beam computed tomography in patients referred for dental implant surgery in the posterior maxilla. Clin. Oral Implant. Res. 2011, 22, 1446–1453. [Google Scholar] [CrossRef]
- Shanbhag, S.; Shanbhag, V.; Stavropoulos, A. Volume changes of maxillary sinus augmentations over time: A systematic review. Int. J. Oral Maxillofac. Implant. 2014, 29, 881–892. [Google Scholar] [CrossRef]
- Nascimento, E.H.; Pontual, M.L.A.; Pontual, A.A.; Freitas, D.Q.; Perez, D.E.C.; Ramos-Perez, F.M. Association between odontogenic conditions and maxillary sinus disease: A study using cone-beam computed tomography. J. Endod. 2016, 42, 1509–1515. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.A.; Ferguson, B.J. Odontogenic sinusitis: An ancient but under-appreciated cause of maxillary sinusitis. Curr. Opin. Otolaryngol. Head Neck Surg. 2012, 20, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Longhini, A.B.; Ferguson, B.J. Clinical aspects of odontogenic maxillary sinusitis: A case series. Int. Forum Allergy Rhinol. 2011, 1, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.A.; Guedes, O.A.; Alencar, A.H.G.; Peters, O.A.; Estrela, C.R.; Estrela, C. Evaluation of periapical lesions and their association with maxillary sinus abnormalities on cone-beam computed tomographic images. J. Endod. 2016, 42, 42–46. [Google Scholar] [CrossRef]
- Liang, X.; Jacobs, R.; Hassan, B.; Li, L.; Pauwels, R.; Corpas, L.; Souza, P.C.; Martens, W.; Shahbazian, M.; Alonso, A.; et al. A comparative evaluation of cone beam computed tomography (CBCT) and multi-slice CT (MSCT): Part I On subjective image quality. Eur. J. Radiol. 2010, 75, 265–269. [Google Scholar] [CrossRef]
- Ezzodini Ardakani, F.; Razavi, S.H.; Tabrizizadeh, M. Diagnostic value of cone-beam computed tomography and periapical radiography in detection of vertical root fracture. Iran. Endod. J. 2015, 10, 122–126. [Google Scholar]
- Cymerman, J.J.; Cymerman, D.H.; O’Dwyer, R.S. Evaluation of odontogenic maxillary sinusitis using cone-beam computed tomography: Three case reports. J. Endod. 2011, 37, 1465–1469. [Google Scholar] [CrossRef] [PubMed]
- Ritter, L.; Lutz, J.; Neugebauer, J.; Scheer, M.; Dreiseidler, T.; Zinser, M.J.; Rothamel, D.; Mischkowski, R.A. Prevalence of pathologic findings in the maxillary sinus in cone-beam computerized tomography. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 111, 634–640. [Google Scholar] [CrossRef] [PubMed]
- Bajoria, A.A.; Sarkar, S.; Sinha, P. Evaluation of Odontogenic Maxillary Sinusitis with Cone Beam Computed Tomography: A Retrospective Study with Review of Literature. J. Int. Soc. Prev. Community Dent. 2019, 9, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Nair, U.P.; Nair, M.K. Maxillary sinusitis of odontogenic origin: Cone-beam volumetric computerized tomography-aided diagnosis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, e53–e57. [Google Scholar] [CrossRef]
- Obayashi, N.; Ariji, Y.; Goto, M.; Izumi, M.; Naitoh, M.; Kurita, K.; Shimozato, K.; Ariji, E. Spread of odontogenic infection originating in the maxillary teeth: Computerized tomographic assessment. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004, 98, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, M.; Jacobs, R. Diagnostic value of 2D and 3D imaging in odontogenic maxillary sinusitis: A review of literature. J. Oral Rehabil. 2012, 39, 294–300. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, J.B.; Timothy, R.; Walker, C.; Hunter, R.; Benavides, E.; Samuelson, D.B.; Scheske, M.J. Effective dose of dental CBCT—A meta analysis of published data and additional data for nine CBCT units. Dentomaxillofac. Radiol. 2015, 44, 20140197. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, J.B.; Ivanovic, M. Comparative dosimetry of dental CBCT devices and 64-slice CT for oral and maxillofacial radiology. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 106, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Drumond, J.P.N.; Allegro, B.B.; Novo, N.F.; Miranda, S.L.; Sendyk, W.R. Evaluation of the Prevalence of Maxillary Sinuses Abnormalities through Spiral Computed Tomography (CT). Int. Arch. Otorhinolaryngol. 2017, 21, 126–133. [Google Scholar] [CrossRef] [PubMed]
- Raghav, M.; Karjodkar, F.R.; Sontakke, S.; Sansare, K. Prevalence of incidental maxillary sinus pathologies in dental patients on conebeam computed tomographic images. Contemp. Clin. Dent. 2014, 5, 361–365. [Google Scholar] [CrossRef]
- Phothikhun, S.; Suphanantachat, S.; Chuenchompoonut, V.; Nisapakultorn, K. Cone-beam computed tomographic evidence of the association between periodontal bone loss and mucosal thickening of the maxillary sinus. J. Periodontol. 2012, 83, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Kasikcioglu, A.; Gulsahi, A. Relationship between maxillary sinus pathologies and maxillary posterior tooth periapical pathologies. Oral Radiol. 2016, 32, 180–186. [Google Scholar] [CrossRef]
- Brook, I. Sinusitis of odontogenic origin. Otolaryngol. Head Neck Surg. 2006, 135, 349–355. [Google Scholar] [CrossRef] [PubMed]
- Kuligowski, P.; Jaroń, A.; Preuss, O.; Gabrysz-Trybek, E.; Bladowska, J.; Trybek, G. Association between Odontogenic and Maxillary Sinus Conditions: A Retrospective Cone-Beam Computed Tomographic Study. J. Clin. Med. 2021, 10, 2849. [Google Scholar] [CrossRef]
- Fry, R.R.; Patidar, D.C.; Goyal, S.; Malhotra, A. Proximity of maxillary posterior teeth roots to maxillary sinus and adjacent structures using Denta scan. Indian J. Dent. 2016, 7, 126–130. [Google Scholar] [CrossRef]
- Kocak, N.; Alpoz, E.; Boyacioglu, H. Evaluation of the effect of apical lesion on mucosal thickening and thickness of apical bone using limited cone-beam computed tomography. Niger. J. Clin. Pract. 2018, 21, 954–959. [Google Scholar]
- Bornstein, M.M.; Lauber, R.; Sendi, P.; von Arx, T. Comparison of periapical radiography and limited cone-beam computed tomography in mandibular molars for analysis of anatomical landmarks before apical surgery. J. Endod. 2011, 37, 151–157. [Google Scholar] [CrossRef]
- Yusufoglu, S.I.; Erbasar, G.N.H.; Gülen, O. Evaluation of the effect of periapical lesions and other odontogenic conditions on maxillary sinus mucosal thickness characteristics and mucosal appearance: A CBCT study. J. Dent. Res. Dent. Clin. Dent. Prospect. 2021, 15, 163–171. [Google Scholar] [CrossRef]
- Alqahtani, S.; Alsheraimi, A.; Alshareef, A.; Alsaban, R.; Alqahtani, A.; Almgran, M.; Eldesouky, M.; Al-Omar, A. Maxillary Sinus Pneumatization following Extractions in Riyadh, Saudi Arabia: A Cross-sectional Study. Cureus 2020, 12, e6611. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Yuan, J. Changes in Maxillary Sinus Mucosal Thickening following the Extraction of Teeth with Advanced Periodontal Disease: A Retrospective Study Using Cone-Beam Computed Tomography. BioMed Res. Int. 2021, 2021, 6688634. [Google Scholar] [CrossRef]
- Psillas, G.; Papaioannou, D.; Petsali, S.; Dimas, G.G.; Constantinidis, J. Odontogenic maxillary sinusitis: A comprehensive review. J. Dent. Sci. 2021, 16, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Shanbhag, S.; Karnik, P.; Shirke, P.; Shanbhag, V. Association between periapical lesions and maxillary sinus mucosal thickening: A retrospective cone-beam computed tomographic study. J. Endod. 2013, 39, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Alhujhuj, R.R.; Jouhar, R.; Ahmed, M.A.; Almujhim, A.A.; Albutayh, M.T.; Adanir, N. Evaluation of Root Canal Configuration of Maxillary and Mandibular First Molar by CBCT: A Retrospective Cross-Sectional Study. Diagnostics 2022, 12, 2121. [Google Scholar] [CrossRef] [PubMed]
- Khojastepour, L.; Movahhedian, N.; Zolghadrpour, M.; Mahjoori-Ghasrodashti, M. Assessment of the relationship between the maxillary sinus and the canine root tip using cone beam computed tomography. BMC Oral Health 2021, 21, 338. [Google Scholar] [CrossRef]
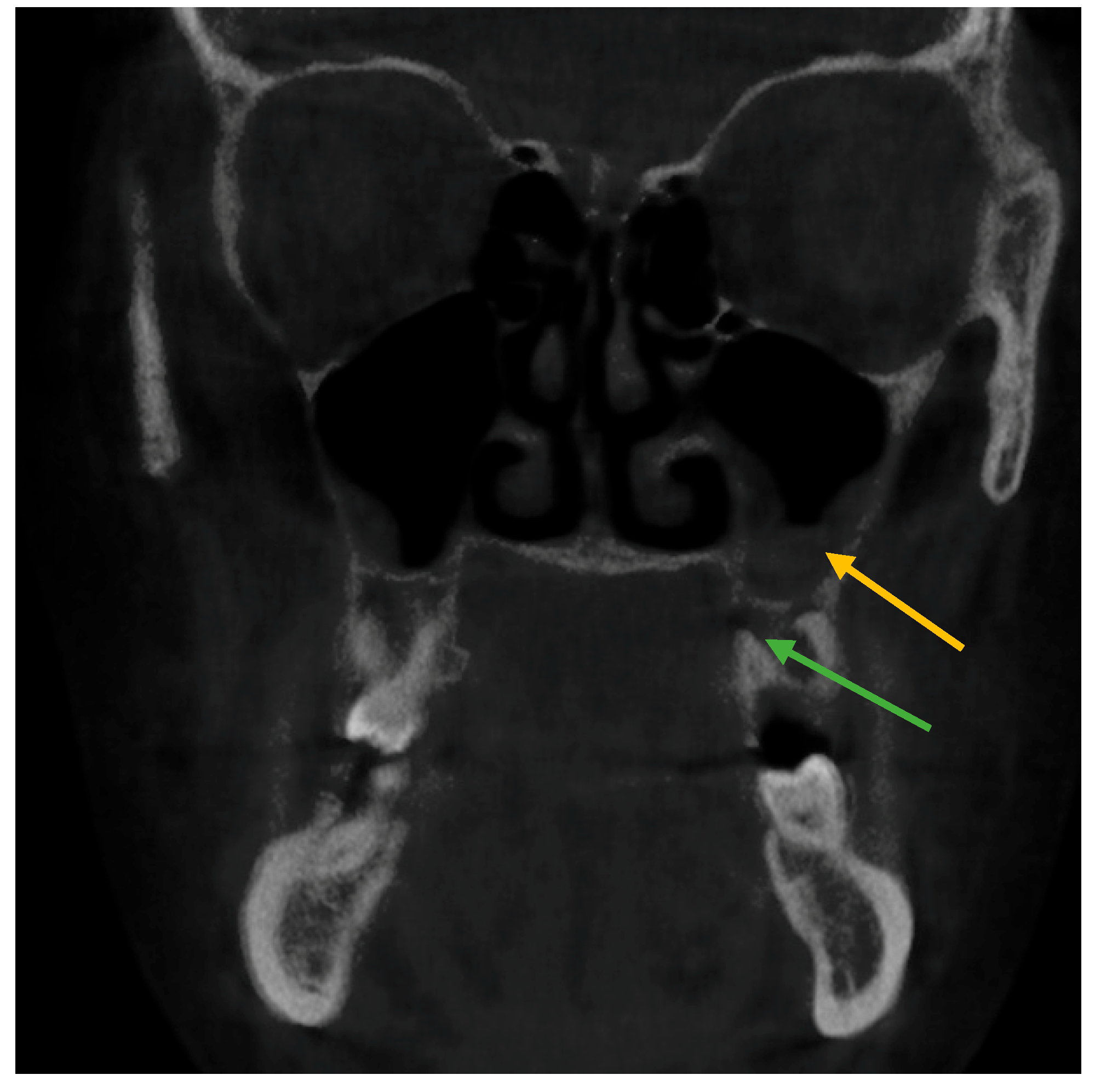
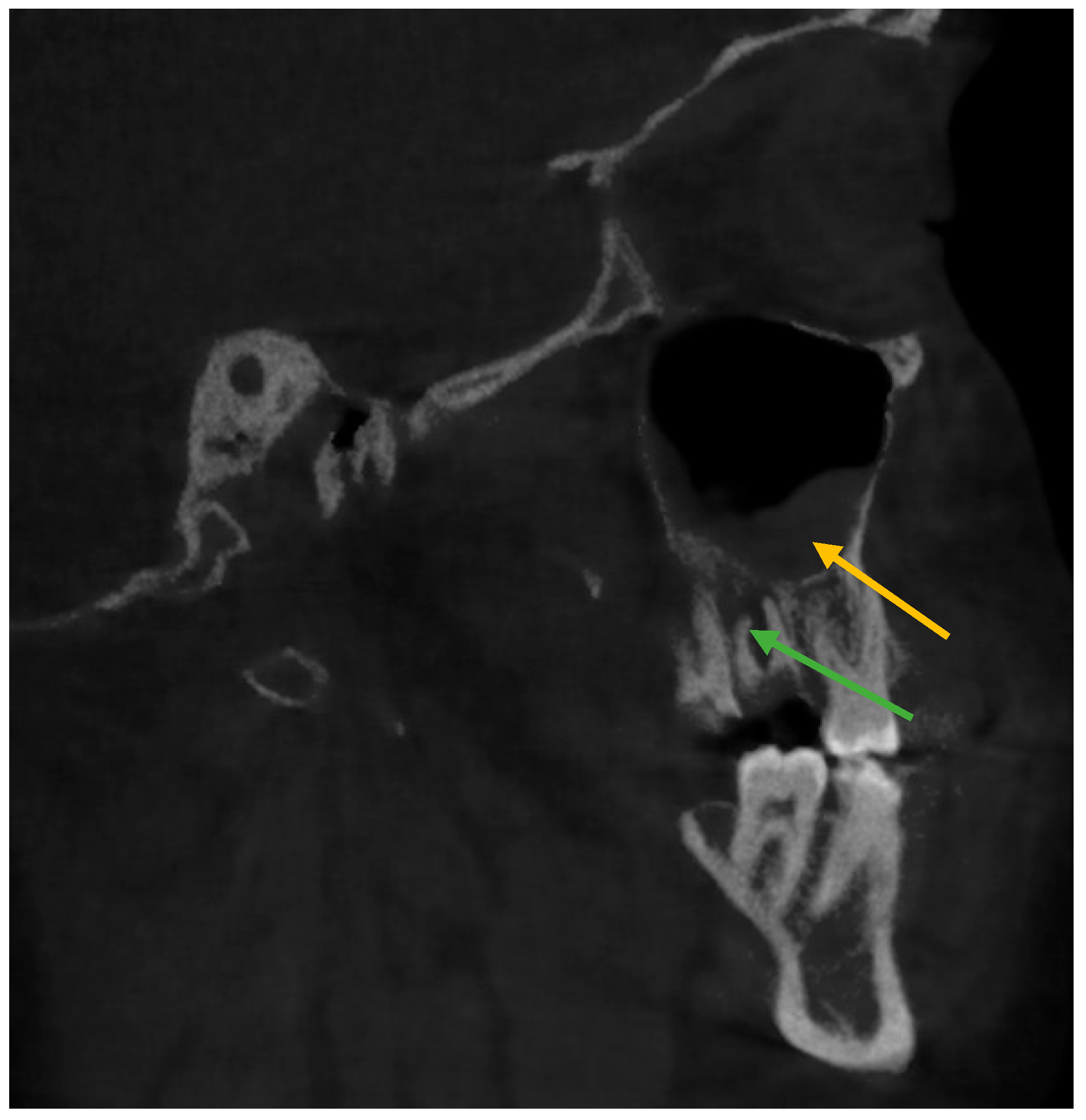
| Right Side | Status of Tooth | Total n (%) | Age Mean ± SD | Gender | ||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| First Premolar | Periapical Pathology | Present | 85(20.2) | 40.8 ± 12 | 55(64.7%) | 30(35.3%) |
| Absent | 291(69.1) | 33.38 ± 12 | 150(51.5) | 141(48.5) | ||
| Missing | 44(10.5) | 44.45 ± 10 | 18 (40.9) | 26(59.1) | ||
| Second Premolar | Periapical Pathology | Present | 103(24.5) | 41.05 ± 13 | 57(55.3) | 46(44.7) |
| Absent | 258(61.3) | 32.39 ± 11 | 131(50.8) | 127(49.2) | ||
| Missing | 59(14) | 43.24 ± 10 | 35(59.3) | 24(40.7) | ||
| First Molar | Periapical Pathology | Present | 145(34.4) | 39.17 ± 12 | 75(51.7) | 70(48.3) |
| Absent | 212(50.4) | 32.17 ± 11 | 109(51.4) | 103(48.6) | ||
| Missing | 63(15) | 41.87 ± 11 | 39(61.9) | 24(38.1) | ||
| Second Molar | Periapical Pathology | Present | 99(23.5) | 39.10 ± 13 | 49(49.5) | 50(50.5) |
| Absent | 284(67.5) | 33.61 ± 12 | 157(55.3) | 127(44.7) | ||
| Missing | 37(8.8) | 46.49 ± 10 | 17(45.9) | 20(54.1) | ||
| Left Side | Status of Tooth | Total n (%) | Age Mean ± SD | Gender | ||
|---|---|---|---|---|---|---|
| Male | Female | |||||
| First Premolar | Periapical Pathology | Present | 66(15.7) | 40.19 ± 12 | 42(63.6) | 24(36.4) |
| Absent | 312(74.3) | 33.92 ± 12 | 164(52.6) | 148(47.4) | ||
| Missing | 42(10) | 45.31 ± 10 | 17(40.5) | 25(59.5) | ||
| Second Premolar | Periapical Pathology | Present | 119(28.3) | 39.40 ± 12 | 71(59.7) | 48(40.3) |
| Absent | 244(58.1) | 32.26 ± 11 | 124(50.8) | 120(49.2) | ||
| Missing | 57(13.6) | 45.21 ± 11 | 28(49.1) | 29(50.9) | ||
| First Molar | Periapical Pathology | Present | 128(30.5) | 38.23 ± 12 | 66(51.5) | 62(48.4) |
| Absent | 207(49.3) | 31.67 ± 11 | 109(52.7) | 98(47.3) | ||
| Missing | 85(20.2) | 43.38 ± 12 | 48(56.5) | 37(43.5) | ||
| Second Molar | Periapical Pathology | Present | 113(26.9) | 41.01 ± 12 | 57(50.4) | 56(49.6) |
| Absent | 270(64.3) | 32.62 ± 11 | 145(53.7) | 125(46.3) | ||
| Missing | 37(8.8) | 45.84 ± 10 | 21(56.8) | 16(43.2) | ||
| Mucosal Thickening of Maxillary Sinus | |||||||
|---|---|---|---|---|---|---|---|
| Present, n (%) | Absent, n (%) | Mean ± SD | T | df | p Value | ||
| Right side | |||||||
| Gender | Male | 126(56.5) | 97(43.5) | 1.43 ± 0.497 | −2.326 | i418 | 0.499 |
| Female | 89(45.2) | 108(54.8) | 1.55 ± 0.499 | ||||
| Total | 215(51.2) | 205(48.8) | |||||
| Left side | |||||||
| Gender | Male | 133(59.6) | 90(40.4) | 1.40 ± 0.492 | −3.630 | 418 | 0.467 |
| Female | 83(42.1) | 114(57.9) | 1.58 ± 0.495 | ||||
| Total | 216(51.4) | 204(48.6) | |||||
| Mucosal Thickening of Maxillary Sinus | ||||||
|---|---|---|---|---|---|---|
| Present | Absent | p-Value | B | |||
| Periapical Pathology | Right Side | Present | 167(80.70) | 48(22.50) | <0.001 | −2.664 |
| Absent | 40(19.30) | 165(77.50) | ||||
| Left Side | Present | 171(79.2) | 45(20.8) | <0.001 | −2.569 | |
| Absent | 46(22.5) | 158(77.5) | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jouhar, R.; Alkhames, H.M.; Ahmed, M.A.; Almadeh, N.M.; Faheemuddin, M.; Umer, M.F. CBCT Evaluation of Periapical Pathologies in Maxillary Posterior Teeth and Their Relationship with Maxillary Sinus Mucosal Thickening. Healthcare 2023, 11, 787. https://doi.org/10.3390/healthcare11060787
Jouhar R, Alkhames HM, Ahmed MA, Almadeh NM, Faheemuddin M, Umer MF. CBCT Evaluation of Periapical Pathologies in Maxillary Posterior Teeth and Their Relationship with Maxillary Sinus Mucosal Thickening. Healthcare. 2023; 11(6):787. https://doi.org/10.3390/healthcare11060787
Chicago/Turabian StyleJouhar, Rizwan, Hussain Mohammed Alkhames, Muhammad Adeel Ahmed, Naji Mohammad Almadeh, Muhammad Faheemuddin, and Muhammad Farooq Umer. 2023. "CBCT Evaluation of Periapical Pathologies in Maxillary Posterior Teeth and Their Relationship with Maxillary Sinus Mucosal Thickening" Healthcare 11, no. 6: 787. https://doi.org/10.3390/healthcare11060787
APA StyleJouhar, R., Alkhames, H. M., Ahmed, M. A., Almadeh, N. M., Faheemuddin, M., & Umer, M. F. (2023). CBCT Evaluation of Periapical Pathologies in Maxillary Posterior Teeth and Their Relationship with Maxillary Sinus Mucosal Thickening. Healthcare, 11(6), 787. https://doi.org/10.3390/healthcare11060787









