Brain Analysis with a Complex Network Approach in Stroke Patients Based on Electroencephalography: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
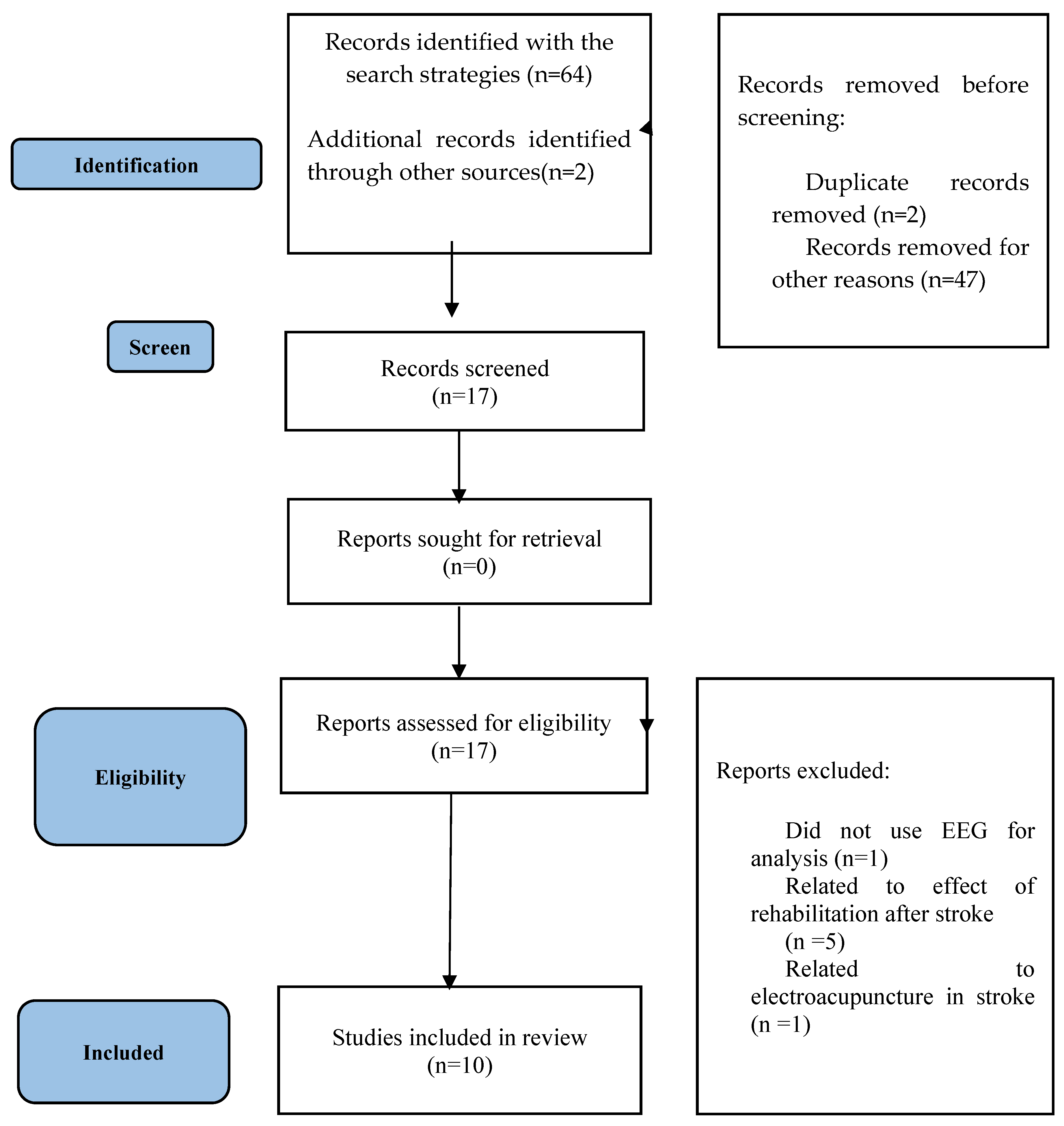
2. Materials and Methods
2.1. Study Design
2.2. Data Sources
2.3. Study Eligibility Criteria
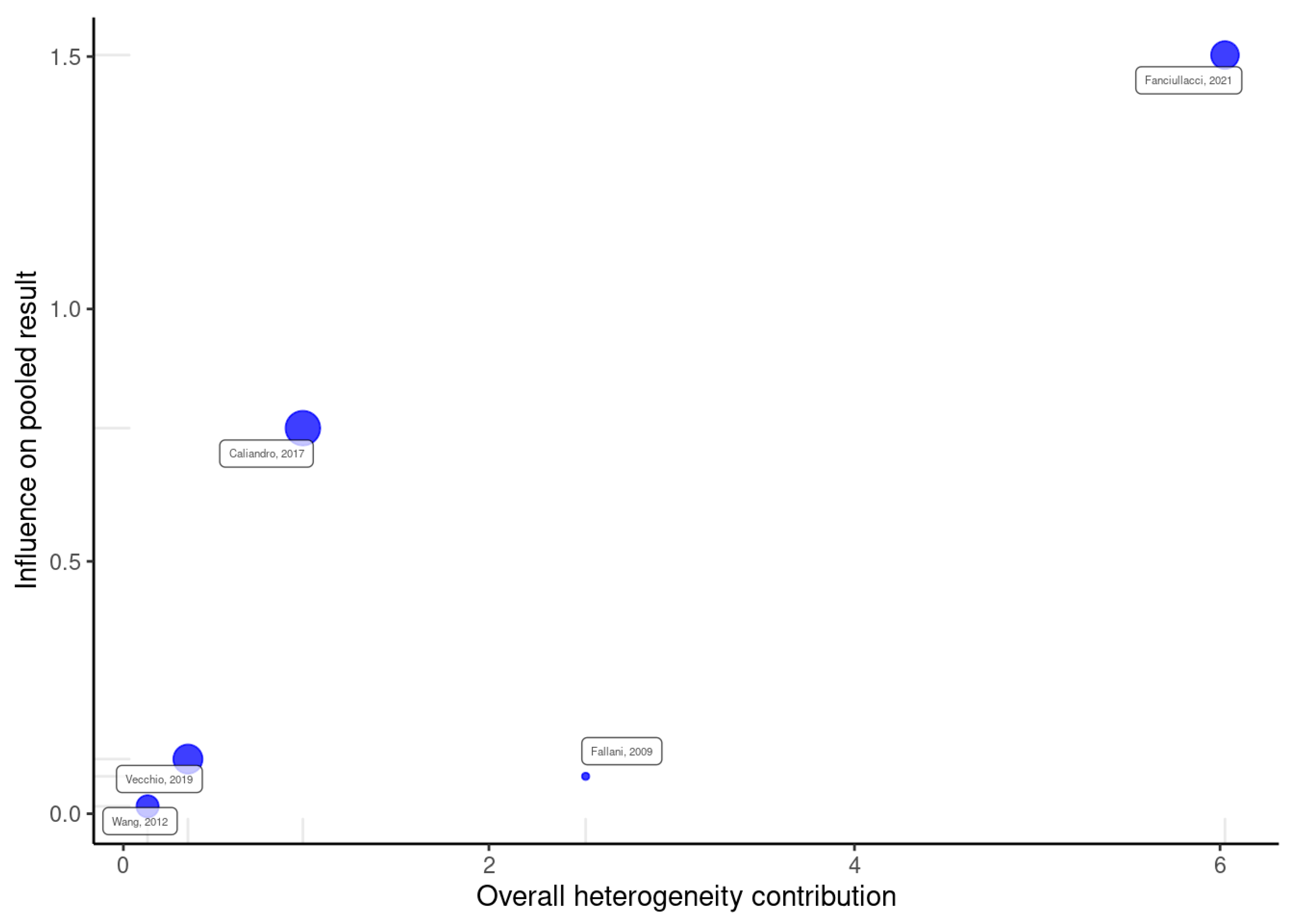
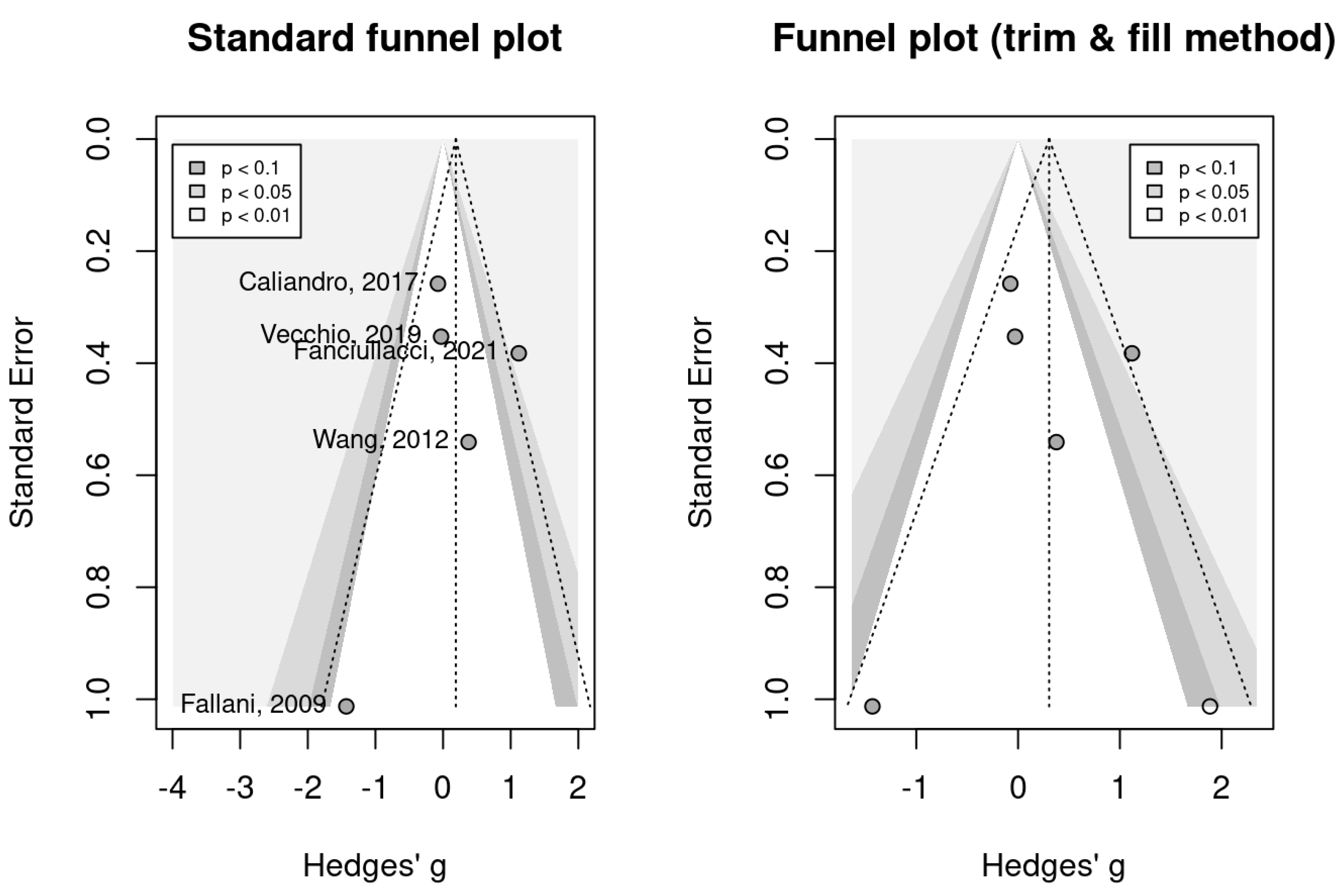
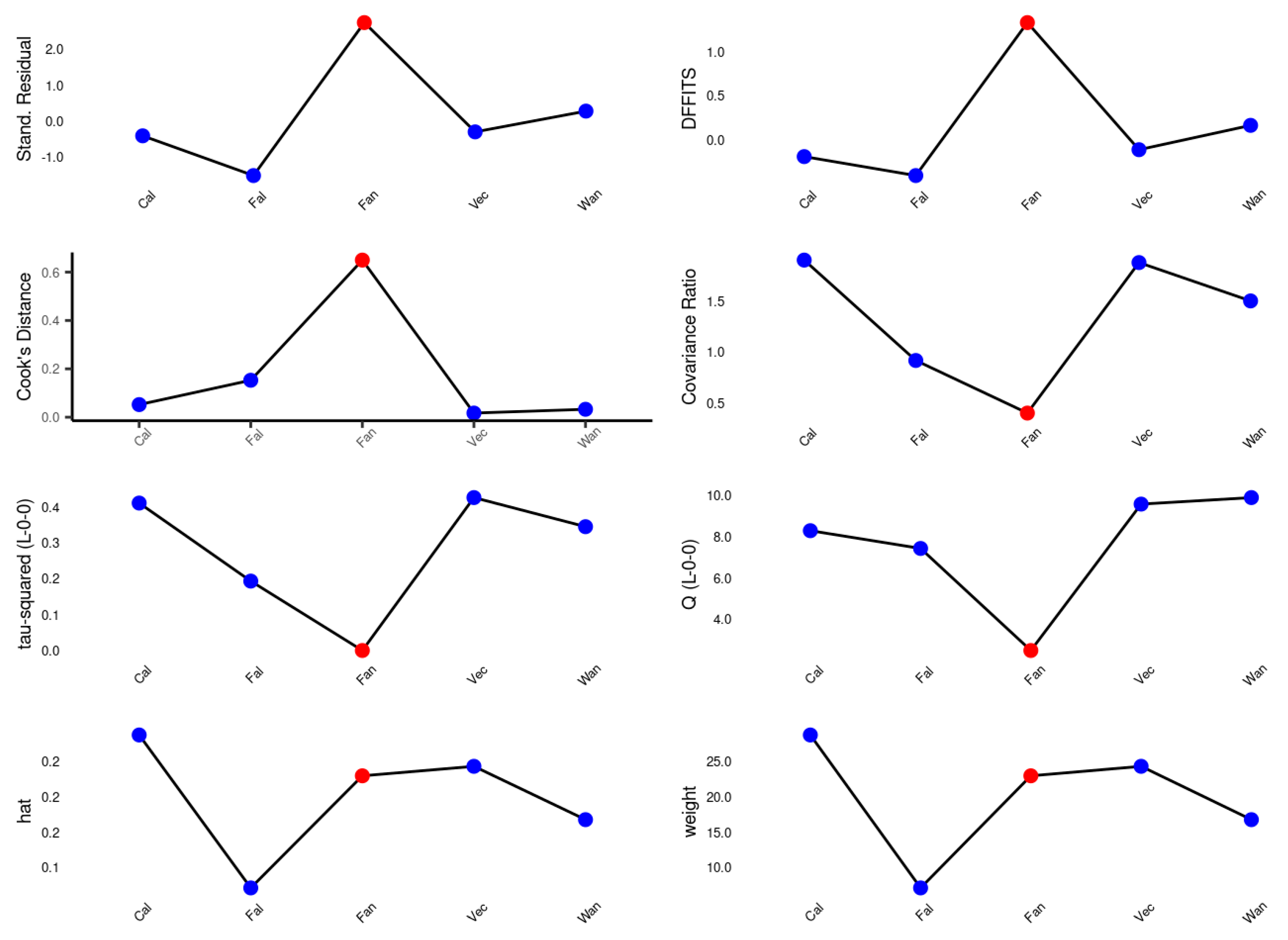
2.4. Data Analysis
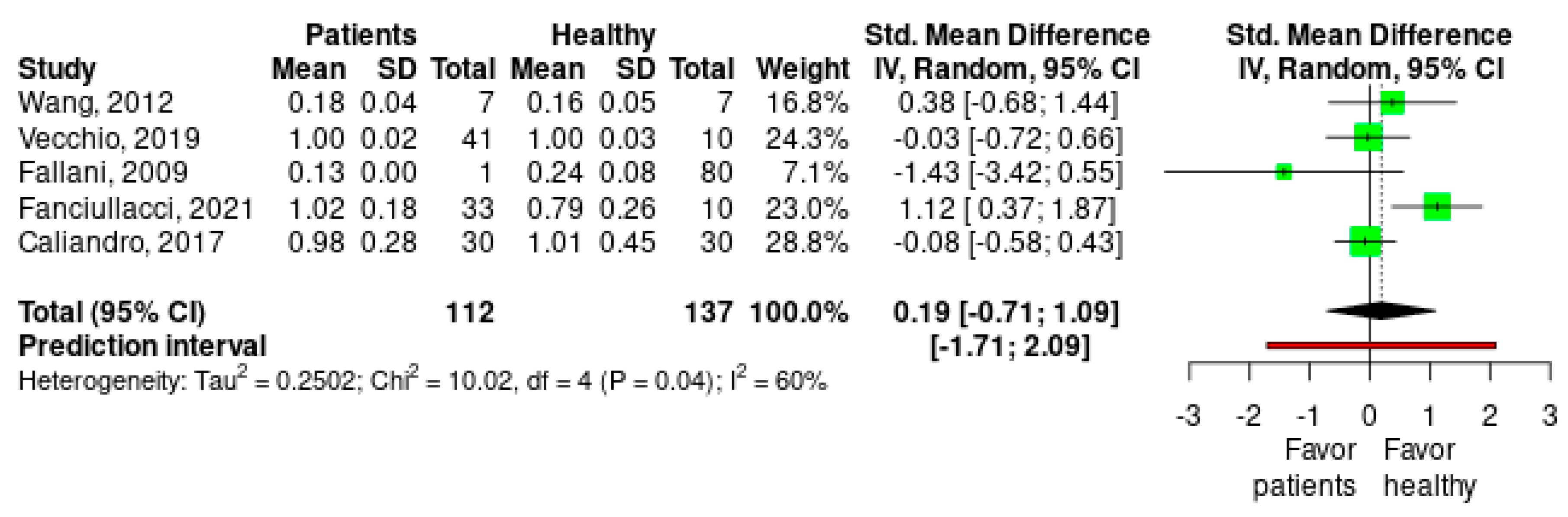
3. Results
| Study | Population | Control | Intervention | Outcome | Results |
|---|---|---|---|---|---|
| Liu, Shuang et al., 2016 [6] | 30 acute thalamic ischemic stroke patients | 30 healthy subjects | EEG in resting condition with eyes closed was recorded. | The functional connectivity was estimated with partial directed coherence (PDC) [46]. | Compared to the control group, the stroke group showed a trend of weaker cortical connectivity and a symmetrical pattern of functional connectivity; that is, there was less information transfer between electrodes on the brain. |
| Vecchio, Fabrizio et al., 2019 [4] | 30 patients with middle cerebral artery stroke and 11 with cerebellar stroke. | 30 healthy subjects | EEG was measured in resting state (at least 5 min) with eyes closed with 19 electrodes in the International 10–20 system position and sampling rate fixed at 256 Hz. | Functional connectivity of EEG data was carried out with eLORETA. The eLORETA algorithm is a linear inverse solution for detection of the EEG signals’ source [47]. | Beta2 and gamma small-world index were increased in the right hemisphere of patients with cerebellar stroke, respectively, compared to healthy subjects, while the alpha 2 small-world index was increased only in patients with middle cerebral stroke. Cerebellar stroke differed from MCA in that it did not cause reorganization of the alpha 2 network, whereas it caused reorganization of the high-frequency network in the beta 2 and gamma bands with small-world index enhancement. |
| Rutar Gorišek, Veronika et al., 2016 [5] | 10 Broca’s patients | 10 healthy subjects | The testing and EEG recordings were performed from 10 to 90 days (mean 54.4 ± SD 30.7) after the ischemic stroke. | Coherences were calculated by using the mscohere function in Matlab. | It was shown that the precise balance between task-related theta synchronization and desynchronization found in healthy subjects was severely disrupted in Broca’s patients, and functional networks in the theta frequency band were significantly altered in the patient group.Gamma desynchronization was widespread in healthy controls, but in Broca patients, task-related desynchronization was less in the right hemisphere, and functional networks in the gamma frequency band were significantly altered in the patient group. |
| Vecchio, Fabrizio et al., 2019 [7] | 139 consecutive patients were enrolled in the acute phase of stroke | 110 healthy subjects | The EEG recording was performed at rest, with closed eyes. | EEG functional connectivity analysis has been performed using the eLORETA. | When comparing the patients with the control group, there were significant differences, with higher levels of SW in the healthy subjects.A strong negative correlation was found between the NIHSS at follow-up and the small-world index gamma index in the acute phase, giving the SW gamma index a predictive weight for recovery. |
| Wang, Lei et al., 2012 [8] | 7 stroke patients with hemianopia | 7 healthy control subjects | EEG data were recorded with 30 scalp electrodes with the patient kept awake with eyes closed throughout the EEG recording for 2 min. | Phase synchronization index (PSI) [48] has been used. | For each case of the brain network with a different number of edges, the weighted clustering coefficient of the network of hemianopia stroke patients seems to be generally higher than that of the normal control group.Hemianopia stroke patients generally had a lower weighted characteristic path length than the control group. |
| Dubovik, Sviatlana et al., 2013 [9] | 20 stroke patients | 19 healthy participants | EEG was recorded with a 128-channel EEG system in an awake, resting condition with eyes closed. | The electromagnetic neural activity at each gray matter voxel was reconstructed with an adaptive spatial filter (beamformer) | Increased functional connectivity (FC) was observed in non-lesioned areas. These changes were mostly related to the alpha frequency band, and FC in the dysfunctional brain regions was consistently reduced in the alpha frequency band. |
| De Vico Fallani, Fabrizio et al., 2009 [10] | 1 stroke patient | 8 healthy subjects | EEG signals were recorded with a sampling frequency of 2048 Hz from 128 scalp electrodes. | Brain functional connectivity is achieved through the computation of task-related coherence. | The differences mainly involved the highest spectral contents (beta and gamma bands). In these bands, the global and local performances of the patient were statistically lower than the control subjects in the PRE (during the planning period) and EXE (movement execution) intervals.Network topology changes were particularly prominent in the beta band, which is already involved in motor tasks [45], as well as in the gamma band. |
| Vecchio, Fabrizio et al., 2017 [11] | A 72-year-old patient with stroke | Before and during a stroke attack | EEG Holter was recorded for evaluating signs of stroke-related epilepsy. | Magnitude squared coherence used (mscohere) | SW decreases in stroke and increases after stroke.SW decrease in the delta band and SW increase in the alpha bands.Coherence decreases during stroke and increases after stroke. |
| Fanciullacci, Chiara et al., 2021 [12] | 33 unilateral post stroke patients in the sub-acute phase: cortico-subcortical (n = 18) and subcortical (n = 15) | 10 healthy subjects | EEG was recorded for 10 min with a 10/20 EEG system in an awake, resting condition with eyes closed. | to explore interconnectivity between the ROIs, and intracortical lagged linear coherence was computed | In both groups of patients, compared to healthy subjects, there was an increase in the small-world index of the resting-state network in the θ band.β-band network measures differed significantly between stroke patients, with greater resolution and small-world index in patients with cortical involvement. |
| Caliandro, Pietro et al., 2017 [13] | 30 patients with ischemic lesion | 30 healthy subjects | The EEG recording was performed at rest, with eyes closed and no task condition for at least 5 min from 19 electrodes. | Connectivity analysis using eLORETA in both hemispheres. | Resting-state network changes were mainly detected in low- and medium-frequency EEG bands, i.e., delta, theta and alpha 2 rhythms, while no network reorganization was found in alpha 1, beta and gamma bands. |
Meta-Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A



References
- Ma, V.Y.; Chan, L.; Carruthers, K.J. Incidence, prevalence, costs, and impact on disability of common conditions requiring rehabilitation in the United States: Stroke, spinal cord injury, traumatic brain injury, multiple sclerosis, osteoarthritis, rheumatoid arthritis, limb loss, and back pain. Arch. Phys. Med. Rehabil. 2014, 95, 986–995.e981. [Google Scholar]
- Béjot, Y.; Bailly, H.; Durier, J.; Giroud, M. Epidemiology of stroke in Europe and trends for the 21st century. La Presse Méd. 2016, 45, e391–e398. [Google Scholar] [CrossRef]
- Ajčević, M.; Furlanis, G.; Miladinović, A.; Buoite Stella, A.; Caruso, P.; Ukmar, M.; Cova, M.A.; Naccarato, M.; Accardo, A.; Manganotti, P. Early EEG alterations correlate with CTP hypoperfused volumes and neurological deficit: A wireless EEG study in hyper-acute ischemic stroke. Ann. Biomed. Eng. 2021, 49, 2150–2158. [Google Scholar] [CrossRef]
- Vecchio, F.; Caliandro, P.; Reale, G.; Miraglia, F.; Piludu, F.; Masi, G.; Iacovelli, C.; Simbolotti, C.; Padua, L.; Leone, E. Acute cerebellar stroke and middle cerebral artery stroke exert distinctive modifications on functional cortical connectivity: A comparative study via EEG graph theory. Clin. Neurophysiol. 2019, 130, 997–1007. [Google Scholar] [CrossRef]
- Gorišek, V.R.; Isoski, V.Z.; Belič, A.; Manouilidou, C.; Koritnik, B.; Bon, J.; Meglič, N.P.; Vrabec, M.; Žibert, J.; Repovš, G. Beyond aphasia: Altered EEG connectivity in Broca’s patients during working memory task. Brain Lang. 2016, 163, 10–21. [Google Scholar] [CrossRef]
- Liu, S.; Guo, J.; Meng, J.; Wang, Z.; Yao, Y.; Yang, J.; Qi, H.; Ming, D. Abnormal EEG complexity and functional connectivity of brain in patients with acute thalamic ischemic stroke. Comput. Math. Methods Med. 2016, 2016, 2582478. [Google Scholar] [CrossRef]
- Vecchio, F.; Tomino, C.; Miraglia, F.; Iodice, F.; Erra, C.; Di Iorio, R.; Judica, E.; Alù, F.; Fini, M.; Rossini, P.M. Cortical connectivity from EEG data in acute stroke: A study via graph theory as a potential biomarker for functional recovery. Int. J. Psychophysiol. 2019, 146, 133–138. [Google Scholar] [CrossRef]
- Wang, L.; Guo, X.; Sun, J.; Jin, Z.; Tong, S. Cortical networks of hemianopia stroke patients: A graph theoretical analysis of EEG signals at resting state. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 49–52. [Google Scholar]
- Dubovik, S.; Ptak, R.; Aboulafia, T.; Magnin, C.; Gillabert, N.; Allet, L.; Pignat, J.-M.; Schnider, A.; Guggisberg, A.G. EEG alpha band synchrony predicts cognitive and motor performance in patients with ischemic stroke. Behav. Neurol. 2013, 26, 187–189. [Google Scholar] [CrossRef]
- De Vico Fallani, F.; Astolfi, L.; Cincotti, F.; Mattia, D.; La Rocca, D.; Maksuti, E.; Salinari, S.; Babiloni, F.; Vegso, B.; Kozmann, G. Evaluation of the brain network organization from EEG signals: A preliminary evidence in stroke patient. Anat. Rec. Adv. Integr. Anat. Evol. Biol. Adv. Integr. Anat. Evol. Biol. 2009, 292, 2023–2031. [Google Scholar] [CrossRef]
- Vecchio, F.; Miraglia, F.; Romano, A.; Bramanti, P.; Rossini, P.M. Small world brain network characteristics during EEG Holter recording of a stroke event. Clin. Neurophysiol. 2017, 128, 1–3. [Google Scholar] [CrossRef]
- Fanciullacci, C.; Panarese, A.; Spina, V.; Lassi, M.; Mazzoni, A.; Artoni, F.; Micera, S.; Chisari, C. Connectivity measures differentiate cortical and subcortical sub-acute ischemic stroke patients. Front. Hum. Neurosci. 2021, 15, 669915. [Google Scholar] [CrossRef]
- Caliandro, P.; Vecchio, F.; Miraglia, F.; Reale, G.; Della Marca, G.; La Torre, G.; Lacidogna, G.; Iacovelli, C.; Padua, L.; Bramanti, P. Small-world characteristics of cortical connectivity changes in acute stroke. Neurorehabilit. Neural Repair 2017, 31, 81–94. [Google Scholar] [CrossRef]
- Sporns, O. Networks of the Brain; MIT press: Cambridge, MA, USA, 2016. [Google Scholar]
- Watts, D.J.; Strogatz, S.H. Collective dynamics of ‘small-world’networks. Nature 1998, 393, 440–442. [Google Scholar] [CrossRef]
- Carter, A.R.; Astafiev, S.V.; Lang, C.E.; Connor, L.T.; Rengachary, J.; Strube, M.J.; Pope, D.L.; Shulman, G.L.; Corbetta, M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann. Neurol. 2010, 67, 365–375. [Google Scholar]
- Yin, D.; Song, F.; Xu, D.; Sun, L.; Men, W.; Zang, L.; Yan, X.; Fan, M. Altered topological properties of the cortical motor-related network in patients with subcortical stroke revealed by graph theoretical analysis. Hum. Brain Mapp. 2014, 35, 3343–3359. [Google Scholar] [CrossRef]
- Siegel, J.S.; Ramsey, L.E.; Snyder, A.Z.; Metcalf, N.V.; Chacko, R.V.; Weinberger, K.; Baldassarre, A.; Hacker, C.D.; Shulman, G.L.; Corbetta, M. Disruptions of network connectivity predict impairment in multiple behavioral domains after stroke. Proc. Natl. Acad. Sci. USA 2016, 113, E4367–E4376. [Google Scholar] [CrossRef]
- Miraglia, F.; Vecchio, F.; Rossini, P.M. Searching for signs of aging and dementia in EEG through network analysis. Behav. Brain Res. 2017, 317, 292–300. [Google Scholar] [CrossRef]
- Van Wijk, B.C.; Stam, C.J.; Daffertshofer, A. Comparing brain networks of different size and connectivity density using graph theory. PLoS ONE 2010, 5, e13701. [Google Scholar] [CrossRef]
- Rubinov, M.; Sporns, O. Complex network measures of brain connectivity: Uses and interpretations. Neuroimage 2010, 52, 1059–1069. [Google Scholar] [CrossRef]
- Onnela, J.-P.; Saramäki, J.; Kertész, J.; Kaski, K. Intensity and coherence of motifs in weighted complex networks. Phys. Rev. E 2005, 71, 065103. [Google Scholar] [CrossRef]
- Eliassen, J.C.; Boespflug, E.L.; Lamy, M.; Allendorfer, J.; Chu, W.-J.; Szaflarski, J.P. Brain-mapping techniques for evaluating poststroke recovery and rehabilitation: A review. Top. Stroke Rehabil. 2008, 15, 427–450. [Google Scholar] [CrossRef]
- Cohen, B.A.; Bravo-Fernandez, E.J.; Sances Jr, A. Quantification of computer analyzed serial EEGs from stroke patients. Electroencephalogr. Clin. Neurophysiol. 1976, 41, 379–386. [Google Scholar] [CrossRef]
- Cohen, B.; Bravo-Fernandez, E.; Sances, A. Automated electroencephalographic analysis as a prognostic indicator in stroke. Med. Biol. Eng. Comput. 1977, 15, 431–437. [Google Scholar] [CrossRef]
- Luu, P.; Tucker, D.M.; Englander, R.; Lockfeld, A.; Lutsep, H.; Oken, B. Localizing acute stroke-related eeg changes:: Assessing the effects of spatial undersampling. J. Clin. Neurophysiol. 2001, 18, 302–317. [Google Scholar] [CrossRef]
- Finnigan, S.P.; Rose, S.E.; Walsh, M.; Griffin, M.; Janke, A.L.; McMahon, K.L.; Gillies, R.; Strudwick, M.W.; Pettigrew, C.M.; Semple, J. Correlation of quantitative EEG in acute ischemic stroke with 30-day NIHSS score: Comparison with diffusion and perfusion MRI. Stroke 2004, 35, 899–903. [Google Scholar] [CrossRef]
- Bullmore, E.; Sporns, O. Complex brain networks: Graph theoretical analysis of structural and functional systems. Nat. Rev. Neurosci. 2009, 10, 186–198. [Google Scholar] [CrossRef]
- Andrew, C.; Pfurtscheller, G. Event-related coherence as a tool for studying dynamic interaction of brain regions. Electroencephalogr. Clin. Neurophysiol. 1996, 98, 144–148. [Google Scholar] [CrossRef]
- Weiss, S.; Mueller, H.M. The contribution of EEG coherence to the investigation of language. Brain Lang. 2003, 85, 325–343. [Google Scholar] [CrossRef]
- Hallett, M. Can EEG coherence help solve the binding problem?: The Adrian Lecture. In Supplements to Clinical Neurophysiology; Elsevier: Amsterdam, The Netherlands, 2000; Volume 53, pp. 19–26. [Google Scholar]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar]
- Quinn, T.; Dawson, J.; Walters, M.; Lees, K. Functional outcome measures in contemporary stroke trials. Int. J. Stroke 2009, 4, 200–205. [Google Scholar] [CrossRef]
- Lees, R.; Fearon, P.; Harrison, J.K.; Broomfield, N.M.; Quinn, T.J. Cognitive and mood assessment in stroke research: Focused review of contemporary studies. Stroke 2012, 43, 1678–1680. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; Group, P. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Int. J. Surg. 2010, 8, 336–341. [Google Scholar] [CrossRef]
- Greenhalgh, T. How to read a paper: The Medline database. BMJ 1997, 315, 180–183. [Google Scholar] [CrossRef]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses; ScienceOpen, Inc.: Burlington, MA, USA, 2000. [Google Scholar]
- Wan, X.; Wang, W.; Liu, J.; Tong, T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med. Res. Methodol. 2014, 14, 135. [Google Scholar] [CrossRef]
- Luo, D.; Wan, X.; Liu, J.; Tong, T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat. Methods Med. Res. 2018, 27, 1785–1805. [Google Scholar] [CrossRef]
- Higgins, J.P.; Thomas, J.; Chandler, J.; Cumpston, M.; Li, T.; Page, M.J.; Welch, V.A. Cochrane Handbook for Systematic Reviews of Interventions; John Wiley & Sons: Hoboken, NJ, USA, 2019. [Google Scholar]
- Drevon, D.; Fursa, S.R.; Malcolm, A.L. Intercoder reliability and validity of WebPlotDigitizer in extracting graphed data. Behav. Modif. 2017, 41, 323–339. [Google Scholar] [CrossRef]
- Viechtbauer, W.; Cheung, M.W.L. Outlier and influence diagnostics for meta-analysis. Res. Synth. Methods 2010, 1, 112–125. [Google Scholar] [CrossRef]
- Baujat, B.; Mahé, C.; Pignon, J.P.; Hill, C. A graphical method for exploring heterogeneity in meta-analyses: Application to a meta-analysis of 65 trials. Stat. Med. 2002, 21, 2641–2652. [Google Scholar] [CrossRef]
- Olkin, I.; Dahabreh, I.J.; Trikalinos, T.A. GOSH–a graphical display of study heterogeneity. Res. Synth. Methods 2012, 3, 214–223. [Google Scholar] [CrossRef]
- Duval, S.; Tweedie, R. Trim and fill: A simple funnel-plot–based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000, 56, 455–463. [Google Scholar] [CrossRef]
- Sakkalis, V. Review of advanced techniques for the estimation of brain connectivity measured with EEG/MEG. Comput. Biol. Med. 2011, 41, 1110–1117. [Google Scholar] [CrossRef]
- Pascual-Marqui, R.D.; Lehmann, D.; Koukkou, M.; Kochi, K.; Anderer, P.; Saletu, B.; Tanaka, H.; Hirata, K.; John, E.R.; Prichep, L. Assessing interactions in the brain with exact low-resolution electromagnetic tomography. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2011, 369, 3768–3784. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Small, M. Unified framework for detecting phase synchronization in coupled time series. Phys. Rev. E 2009, 80, 046219. [Google Scholar] [CrossRef] [PubMed]
- Asadi, B.; Fard, K.R.; Ansari, N.N.; Marco, Á.; Calvo, S.; Herrero, P. The Effect of dry Needling in Chronic Stroke with a complex Network Approach: A Case Study. Clin. EEG Neurosci. 2023, 54, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Rabiller, G.; He, J.-W.; Nishijima, Y.; Wong, A.; Liu, J. Perturbation of brain oscillations after ischemic stroke: A potential biomarker for post-stroke function and therapy. Int. J. Mol. Sci. 2015, 16, 25605–25640. [Google Scholar] [CrossRef]
- Felling, R.J.; Song, H. Epigenetic mechanisms of neuroplasticity and the implications for stroke recovery. Exp. Neurol. 2015, 268, 37–45. [Google Scholar] [CrossRef]
- Cramer, S.C.; Sur, M.; Dobkin, B.H.; O’Brien, C.; Sanger, T.D.; Trojanowski, J.Q.; Rumsey, J.M.; Hicks, R.; Cameron, J.; Chen, D. Harnessing neuroplasticity for clinical applications. Brain 2011, 134, 1591–1609. [Google Scholar] [CrossRef]
- Colcombe, S.J.; Kramer, A.F.; Erickson, K.I.; Scalf, P.; McAuley, E.; Cohen, N.J.; Webb, A.; Jerome, G.J.; Marquez, D.X.; Elavsky, S. Cardiovascular fitness, cortical plasticity, and aging. Proc. Natl. Acad. Sci. USA 2004, 101, 3316–3321. [Google Scholar] [CrossRef]
- Keil, A.; Müller, M.M.; Ray, W.J.; Gruber, T.; Elbert, T. Human gamma band activity and perception of a gestalt. J. Neurosci. 1999, 19, 7152–7161. [Google Scholar] [CrossRef]
- Gruber, T.; Müller, M.M. Oscillatory brain activity dissociates between associative stimulus content in a repetition priming task in the human EEG. Cereb. Cortex 2005, 15, 109–116. [Google Scholar] [CrossRef]
- Herrmann, C.S.; Mecklinger, A.; Pfeifer, E. Gamma responses and ERPs in a visual classification task. Clin. Neurophysiol. 1999, 110, 636–642. [Google Scholar] [CrossRef]
- Gruber, T.; Müller, M.M.; Keil, A.; Elbert, T. Selective visual-spatial attention alters induced gamma band responses in the human EEG. Clin. Neurophysiol. 1999, 110, 2074–2085. [Google Scholar] [CrossRef] [PubMed]
- Tallon-Baudry, C.; Bertrand, O.; Peronnet, F.; Pernier, J. Induced γ-band activity during the delay of a visual short-term memory task in humans. J. Neurosci. 1998, 18, 4244–4254. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.S.; Munk, M.H.; Engel, A.K. Cognitive functions of gamma-band activity: Memory match and utilization. Trends Cogn. Sci. 2004, 8, 347–355. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.; Tsivilis, D.; Montaldi, D.; Müller, M.M. Induced gamma band responses: An early marker of memory encoding and retrieval. Neuroreport 2004, 15, 1837–1841. [Google Scholar] [CrossRef] [PubMed]
- Osipova, D.; Takashima, A.; Oostenveld, R.; Fernández, G.; Maris, E.; Jensen, O. Theta and gamma oscillations predict encoding and retrieval of declarative memory. J. Neurosci. 2006, 26, 7523–7531. [Google Scholar] [CrossRef]
- Busch, N.A.; Groh-Bordin, C.; Zimmer, H.D.; Herrmann, C.S. Modes of memory: Early electrophysiological markers of repetition suppression and recognition enhancement predict behavioral performance. Psychophysiology 2008, 45, 25–35. [Google Scholar] [CrossRef]
- Park, J.Y.; Lee, K.S.; An, S.K.; Lee, J.; Kim, J.-J.; Kim, K.H.; Namkoong, K. Gamma oscillatory activity in relation to memory ability in older adults. Int. J. Psychophysiol. 2012, 86, 58–65. [Google Scholar] [CrossRef]
- Lenz, D.; Jeschke, M.; Schadow, J.; Naue, N.; Ohl, F.W.; Herrmann, C.S. Human EEG very high frequency oscillations reflect the number of matches with a template in auditory short-term memory. Brain Res. 2008, 1220, 81–92. [Google Scholar] [CrossRef]
- Achard, S.; Bullmore, E. Efficiency and cost of economical brain functional networks. PLoS Comput. Biol. 2007, 3, e17. [Google Scholar] [CrossRef]


| Study | Selection | Comparability | Outcome | Quality Score | |||||
|---|---|---|---|---|---|---|---|---|---|
| Representativeness of Exposed Cohort | Selection of the Non-Exposed Cohort from Same Source as Exposed Cohort | Ascertainment of Exposure | Outcome of Interest Was Not Present at Start of Study | Comparability of Cohorts | Assessment of Outcome | Follow-up Long Enough for Outcome to Occur | Adequacy of Follow-Up | ||
| Liu, Shuang et al., 2016 [6] | Participants were in two groups: ischemic thalamic stroke (n = 30) and the healthy group (n = 30). ★ | Yes ★ | Inclusion criteria of the patients consisted of focal ischemic lesion of the thalamus and hand numbness as symptoms. | Yes ★ | Nothing matched | Comparison of parameters of brain network between ischemic thalamic stroke patients and healthy group. | Yes ★ | All stroke patients from whom EEG was taken participated in the study. ★ | Fair |
| Vecchio, Fabrizio et al., 2019 [4] | Patients were in two groups: cerebellar and middle cerebral artery strokes (n = 30) and healthy group (n = 30). ★ | Yes ★ | The patients were clinically assessed by the National Institutes of Health Stroke Scale (NIHSS) during the acute phase. | Yes ★ | Age and gender matched ★ | Comparison of parameters of brain network between stroke patients and healthy group. | Yes ★ | All stroke patients from whom EEG was taken participated in the study. ★ | Good |
| Rutar Gorišek, Veronika et al., 2016 [5] | Participants were in two groups: Broca’s patients (n = 10) and healthy group (n = 10). ★ | Yes ★ | Boston Diagnostic Aphasia Evaluation (BDAE) | Yes ★ | Sex and education matched ★ | Comparison of parameters of brain network between stroke patients and healthy group. | Yes ★ | All patients from whom EEG was taken participated in the study. ★ | Good |
| Vecchio, Fabrizio et al., 2019 [7] | Participants were in two groups: patients with stroke in the acute phase (n = 139) and healthy group (n = 110). ★ | Yes ★ | All patients were clinically evaluated with three scales for stroke: NIHSS, Barthel and ARAT. | Yes ★ | Sex and age matched ★ | Comparison of parameters of brain network between stroke patients and healthy group. | Yes ★ | All patients from whom EEG was taken participated in the study. ★ | Good |
| Wang, Lei et al., 2012 [8] | Participants were in two groups: stroke patients (n = 7) and healthy controls (n = 7). ★ | Yes ★ | All patients were diagnosed with hemianopia stroke according to visual threshold test and MRI/CT scanning. | Yes ★ | Sex and age matched ★ | Comparison of parameters of brain network between stroke patients and healthy group. | Yes ★ | All patients from whom EEG was taken participated in the study. ★ | Good |
| Dubovik, Sviatlana et al., 2013 [9] | Participants were in two groups: patients with ischemic stroke (n = 20) and healthy participants (n = 19). ★ | Yes ★ | Motor function was evaluated by means of the Jamar dynamometer, the Nine Hole Peg test, the Stroke Rehabilitation Assessment of Movement (STREAM) and the Fugl–Meyer score. | Yes ★ | Age matched | Assessment resting-state functional connectivity with (EEG). | Yes ★ | All patients from whom EEG was taken participated in the study. ★ | Fair |
| de Vico Fallani, Fabrizio et al., 2009 [10] | Participants were in two groups: healthy subjects (n = 8) and one patient with stroke. ★ | Yes ★ | No information | Yes ★ | Nothing matched | Analysis of cerebral electro-physiological activity during planning or execution of movement in in stroke patients. | Yes ★ | All patients and healthy people from whom EEG was taken participated in the study. ★ | Fair |
| Fanciullacci, Chiara et al., 2021 [12] | Participants were in two groups: stroke patients in the sub-acute phase (n = 33) and healthy subjects (n = 10). ★ | Yes ★ | Brain injury was assessed by means of a standard CT scan. | Yes ★ | Age matched | Characterizing resting-state EEG activity and functional connectivity changes in a cohort of unilateral ischemic patients compared with the healthy group. | Yes ★ | All patients from whom EEG was taken participated in the study. ★ | Fair |
| Caliandro, Pietro et al., 2017 [13] | Participants were in 2 groups: patients with ischemic lesion (n = 30) and healthy subjects (n = 30). ★ | Yes ★ | Patients were clinically evaluated by the National Institutes of Health Stroke Scale. | Yes ★ | Age and sex matched ★ | Whether and how ischemic stroke in the acute stage may determine changes in the small-world index of cortical networks. | Yes ★ | All patients from whom EEG was taken participated in the study. ★ | Good |
| Vecchio, Fabrizio et al., 2017 [11] | |
|---|---|
| Did the study address a clearly focused question/issue? | Yes |
| Is the study design appropriate for answering the research question? | Yes |
| Does the study have a well-defined protocol? | No |
| Are both the setting and the subjects representative with regard to the population to which the findings will correlate? | Yes |
| Is the researcher’s perspective clearly described and taken into account? | Yes |
| Are the methods for collecting data clearly described? | Yes |
| Are the methods for analyzing the data likely to be valid and reliable? Are quality control measures used? | Yes |
| Was the analysis repeated by more than one researcher to ensure reliability? | No |
| Are the results credible, and if so, are they relevant for practice? Are results easy to understand? | Yes |
| Were there clinically relevant outcomes? | Yes |
| Are the conclusions drawn justified by the results? | Yes |
| Are the findings of the study transferable to other settings? | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Asadi, B.; Cuenca-Zaldivar, J.N.; Nakhostin Ansari, N.; Ibáñez, J.; Herrero, P.; Calvo, S. Brain Analysis with a Complex Network Approach in Stroke Patients Based on Electroencephalography: A Systematic Review and Meta-Analysis. Healthcare 2023, 11, 666. https://doi.org/10.3390/healthcare11050666
Asadi B, Cuenca-Zaldivar JN, Nakhostin Ansari N, Ibáñez J, Herrero P, Calvo S. Brain Analysis with a Complex Network Approach in Stroke Patients Based on Electroencephalography: A Systematic Review and Meta-Analysis. Healthcare. 2023; 11(5):666. https://doi.org/10.3390/healthcare11050666
Chicago/Turabian StyleAsadi, Borhan, Juan Nicolás Cuenca-Zaldivar, Noureddin Nakhostin Ansari, Jaime Ibáñez, Pablo Herrero, and Sandra Calvo. 2023. "Brain Analysis with a Complex Network Approach in Stroke Patients Based on Electroencephalography: A Systematic Review and Meta-Analysis" Healthcare 11, no. 5: 666. https://doi.org/10.3390/healthcare11050666
APA StyleAsadi, B., Cuenca-Zaldivar, J. N., Nakhostin Ansari, N., Ibáñez, J., Herrero, P., & Calvo, S. (2023). Brain Analysis with a Complex Network Approach in Stroke Patients Based on Electroencephalography: A Systematic Review and Meta-Analysis. Healthcare, 11(5), 666. https://doi.org/10.3390/healthcare11050666