Abstract
Spinal cord injury has a major impact on both the individual and society. This damage can cause permanent loss of sensorimotor functions, leading to structural and functional changes in somatotopic regions of the spinal cord. The combined use of a brain–machine interface and virtual reality offers a therapeutic alternative to be considered in the treatment of this pathology. This systematic review aimed to evaluate the effectiveness of the combined use of virtual reality and the brain–machine interface in the treatment of spinal cord injuries. A search was performed in PubMed, Web of Science, PEDro, Cochrane Central Register of Controlled Trials, CINAHL, Scopus, and Medline, including articles published from the beginning of each database until January 2023. Articles were selected based on strict inclusion and exclusion criteria. The Cochrane Collaboration’s tool was used to assess the risk of bias and the PEDro scale and SCIRE systems were used to evaluate the methodological quality of the studies. Eleven articles were selected from a total of eighty-two. Statistically significant changes were found in the upper limb, involving improvements in shoulder and upper arm mobility, and weaker muscles were strengthened. In conclusion, most of the articles analyzed used the electroencephalogram as a measurement instrument for the assessment of various parameters, and most studies have shown improvements. Nonetheless, further research is needed with a larger sample size and long-term follow-up to establish conclusive results regarding the effect size of these interventions.
1. Introduction
Spinal cord injury (SCI) has a major impact on both the individual and society. Hence, an increasing number of professionals are involved in the treatment of affected individuals, seeking the most advanced techniques for the enhancement of patient recovery [1]. Considering that a SCI entails a chronic life situation, the impact on the health system is not limited to the acute phase of the injury, rather, the person with SCI must face chronic diseases derived from the injury during their entire lifetime [2]. Spinal cord damage can cause a permanent loss of sensorimotor functions and persistent neuropathic pain, leading to structural and functional changes in the spinal cord [3]. In addition, most patients experience difficulties performing certain activities of daily living, which may lead to a poorer perception of quality of life [4,5].
Complete or incomplete SCI causes persistent neurological deficits because of the interruption of nerve impulses. The creation of a glial scar from the continuous deposition of fibrous tissue generates a physical barrier for axonal regeneration because of the main damage resulting from the injury [6,7]. The neurological severity of a SCI is commonly graded according to the American Spinal Injury Association Impairment Scale (AIS). This scale assesses motor and sensory functions and groups patients with SCI into five functional categories from A (absence of both functions) to E (normal function or with minimal neurological deficit) [8].
A brain–machine interface (BMI) can help restore independence to people with paralysis by using brain signals to control prostheses or trigger functional electrical stimulation [9]. The possibility of establishing a direct channel of communication and control between the human brain and computers or robots has been the subject of scientific speculation and even science fiction for many years [10]. This technology, called brain–machine interface (BMI) technology, provides a new output channel for brain signals to communicate with, or control external devices without using the normal output pathways of peripheral nerves and muscles. A BMI recognizes the user’s intent through electrophysiological or other brain signals. Electrophysiological signals may be recorded on the scalp, under the scalp, or within the brain; other types of physiological signals may be recorded by magnetic sensors or other means [11]. Other methods of assessment based on clinical neurophysiology, such as evoked potentials or event-related potential have demonstrated sensitivity for a range of cognitive functions, including attention, language processing, and memory [12]. In real time, a brain signal is translated into output commands that fulfill the user’s wish. The most common example of the use of such technology is the direct control of a computer cursor by a BMI based on electrophysiological signals [13]. The development of BMI systems has largely focused on improving the functional independence of people with severe motor disabilities, including the provision of tools for communication and mobility [14].
Virtual reality (VR) is another revolutionary therapy that can help address certain impairments caused by a SCI. Thus, VR can offer patients novel challenges and difficulties, offering a “training” that may enable people to learn possible responses that can be applied in their daily lives [15]. The use of virtual reality training can play an important role in improving cognitive functions and motor disabilities [16,17,18]. Virtual environments are offered with different degrees of immersion: non-immersive, partial, and total [19,20,21,22,23]. Total immersive VR has been gaining attention following explosive growth in VR technologies over the past decade. The key to such success is attributed to the realistic immersive settings that the head-mounted displays can produce and provide users [24]. These screens display the scene in first person, and each eye is shown slightly different two-dimensional images, thus creating the illusion that the person is seeing a three-dimensional environment [25].
Neuroprostheses that combine a BMI with functional electrical stimulation (FES) can restore voluntary control of patients’ paralyzed limbs [26]. After years of research, there is evidence that subjects can improve with VR using an appropriate BMI that is able to adapt to the patient and which, in turn, the patient is able to adapt to; however, this therapy has focused mainly on people with cerebral palsy and stroke [27,28]. Thanks to the latest technological advances, this treatment can be performed from home, which means that patients will be able to improve more rapidly with the help of their physiotherapists, occupational therapists, and family members [29]. Therefore, the aim of this systematic review was to evaluate the effectiveness of the combined use of VR and BMI in patients with SCI.
2. Materials and Methods
2.1. Literature Search
The PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) were followed in this SR [30]. Supplementary Material Figure S1 features a complete PRISMA checklist. The search protocol was registered in the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42023352246).
The literature search was conducted in the following databases: PubMed, Web of Science, PEDro, Cochrane Central Register of Controlled Trials, CINAHL, Scopus, and Medline, including articles published from the beginning of each database until January 2023. The search strategy was as follows: (“spinal cord injury” OR “spinal cord injuries” [MeSH] OR “paraplegia” [MeSH] OR “tetraplegia” OR “wheelchair”) AND (“virtual reality” [MeSH] OR “virtual reality exposure therapy” [MeSH] OR “virtual systems” OR “augmented reality” [MeSH] OR “virtual environments” OR “video games” [MeSH] OR “exergames”) AND (“brain computer interfaces” [MeSH] OR “body machine interface”). The PubMed search was performed using the MeSH descriptors (Supplementary Material Figure S2). Hand searches were also performed, by searching the reference list of studies included in the review, and adding those studies that met the inclusion criteria.
2.2. Selection Criteria
The PICOS model was used [31] (Population, Intervention, Comparison, Outcome/Outcome, Study design), to define the inclusion criteria: (P) Population; adults diagnosed with SCI. (I) Intervention; immersive, semi-immersive, or non-immersive VR combined with a BMI system connected to SCI patients. (C) Comparison; other intervention: no treatment, usual care/activities, conventional rehabilitation program, traditional physical, or occupational therapy treatment. (O) Outcome; proprioception. level of pain. Kinesthetic motor imagery. Upper extremity muscle strength. General shoulder function. Range of motion of the upper limb joints. (S) Study design; no restrictions related to study design.
Exclusion criteria; studies involving other pathologies in addition to SCI without providing separate details of the results between populations. Publications in the form of summaries and reviews.
Two reviewers (A.D.M.-R. and I.G.-A.) independently screened, reviewed, and extracted data from the final studies. In the event of any doubts or discrepancies, a third reviewer (A.A.-R.) participated in this process.
2.3. Data Extraction
The information extracted from each article included: author, country, number of participants, age and sex, AIS grade, level of injury, time since injury, type of study, level of evidence, type of intervention, session intensity, session duration, intervention duration, study variables, measurement instruments, and results.
2.4. Quality Assessment
Risk of bias was evaluated using the Cochrane Collaboration’s tool [32], developed by the Review Manager 5.3 software (Copenhagen, Denmark). This tool provides an evaluation of different items according to risk of bias. Studies are categorized as: “unclear risk”, “low risk”, and “high risk”. The risk of bias assessment was conducted by two reviewers. When in doubt, a third assessor was involved in the final decision. In order to evaluate the methodological quality of the studies, the Spinal Cord Injury Rehabilitation Evidence (SCIRE) system [33] and the Physiotherapy Evidence Database (PEDro) scale [34] were used. Moreover, the level of evidence of the included studies was classified using the combination of the SCIRE and PEDro systems. This combined score (SCIRE-PEDro) uses different categories to analyze the research design and methodological quality, grading from level 1 (highest quality) to 5 (lowest quality). The methodological quality of the studies was assessed using the PEDro scale. This scale features items related to selection, performance, detection, information, and attribution bases. According to the PEDro scale, research with a score of 9–10 is considered methodologically excellent, while a score of 6–8 is good.
2.5. Data Synthesis
A systematic review was conducted using qualitative synthesis, considering the heterogeneity of the variables studied and the treatments included in the trials. For this reason, a meta-analysis (quantitative synthesis) could not be performed.
3. Results
The literature search yielded a total of 82 articles from the electronic databases, of which 31 were duplicates. Of the 51 remaining articles, those that were unrelated to the study aim were removed (19), which resulted in a total of 32 articles. Those studies that did not combine VR with BMI, treated other pathologies, or were incomplete were removed, leaving 10 articles, which, together with those found by other means (1), yielded 11 final papers. The flow chart for the selection of the articles included in this SR was based on the PRISMA recommendations [30], displayed in Figure 1.
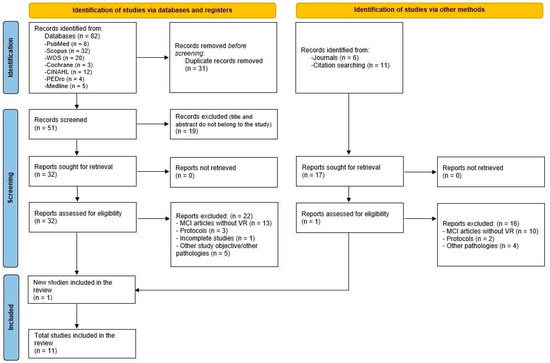
Figure 1.
Flow diagram of the selection process of the systematic review following the PRISMA recommendations [30].
The 11 selected articles were those by: Abdollahi et al. [35], Bayon-Calatayud et al. [36], Casadio et al. [37], King et al. [38], Leeb et al. [39], Mason et al. [40], Nicolelis et al. [41], Pais-Vieira et al. [42], Salisbury et al. [43], Tidoni et al. [11], and Wang et al. [44]. Table 1 and Table 2 show the main characteristics analyzed in the 11 selected articles.

Table 1.
Demographic and clinical characteristics of the studies.

Table 2.
Main characteristics of the interventions.
3.1. Summary of the Main Results
Nine articles of the eleven selected used electroencephalogram (EEG) as a measurement tool, with the exception of Abdollahi et al. [35] and Casadio et al. [37], who used the BoMI Controller and MMT. In addition, all articles except Mason et al. [40] used the AIS scale to clarify the level of SCI; however, this article does not provide the necessary information to classify its participants on this scale. The most relevant results were greater precision in the movements requested [35,39,40], improved grip in the affected arm [36,40], improved online (VR) performance of participants [38], progress was made in terms of the initial classification of SCI, which evolved from AIS A to AIS C scale [41] improved performance during the sessions [42], viability [43], the patients’ sense of embodiment within the VR [11], and a realistic approach to the treatment of patients with SCI was appreciated [44].
3.2. Assessment of the Risk of Bias and Methodological Quality of the Studies Included in the Review
Figure 2 and Figure 3 show a summary of the risk of bias assessment of the included studies, both globally and individually for each study. When analyzed individually (Figure 2), the study by Nicolelis et al. [41] has the lowest risk of bias; conversely, the studies with the highest risk of bias are those by Bayon-Calatayud et al. [36], Leeb et al. [39], and Pais-Vieira et al. [42]. Overall (Figure 3), 100% of the biases appear when assessing performance biases. Furthermore, regarding the risk of bias among the analyzed studies, the lowest biases were found with the selective reporting of results (0%) and partial reporting (18%), while the highest value (100%) was found for allocation concealment.
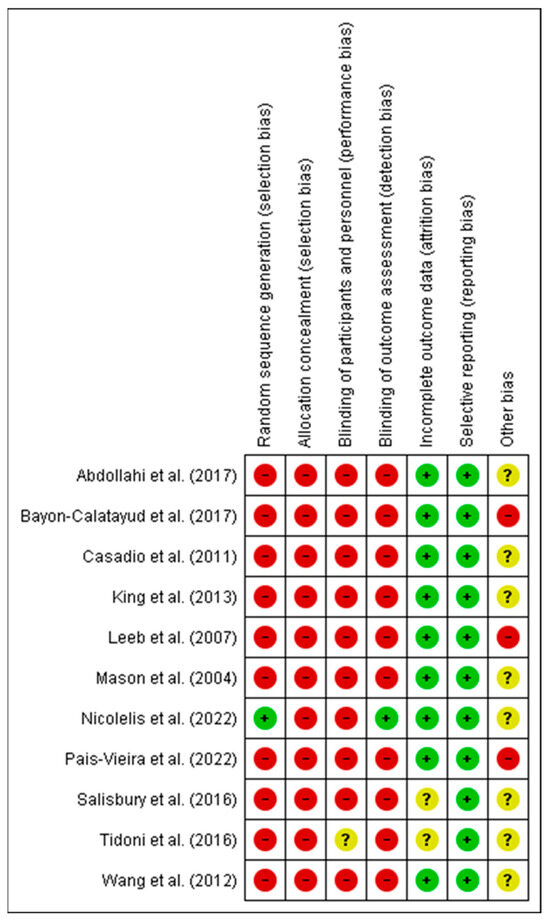
Figure 2.
Risk of bias of the studies included in the systematic review. The green circle (+) indicates low risk of bias, the yellow circle (?) unclear risk of bias, and the red circle (-) high risk of bias [11,35,36,37,38,39,40,41,42,43,44].
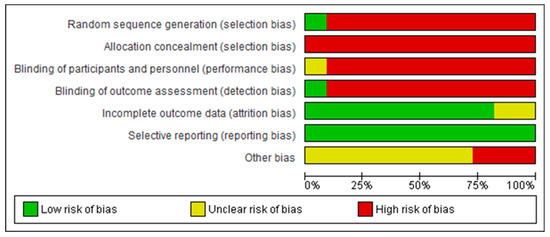
Figure 3.
Overall risk of bias, with each category presented as percentages.
The methodological quality of the only RCT found in this SR was good (total PEDro score = 7) (Supplementary Material Table S1). The remaining studies obtained a level four and five level of evidence according to the SCIRE-PEDro criteria (Table 2).
4. Discussion
The aim of the present SR was to estimate the feasibility of treatment combining VR with BCI in patients with SCI. Eleven articles were selected for this study, of which only one was a randomized controlled trial (RCT) [41], three were case studies [36,39,42], two were post-test [11,44], and five were pre-post-test [35,37,38,40,43] studies. All articles share a series of common characteristics useful for the present study, which, when compared, help us to answer our question. Of the total sample (n = 93), the number of participants per study were between a minimum of five and a maximum of twenty-five, without counting the articles that only analyzed one case, such as Bayon-Calatayud et al., Leeb et al., and Pais-Vieira et al. [36,39,42]. The participants included 75 men and 18 women. The mean age was above 18 years in all cases, with the lowest mean age belonging to the intervention groups (IG) in the studies of Tidoni et al. [11] and Wang et al. [44]. Participants in most papers were between 21 and 64 years of age, except for the study by Nicolelis et al. [41], which only reports that the participants are over 18 years of age. A total of fifty-one patients presented a complete SCI, four were incomplete, and one was not described. Of the complete lesions, 62.7% were cervical (32 lesions), 27.4% were thoracic, and 1.9% were lumbar. There were 34 healthy patients among all the articles, representing individuals who were part of the control group (CG), which helped to validate the results.
In all the studies, the CG and IG underwent the same treatment, and therefore the results validate the true effect in individuals with SCI compared to healthy individuals. The total number of sessions received ranged from 5 to 28, divided between 10 days and 4 months, although some authors fail to specify this information, concretely: Salisbury et al. [43], Tidoni et al. [11], and Wang et al. [44].
VR and BMI have been supported by other innovative techniques that provide a more realistic and differentiated view of the treatment given to patients today, such as BoMI, a customized cervical LM BMI system [35], treatment using BMI with VR, and electro-functional electrostimulation (BCI + FES + VR) combined with occupational therapy [36], LF-ASD, an EEG-based brain switch that allows the patient to turn a video game character in real time by thinking that character is moving in that direction [40], a treatment that integrates assisted locomotion with noninvasive BMI, VR, and tactile feedback [41], a protocol comprising VR goggles, tactile and thermal feedback sleeves, headsets and controllers to provide a much more immersive experience [42], and finally, treatment using kinesthetic motor imagery (KMI) to move an avatar forward in a VR environment, and inactivity to stop [44].
BMI systems are used for severe motor restrictions, combined with the use of external movement aids, although they can also be used for basic rehabilitation purposes [45]. Thus, BMI provides us with a new tool to restore mobility in paralyzed limbs [46]. In BMI, most closed-loop stimulation applications act on peripheral nerves or muscles, resulting in rapid muscle fatigue [47].
The AIS scale has been chosen by 10 of the 11 articles to measure the degree of SCI, with the exception of Mason et al. [40], who does not mention this scale at any time, nor does it label its participants in any of its grades. Another key measurement tool of the selected articles is the EEG, which appears in 9 of the 11 articles, Abdollahi et al. [35] and Casadio et al. [37] are the only two that do not use it. The EEG can be helpful for perceiving the nervous response that the patient is going to have in the area of interest while immersed in VR by means of the BMI [48].
An important consideration in the selected articles is the measurement of parameters using the upper extremities as a reference point, since in Abdollahi et al. [35], Bayon-Calatayud et al. [36], Casadio et al. [37], Leeb et al. [39], Pais-Vieira et al. [42], and Tidoni et al. [11], a sensor is placed on the hand, shoulder, biceps, or even the sleeve to capture information about the movements and their location.
Abdollahi et al. [35] uses inertial measurement units (inserted into a custom-made vest) to capture localized body movements, allowing the patients in this study (with cervical injury) much greater accuracy in detecting their movements, which will help them through practice to be more precise in their tasks. The BoMI used in the study can simulate motor learning and potentially overcome established barriers for independence and partial recovery in patients with cervical SCI. Treatment with BoMI offers patients the possibility of controlling their chairs by means of residual movements of the upper third of the body. Thus, although the process followed by Casadio et al. [37] for obtaining responses is somewhat different, using four infrared video cameras (two-dimensional each) to track four active light markers, both studies analyze the residual movements that SCI patients have, using them to their advantage by assigning meaning to them.
One of the most innovative therapies of the selected articles is the combination of BMI, functional electrical stimulation, and virtual feedback proposed by Bayon-Calatayud et al. [36]. At the end of the treatment intervention, the patient completed a usability questionnaire to evaluate the feasibility of the project. The accuracy of the patients with this technique after finishing the five indicated sessions was 85.8 ± 11.8%. Both this study and those by Leeb et al. [39] and Pais-Vieira et al. [42] reveal favorable and promising results; however, for greater reliability, more studies are needed with larger sample numbers to demonstrate the results in a larger number of patients.
Both Leeb et al. [39] and King et al. [38] used similar methods regarding the information given to the patient when involved in VR and BMI. In both studies, the patient should start by creating an idling and walking KMI; these data are then recorded with the EEG. Upon beginning data collection, Leeb et al. [39] use a form of immersion with three “cave” projections on the three walls surrounding the patient and a screen in the patient’s frontal field. This multi-wall projection system has a special feature in that the images on adjacent walls are seamlessly joined together without leaving sharp corners. Moreover, King et al. [38] uses a screen to project the image on a monitor. The high level of control achieved in both studies of SCI patients gives us some optimism for the development of lower limb prostheses controlled by an BMI system, and the protocols used for these studies can be used as tools for greater accuracy and control of BMI [38,39]. These authors are joined by Wang et al. [44] with their KMI system including idling and running moments.
A relevant aspect of the study by Salisbury et al. [43] are the headsets used (Emotiv EPOC), initially geared towards video games, but also incorporated in numerous studies; these are compact wireless headsets that require minimal effort to set up and allow much more flexibility and mobility than traditional EEG, and even analyze the patient’s facial features during their required activity within the study. Both this article and those by Abdollahi et al. [35], Tidoni et al. [11], and Mason et al. [40] modify their treatment protocol and instead of performing processed gait in VR, they practice fine motor techniques.
Pais-Vieira et al. [42] employed a BMI configuration for neurorehabilitation, combining EEG activity, VR (visual and auditory), and tactile and thermal feedback sleeves in patients with SCI to determine whether this combination of multimodal feedback would prevent brain control of an avatar. The patient was able to modulate neural activity to generate “Walk” and “Do not walk” commands according to the cues presented, supporting the hypothesis that this multimodal feedback did not impede the avatar’s brain control. An interesting finding of this study is that the patient reported feeling cold in the lower extremities when his avatar was placed in a water setting [42].
In the study by Nicolelis et al. [41], after following the action protocol prepared by the physicians, the participants managed to improve their status on the AIS scale, which is quite encouraging, since it means a significant improvement in terms of the patient’s neurological activity.
Limitations
It was difficult to find high-quality articles that combined VR treatment with a BMI system in patients with SCI in the same study. Moreover, most of the studies did not have a CG, which reduces the quality of the study, and they also included a rather small sample of patients, making it difficult to generalize the study to the entire population. The levels of SCI differ greatly from paper to paper, thus modifying the approach of the study methodology, which may be aimed at improving the patient’s gait or at improving their function by optimizing their voluntary movement of the upper extremities. Also, none of the studies provide information on the patient’s injury status months after the intervention, and therefore the long-term effect of the treatment is unknown, which may also be influenced by the novelty of this technique. Finally, it is important to consider that although an artificial intelligence tool may be useful for use in systematic reviews, it may also have inherent limitations regarding its ability to retrieve all works relating to the problem. This should be considered as a potential limitation and, therefore, human oversight is potentially necessary.
5. Conclusions
In the studies analyzed in this SR, the combined treatment with VR and BMI can be carried out in two manners, depending on the purpose of therapy.
A first aspect is more related to the recovery of the patient’s gait, which is the patient’s main concern, and for this purpose, a neurological response below the level of injury has been sought by means of BMI systems immersed in VR, which forces the patient to have intentionality of gait, and this reactivation can be favored.
The other aspect of treatment focuses on wheelchair-bound patients who have low motor activity, even in the upper limbs, and who, through their residual upper trunk skills and a trained BMI in a VR environment, may have sufficient autonomy to not rely on a third person to carry out their daily functions.
Most of the articles analyzed have used the EEG as a measurement tool for the assessment of various parameters, using the upper limbs as a reference point. With all the systems used, improvements have been obtained in most of the parameters analyzed, although the statistically significant changes have occurred in the upper limb, where the mobility of the shoulder and upper arm has improved, and the weakest muscles have been strengthened.
The improvement of patients in terms of BMI connection over the course of the sessions is clear and encourages us to be very optimistic about this therapy, as good results have been obtained every time it has been used. However, it would be interesting for future research to group the different patients according to their degree of SCI to determine the most appropriate type of treatment, analyze protocols with larger samples, and to increase the number of intervention sessions.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/healthcare11243189/s1, Figure S1: PRISMA Checklist; Figure S2: Pubmed Database Search [49,50,51,52,53,54]; Table S1: PEDro scores obtained of the randomized controlled trials included in the systematic review.
Author Contributions
Conceptualization and methodology, A.D.M.-R., I.G.-A. and A.A.-R.; software, A.A.-R.; writing—original draft preparation, A.D.M.-R., I.G.-A., M.D.D.M.-R. and A.A.-R.; writing—review and editing A.D.M.-R., M.A.-A., D.L.-A. and A.A.-R.; visualization D.L.-A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets analyzed for this study can be found in the manuscript and Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Müller-Putz, G.R.; Daly, I.; Kaiser, V. Motor imagery-induced EEG patterns in individuals with spinal cord injury and their impact on brain-computer interface accuracy. J. NeuroEng. Rehabil. 2014, 11, 035011. [Google Scholar] [CrossRef] [PubMed]
- Gil Agudo, A.M. Uso de exoesqueletos en la reeducación de la marcha en pacientes con lesión medular. In Nuevas Tecnologías Aplicadas en Fisioterapia; Escuela Universitaria de Fisioterapia de la ONCE: Madrid, Spain, 2021; ISBN 978-84-484-0302-7. [Google Scholar]
- Pozeg, P.; Palluel, E.; Ronchi, R.; Solcà, M.; Al-Khodairy, A.W.; Jordan, X. Virtual reality improves embodiment and neuropathic pain caused by spinal cord injury. Neurology 2017, 89, 1894–1903. [Google Scholar] [CrossRef] [PubMed]
- Khurana, M.; Walia, S.; Noohu, M.M. Study on the Effectiveness of Virtual Reality Game-Based Training on Balance and Functional Performance in Individuals with Paraplegia. Top. Spinal Cord Inj. Rehabil. 2017, 23, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Dimbwadyo-Terrer, I.; Gil-Agudo, A.; Segura-Fragoso, A.; de los Reyes-Guzmán, A.; Trincado-Alonso, F.; Piazza, S.; Polonio-López, B. Effectiveness of the Virtual Reality System Toyra on Upper Limb Function in People with Tetraplegia: A Pilot Randomized Clinical Trial. BioMed Res. Int. 2016, 2016, 6397828. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, F.C.; Silva, T.; Gomes, A.O.; Da Costa-Palácio, P.R.; Andreo, L.; Gonçalves, M.L.L.; Teixeira-Silva, D.F.; Horliana, A.C.R.T.; Motta, L.J.; Mesquita-Ferrari, R.A.; et al. Sensory and motor responses after photobiomodulation associated with physiotherapy in patients with incomplete spinal cord injury: Clinical, randomized trial. Lasers Med. Sci. 2020, 5, 1751–1758. [Google Scholar] [CrossRef] [PubMed]
- Filipp, M.E.; Travis, B.J.; Henry, S.S.; Idzikowski, E.C.; Magnuson, S.A.; Loh, M.Y.; Hanna, A.S. Differences in neuroplasticity after spinal cord injury in varying animal models and humans. Neural Regen. Res. 2019, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Spiess, M.R.; Müller, R.M.; Rupp, R.; Schuld, C.; Van Hedel, H.J. Conversion in ASIA impairment scale during the first year after traumatic spinal cord injury. J. Neurotrauma 2009, 26, 2027–2036. [Google Scholar] [CrossRef]
- Davis, K.C.; Meschede-Krasa, B.; Cajigas, I.; Prins, N.W.; Alver, C.; Gallo, S. Design-development of an at-home modular brain-computer interface (BCI) platform in a case study of cervical spinal cord injury. J. NeuroEng. Rehabil. 2022, 19, 53. [Google Scholar] [CrossRef]
- Hadjiaros, M.; Neokleous, K.; Shimi, A.; Avraamides, M.N.; Pattichis, C.S. Juegos cognitivos de realidad virtual basados en la interfaz cerebro-computadora: Una revisión narrativa. IEEE Access 2023, 11, 18399–18416. [Google Scholar] [CrossRef]
- Tidoni, E.; Abu-Alqumsan, M.; Leonardis, D.; Kapeller, C.; Fusco, G.; Guger, C.; Hintermuller, C.; Peer, A.; Frisoli, A.; Tecchia, F.; et al. Local and Remote Cooperation with Virtual and Robotic Agents: A P300 BCI Study in Healthy and People Living with Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1622–1632. [Google Scholar] [CrossRef]
- Connolly, J.F. Clinical neurophysiology: Research methods and event-related potential components as assessment tools. In Handbook of Clinical Neurology; Elsevier: Amsterdam, The Netherlands, 2020; pp. 277–287. [Google Scholar] [CrossRef]
- Van den Berg, M.E.; Castellote, J.M.; Mahillo-Fernandez, I.; Pedro-Cuesta, J. Incidence of spinal cord injury worldwide: A systematic review. Neuroepidemiology 2010, 34, 184–192. [Google Scholar] [CrossRef] [PubMed]
- Brandman, D.M.; Cash, S.S.; Hochberg, L.R. Review: Human Intracortical Recording and Neural Decoding for Brain-Computer Interfaces. IEEE Trans. Neural Syst. Rehabil. Eng. 2017, 25, 1687–1696. [Google Scholar] [CrossRef] [PubMed]
- Climent, G.; Luna-Lario, P.; Bombin-González, I.; Cifuentes-Rodríguez, A.; Tirapu-Ustárroz, J.; Díaz-Orueta, U. Evaluación neuropsicológica de las funciones ejecutivas mediante realidad virtual. Rev. Neurol. 2014, 58, 465. [Google Scholar] [CrossRef]
- Zając-Lamparska, L.; Wiłkość-Dębczyńska, M.; Wojciechowski, A.; Podhorecka, M.; Polak-Szabela, A.; Warchoł, L.; Kędziora- Kornatowska, K.; Araszkiewicz, A.; Izdebski, P. Effects of virtual reality-based cognitive training in older adults living without and with mild dementia: A pretest-posttest design pilot study. BMC Res. Notes 2019, 12, 776. [Google Scholar] [CrossRef] [PubMed]
- Wenk, N.; Penalver-Andres, J.; Buetler, K.A.; Nef, T.; Müri, R.M.; Marchal-Crespo, L. Effect of immersive visualization technologies on cognitive load, motivation, usability, and embodiment. Virtual Real. 2023, 27, 307–331. [Google Scholar] [CrossRef] [PubMed]
- Stryla, W.; Banas, A. The Use of Virtual Reality Technologies during Physiotherapy of the Paretic Upper Limb in Patients after Ischemic Stroke. J. Neurol. Neurosci. 2015, 6, 33. [Google Scholar] [CrossRef]
- Vrigkas, M.; Nikou, C. A virtual reality 3D game: A comparison between an immersive virtual reality application and a desktop experience. In Proceedings of the Conference Paper Proc. 1st Workshop on 3D Computer Vision and Photogrammetry, Kuala Lumpur, Malaisia, 8–11 October 2023; pp. 1–5. [Google Scholar]
- Sokołowska, B. Impact of Virtual Reality Cognitive and Motor Exercises on Brain Health. Int. J. Environ. Res. Public Health 2023, 20, 4150. [Google Scholar] [CrossRef] [PubMed]
- Martirosov, S.; Bureš, M.; Zítka, T. Cyber sickness in low-immersive, semi-immersive, and fully immersive virtual reality. Virtual Real. 2022, 26, 15–32. [Google Scholar] [CrossRef]
- Wenk, N.; Buetler, K.A.; Penalver-Andres, J.; Müri, R.M.; Marchal-Crespo, L. Naturalistic visualization of reaching movements using head-mounted displays improves movement quality compared to conventional computer screens and proves high usability. J. Neuroeng. Rehabil. 2022, 19, 137. [Google Scholar] [CrossRef]
- Kourtesis, P.; Collina, S.; Doumas, L.A.A.; MacPherson, S.E. Validation of the Virtual Reality Everyday Assessment Lab (VREAL): An Immersive Virtual Reality Neuropsychological Battery with Enhanced Ecological Validity. J. Int. Neuropsychol. Soc. 2021, 27, 181–196. [Google Scholar] [CrossRef]
- Doré, B.; Gaudreault, A.; Everard, G.; Ayena, J.C.; Abboud, A.; Robitaille, N.; Batcho, C.S. Acceptability, Feasibility, and Effectiveness of Immersive Virtual Technologies to Promote Exercise in Older Adults: A Systematic Review and Meta-Analysis. Sensors 2023, 23, 2506. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Kwon, H.; Choi, J.; Kaongoen, N.; Hwang, C.; Kim, M. Neural Applications Using Immersive Virtual Reality: A Review on EEG Studies. IEEE Trans. Neural Syst. Rehabil. Eng. 2023, 31, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Friedenberg, D.A.; Schwemmer, M.A.; Landgraf, A.J.; Annetta, N.V.; Bockbrader, M.A.; Bouton, C.E. Neuroprosthetic-enabled control of graded arm muscle contraction in a paralyzed human. Sci. Rep. 2017, 7, 8386. [Google Scholar] [CrossRef] [PubMed]
- Foldes, S.T.; Weber, D.J.; Collinger, J.L. MEG-based neurofeedback for hand rehabilitation. J. NeuroEng. Rehabil. 2015, 12, 85. [Google Scholar] [CrossRef] [PubMed]
- Tamburella, F.; Moreno, J.C.; Herrera-Valenzuela, D.S.; Pisotta, I.; Iosa, M.; Cincott, F. Influences of the biofeedback content on robotic post-stroke gait rehabilitation: Electromyographic vs joint torque biofeedback. J. NeuroEng. Rehabil. 2019, 16, 95. [Google Scholar] [CrossRef] [PubMed]
- Zulauf-Czaja, A.; Al-Taleb, M.K.H.; Purcell, M.; Petric-Gray, N.; Cloughley, J.; Vuckovic, A. On the way home: A BCI-FES hand therapy self-managed by sub-acute SCI participants and their caregivers: A usability study. J. NeuroEng. Rehabil. 2021, 18, 44. [Google Scholar] [CrossRef]
- Page, J.M.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Costantino, G.; Montano, N.; Casazza, G. When should we change our clinical practice based on the results of a clinical study? The hierarchy of evidence. Intern. Emerg. Med. 2015, 10, 745–747. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Eng, J.J.; Teasell, R.W.; Miller, W.C.; Wolfe, D.L.; Townson, A.F.; Aubut, J.A.; Abramson, C.; Hsieh, J.T.; Connoly, S.; Konnyu, K. Spinal cord injury rehabilitation evidence: Method of the SCIRE systematic review. Top. Spinal Cord Inj. Rehabil. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Maher, C.G.; Sherrington, C.; Herbert, R.D.; Moseley, A.M.; Elkins, M. Reliability of the PEDro Scale for Rating Quality of Randomized Controlled Trials. Phys. Ther. 2003, 83, 713–721. [Google Scholar] [CrossRef]
- Abdollahi, F.; Farshchiansadegh, A.; Pierella, C.; Seáñez-González, I.; Thorp, E.; Lee, M.H. Body-Machine Interface Enables People with Cervical Spinal Cord Injury to Control Devices with Available Body Movements: Proof of Concept. Neurorehabilit. Neural Repair 2017, 31, 487–493. [Google Scholar] [CrossRef] [PubMed]
- Bayon-Calatayud, M.; Trincado-Alonso, F.; López-Larraz, E.; Montesano, L.; Pons, J.L.; Gil-Agudo, Á. Usability of the combination of brain-computer interface, functional electrical stimulation and virtual reality for improving hand function in spinal cord injured patients. In Converging Clinical and Engineering Research on Neurorehabilitation II; Biosystems & Biorobotics; Springer International Publishing: Cham, Switzerland, 2017; pp. 331–335. [Google Scholar] [CrossRef]
- Casadio, M.; Pressman, A.; Acosta, S.; Danziger, Z.; Fishbach, A.; Mussa-Ivaldi, F.A.; Muir, K.; Tseng, H.; Chen, D. Body machine interface: Remapping motor skills after spinal cord injury. In Proceedings of the IEEE International Conference on Rehabilitation Robotics, Zurich, Switzerland, 29 June–1 July 2011; pp. 1–6. [Google Scholar] [CrossRef]
- King, C.E.; Wang, P.T.; Chui, L.A.; Do, A.H.; Nenadik, Z. Operation of a brain-computer interface walking simulator for individuals with spinal cord injury. J. NeuroEng. Rehabil. 2013, 10, 77. [Google Scholar] [CrossRef] [PubMed]
- Leeb, R.; Friedman, D.; Müller-Putz, G.R.; Scherer, R.; Slater, M.; Pfurtscheller, G. Self-paced (asynchronous) BCI control of a wheelchair in virtual environments: A case study with a tetraplegic. Comput. Intell. Neurosci. 2007, 2007, 79642. [Google Scholar] [CrossRef] [PubMed]
- Mason, S.G.; Bohringer, R.; Borisoff, J.F.; Birch, G.E. Real-time control of a video game with a direct brain-computer interface. J. Clin. Neurophysiol. 2004, 21, 404–408. [Google Scholar] [CrossRef] [PubMed]
- Nicolelis, M.; Alho, E.J.L.; Donati, A.R.C.; Yonamine, S.; Aratanha, M.A.; Bao, G. Training with noninvasive brain–machine interface, tactile feedback, and locomotion to enhance neurological recovery in individuals with complete paraplegia: A randomized pilot study. Sci. Rep. 2022, 12, 20545. [Google Scholar] [CrossRef] [PubMed]
- Pais-Vieira, C.; Gaspar, P.; Matos, D.; Alves, L.P.; da Cruz, B.M.; Azevedo, M.J. Embodiment Comfort Levels During Motor Imagery Training Combined with Immersive Virtual Reality in a Spinal Cord Injury Patient. Front. Hum. Neurosci. 2022, 16, 909112. [Google Scholar] [CrossRef] [PubMed]
- Salisbury, D.B.; Parsons, T.D.; Monden, K.R.; Trost, Z.; Driver, S.J. Brain–computer interface for individuals after spinal cord injury. Rehabil. Psychol. 2016, 61, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.T.; King, C.E.; Chui, L.A.; Do, A.H.; Nenadic, Z. Self-paced brain-computer interface control of ambulation in a virtual reality environment. J. NeuroEng. Rehabil. 2012, 9, 056016. [Google Scholar] [CrossRef]
- Pierella, C.; De Luca, A.; Tasso, E.; Cervetto, F.; Gamba, S.; Losio, L. Changes in neuromuscular activity during motor training with a body-machine interface after spinal cord injury. In Proceedings of the 2017 International Conference on Rehabilitation Robotics (ICORR), London, UK, 17–20 July 2017; pp. 1100–1105. [Google Scholar] [CrossRef]
- Donati, A.R.C.; Shokur, S.; Morya, E.; Campos, D.S.F.; Moioli, R.C.; Gitti, C.M. Long-term training with a brain-machine interface-based gait protocol induces partial neurological recovery in paraplegic patients. Sci. Rep. 2016, 6, 30383. [Google Scholar] [CrossRef]
- Samejima, S.; Khorasani, A.; Ranganathan, V.; Nakahara, J.; Tolley, N.M.; Boissenin, A. La interfaz cerebro-computadora-espinal restaura la función de las extremidades superiores después de una lesión de la médula espinal. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 1233–1242. [Google Scholar] [CrossRef]
- Wan, Z.; Yang, R.; Huang, M.; Zeng, N.; Liu, X. A review on transfer learning in EEG signal analysis. Neurocomputing 2021, 421, 1–14. [Google Scholar] [CrossRef]
- Richardson, E.J.; McKinley, E.C.; Rahman, A.K.M.F.; Klebine, P.; Redden, D.T.; Richards, J.S. Effects of virtual walking on spinal cord injury-related neuropathic pain: A randomized, controlled trial. Rehabil. Psychol. 2019, 64, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Lakhani, A.; Martin, K.; Gray, L.; Mallison, J.; Grimbeek, P.; Hollins, I.; Mackareth, C. What Is the Impact of Engaging with Natural Environments Delivered Via Virtual Reality on the Psycho-emotional Health of People with Spinal Cord Injury Receiving Rehabilitation in Hospital? Findings From a Pilot Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2020, 101, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Austin, P.D.; Craig, A.; Middleton, J.W.; Tran, Y.; Costa, D.S.J.; Wrigley, P.J.; Siddall, P.J. The short-term effects of head-mounted virtual-reality on neuropathic pain intensity in people with spinal cord injury pain: A randomised cross-over pilot study. Spinal Cord 2021, 59, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Tran, Y.; Austin, P.; Lo, C.; Craig, A.; Middleton, J.W.; Wrigley, P.J.; Siddall, P. An Exploratory EEG Analysis on the Effects of Virtual Reality in People with Neuropathic Pain Following Spinal Cord Injury. Sensors 2022, 22, 2629. [Google Scholar] [CrossRef]
- Dimbwadyo-Terrer, I.; Trincado-Alonso, F.; de Los Reyes-Guzmán, A.; Aznar, M.A.; Alcubilla, C.; Pérez-Nombela, S.; Del Ama-Espinosa, A.; Polonio-López, B.; Gil-Agudo, Á. Upper limb rehabilitation after spinal cord injury: A treatment based on a data glove and an immersive virtual reality environment. Disabil. Rehabil. Assist. Technol. 2016, 11, 462–467. [Google Scholar] [CrossRef] [PubMed]
- Duffell, L.D.; Paddison, S.; Alahmary, A.F.; Donaldson, N.; Burridge, J. The effects of FES cycling combined with virtual reality racing biofeedback on voluntary function after incomplete SCI: A pilot study. J. Neuroeng. Rehabil. 2019, 16, 149. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).