Cancer-Related Neuropathic Pain, Chemotherapy-Induced Peripheral Neuropathy and Cognitive Decline in a 5-Year Prospective Study of Patients with Breast Cancer—NEON-BC
Abstract
:1. Introduction
2. Materials and Methods
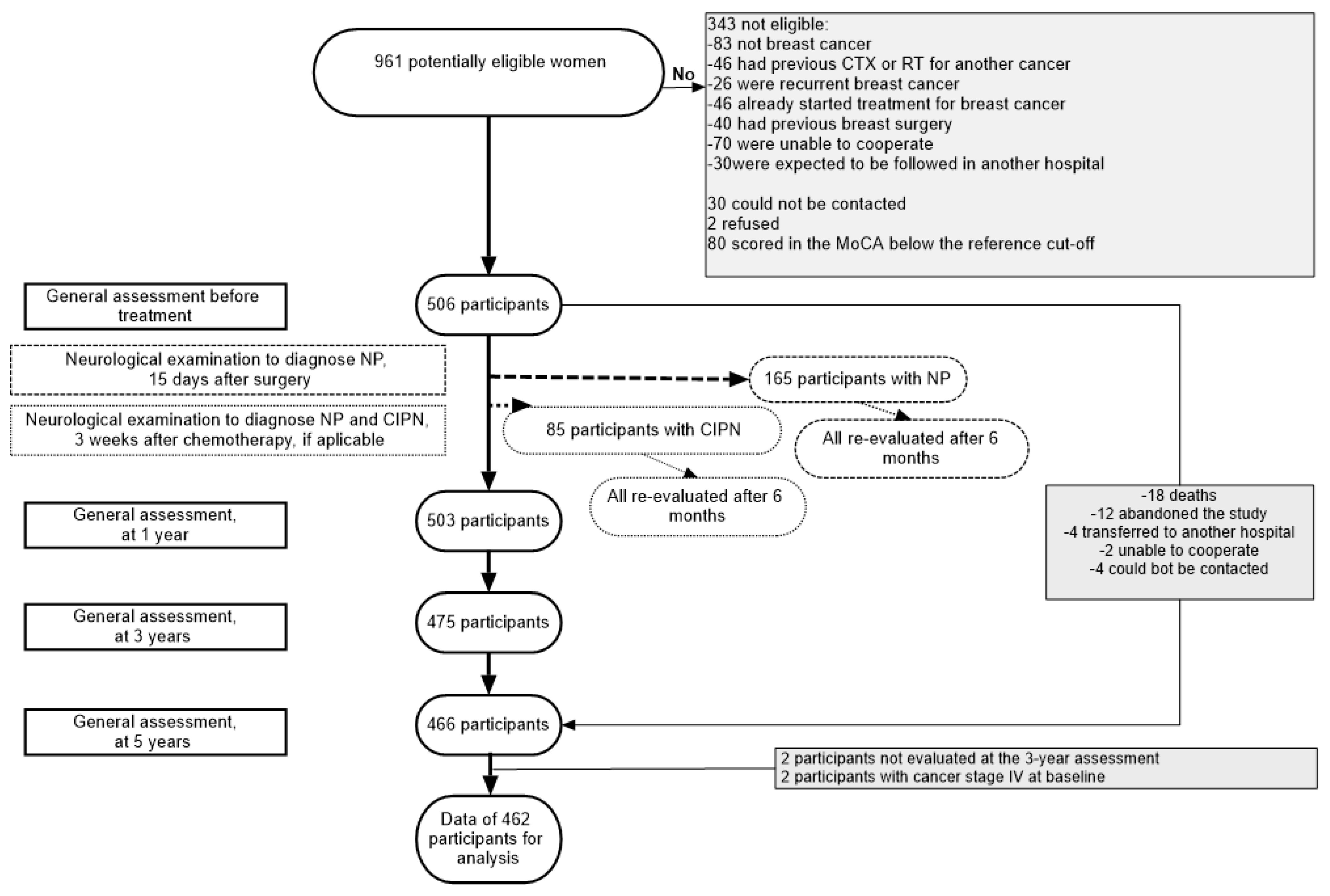
2.1. Patients and Setting
2.2. Data Collection
2.3. Assessment of Neurological Complications
2.4. Statistical Analysis
2.5. Ethics
3. Results
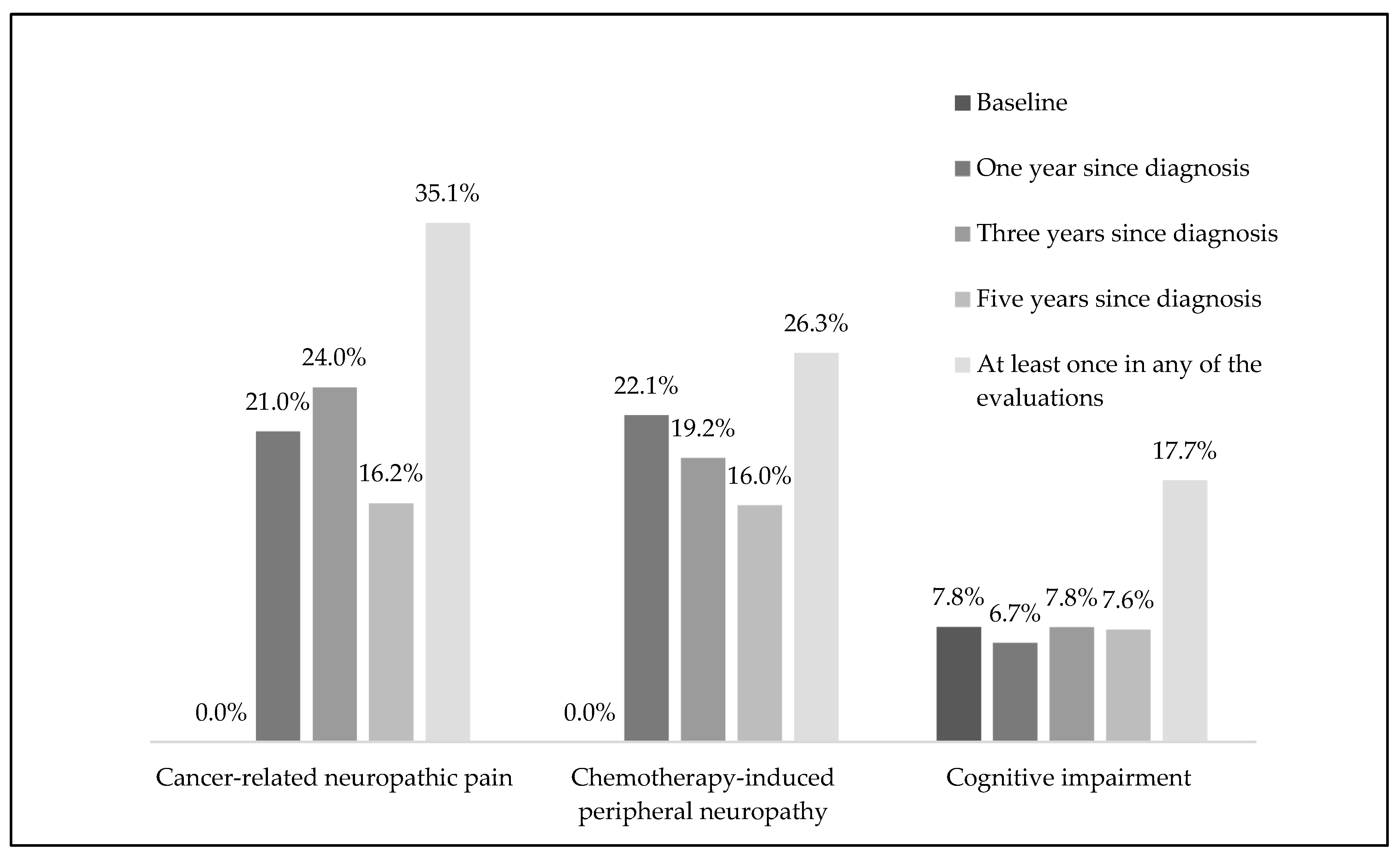
3.1. Neuropathic Pain
3.2. Chemotherapy-Induced Peripheral Neuropathy
3.3. Cognitive Performance Assessed Using MoCA
3.4. Factors Associated with CRNP, CIPN and Variation in Cognitive Performance
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Bonaventure, A.; Valkov, M.; Johnson, C.J.; Estève, J. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef] [PubMed]
- Hamer, J.; McDonald, R.; Zhang, L.; Verma, S.; Leahey, A.; Ecclestone, C.; Bedard, G.; Pulenzas, N.; Bhatia, A.; Chow, R.; et al. Quality of life (QOL) and symptom burden (SB) in patients with breast cancer. Support. Care Cancer 2017, 25, 409–419. [Google Scholar] [CrossRef] [PubMed]
- Eckhoff, L.; Knoop, A.; Jensen, M.; Ewertz, M. Persistence of docetaxel-induced neuropathy and impact on quality of life among breast cancer survivors. Eur. J. Cancer 2015, 51, 292–300. [Google Scholar] [CrossRef]
- Speck, R.M.; Sammel, M.D.; Farrar, J.T.; Hennessy, S.; Mao, J.J.; Stineman, M.G.; DeMichele, A. Impact of chemotherapy-induced peripheral neuropathy on treatment delivery in nonmetastatic breast cancer. J. Oncol. Pract. 2013, 9, e234–e240. [Google Scholar] [CrossRef]
- Ilhan, E.; Chee, E.; Hush, J.; Moloney, N. The prevalence of neuropathic pain is high after treatment for breast cancer: A systematic review. PAIN 2017, 158, 2082–2091. [Google Scholar] [CrossRef] [PubMed]
- Janelsins, M.C.; Kesler, S.R.; Ahles, T.A.; Morrow, G.R. Prevalence, mechanisms, and management of cancer-related cognitive impairment. Int. Rev. Psychiatry 2014, 26, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Seretny, M.; Currie, G.L.; Sena, E.S.; Ramnarine, S.; Grant, R.; MacLeod, M.R.; Colvin, L.A.; Fallon, M. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: A systematic review and meta-analysis. PAIN® 2014, 155, 2461–2470. [Google Scholar] [CrossRef]
- Wang, L.; Cohen, J.C.; Devasenapathy, N.; Hong, B.Y.; Kheyson, S.; Lu, D.; Oparin, Y.; Kennedy, S.A.; Romerosa, B.; Arora, N.; et al. Prevalence and intensity of persistent post-surgical pain following breast cancer surgery: A systematic review and meta-analysis of observational studies. Br. J. Anaesth. 2020, 125, 346–357. [Google Scholar] [CrossRef]
- Bandos, H.; Melnikow, J.; Rivera, D.R.; Swain, S.M.; Sturtz, K.; Fehrenbacher, L.; Wade, J.L., III.; Brufsky, A.M.; Julian, T.B.; Margolese, R.G.; et al. Long-term Peripheral Neuropathy in Breast Cancer Patients Treated With Adjuvant Chemotherapy: NRG Oncology/NSABP B-30. JNCI J. Natl. Cancer Inst. 2017, 110, 149–156. [Google Scholar] [CrossRef]
- Burgess, J.; Ferdousi, M.; Gosal, D.; Boon, C.; Matsumoto, K.; Marshall, A.; Mak, T.; Marshall, A.; Frank, B.; Malik, R.A.; et al. Chemotherapy-Induced Peripheral Neuropathy: Epidemiology, Pathomechanisms and Treatment. Oncol. Ther. 2021, 9, 385–450. [Google Scholar] [CrossRef]
- Jordan, B.; Margulies, A.; Cardoso, F.; Cavaletti, G.; Haugnes, H.S.; Jahn, P.; Le Rhun, E.; Preusser, M.; Scotté, F.; Taphoorn, M.J.B.; et al. Systemic anticancer therapy-induced peripheral and central neurotoxicity: ESMO–EONS–EANO Clinical Practice Guidelines for diagnosis, prevention, treatment and follow-up. Ann. Oncol. 2020, 31, 1306–1319. [Google Scholar] [CrossRef]
- Rivera, D.R.; Ganz, P.A.; Weyrich, M.S.; Bandos, H.; Melnikow, J. Chemotherapy-Associated Peripheral Neuropathy in Patients With Early-Stage Breast Cancer: A Systematic Review. JNCI J. Natl. Cancer Inst. 2017, 110, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Cheng, H.L.; Lopez, V.; Au, J.S.K.; Chan, A.; Bandla, A.; Leung, K.T.; Li, Y.C.; Wong, K.H.; Suen, L.K.P.; et al. Are we mis-estimating chemotherapy-induced peripheral neuropathy? Analysis of assessment methodologies from a prospective, multinational, longitudinal cohort study of patients receiving neurotoxic chemotherapy. BMC Cancer 2019, 19, 132. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, P.; Lisanti, C.; Garutti, M.; Buriolla, S.; Alberti, M.; Mazzeo, R.; Puglisi, F. Chemotherapy in patients with early breast cancer: Clinical overview and management of long-term side effects. Expert Opin. Drug Saf. 2022, 21, 1341–1355. [Google Scholar] [CrossRef]
- Greenlee, H.; Hershman, D.L.; Shi, Z.; Kwan, M.L.; Ergas, I.J.; Roh, J.M.; Kushi, L.H. BMI, Lifestyle Factors and Taxane-Induced Neuropathy in Breast Cancer Patients: The Pathways Study. JNCI J. Natl. Cancer Inst. 2017, 109, 1–8. [Google Scholar] [CrossRef]
- Winters-Stone, K.M.; Horak, F.; Jacobs, P.G.; Trubowitz, P.; Dieckmann, N.F.; Stoyles, S.; Faithfull, S. Falls, Functioning, and Disability Among Women With Persistent Symptoms of Chemotherapy-Induced Peripheral Neuropathy. J. Clin. Oncol. 2017, 35, 2604–2612. [Google Scholar] [CrossRef]
- Jenkins, V.; Shilling, V.; Deutsch, G.; Bloomfield, D.; Morris, R.; Allan, S.; Bishop, H.; Hodson, N.; Mitra, S.; Sadler, G.; et al. A 3-year prospective study of the effects of adjuvant treatments on cognition in women with early stage breast cancer. Br. J. Cancer 2006, 94, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Ono, M.; Ogilvie, J.M.; Wilson, J.S.; Green, H.J.; Chambers, S.K.; Ownsworth, T.; Shum, D.H.K. A Meta-Analysis of Cognitive Impairment and Decline Associated with Adjuvant Chemotherapy in Women with Breast Cancer. Front. Oncol. 2015, 5, 59. [Google Scholar] [CrossRef] [PubMed]
- Van Dyk, K.; Crespi, C.M.; Bower, J.E.; Castellon, S.A.; Petersen, L.; Ganz, P.A. The cognitive effects of endocrine therapy in survivors of breast cancer: A prospective longitudinal study up to 6 years after treatment. Cancer 2019, 125, 681–689. [Google Scholar] [CrossRef]
- Cerulla, N.; Arcusa, À.; Navarro, J.-B.; de la Osa, N.; Garolera, M.; Enero, C.; Chico, G.; Fernández-Morales, L. Cognitive impairment following chemotherapy for breast cancer: The impact of practice effect on results. J. Clin. Exp. Neuropsychol. 2019, 41, 290–299. [Google Scholar] [CrossRef]
- Cerulla, N.; Arcusa, À.; Navarro, J.-B.; Garolera, M.; Enero, C.; Chico, G.; Fernández-Morales, L. Role of taxanes in chemotherapy-related cognitive impairment: A prospective longitudinal study. Breast Cancer Res. Treat. 2017, 164, 179–187. [Google Scholar] [CrossRef] [PubMed]
- Bender, C.M.; Merriman, J.D.; Gentry, A.L.; Ahrendt, G.M.; Berga, S.L.; Brufsky, A.M.; Casillo, F.E.; Dailey, M.M.; Erickson, K.I.; Kratofil, F.M.; et al. Patterns of change in cognitive function with anastrozole therapy. Cancer 2015, 121, 2627–2636. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Fontes, F.; Sonin, T.; Dias, T.; Fragoso, M.; Castro-Lopes, J.M.; Lunet, N. Neurological complications of breast cancer: A prospective cohort study. Breast 2015, 24, 582–587. [Google Scholar] [CrossRef] [PubMed]
- Fontes, F.; Pereira, S.; Castro-Lopes, J.M.; Lunet, N. A prospective study on the neurological complications of breast cancer and its treatment: Updated analysis three years after cancer diagnosis. Breast 2016, 29, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Pereira, S.; Fontes, F.; Sonin, T.; Dias, T.; Fragoso, M.; Castro-Lopes, J.; Lunet, N. Neurological complications of breast cancer: Study protocol of a prospective cohort study. BMJ Open 2014, 4, e006301. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.; Simões, M.R.; Alves, L.; Santana, I. Montreal Cognitive Assessment (MoCA): Normative study for the Portuguese population. J. Clin. Exp. Neuropsychol. 2011, 33, 989–996. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Freitas, S.; Simões, M.; Martins, C.; Vilar, M.; Santana, I. Adaptation studies of the Montreal Cognitive Assessment (MoCA) to the Portuguese population. Avaliaçã Psicológica 2010, 9, 345–357. [Google Scholar]
- Pais-Ribeiro, J.; Silva, I.; Ferreira, T.; Martins, A.; Meneses, R.; Baltar, M. Validation study of a Portuguese version of the Hospital Anxiety and Depression Scale. Psychol. Health Med. 2007, 12, 225–237. [Google Scholar] [CrossRef]
- Zigmond, A.S.; Snaith, R.P. The hospital anxiety and depression scale. Acta Psychiatr. Scand. 1983, 67, 361–370. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, J.S.; Andrykowski, M.A. Psychometric evaluation of the Pittsburgh sleep quality index. J. Psychosom. Res. 1998, 45, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Fontes, F.; Goncalves, M.; Maia, S.; Pereira, S.; Severo, M.; Lunet, N. Reliability and validity of the Pittsburgh Sleep Quality Index in breast cancer patients. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2017, 25, 3059–3066. [Google Scholar] [CrossRef] [PubMed]
- João, K.A.D.R.; Becker, N.B.; de Neves Jesus, S.; Martins, R.I.S. Validation of the Portuguese version of the Pittsburgh sleep quality index (PSQI-PT). Psychiatry Res. 2017, 247, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Edge, S.B.; Byrd, D.R.; Compton, C.C.; Fritz, A.; Greene, F.; Trotti, A. AJCC Cancer Staging Manual, 7th ed.; Springer: New York, NY, USA, 2010; Volume 649. [Google Scholar]
- Cornblath, D.; Chaudhry, V.; Carter, K.; Lee, D.; Seysedadr, M.; Miernicki, M.; Joh, T. Total neuropathy score: Validation and reliability study. Neurology 1999, 53, 1660. [Google Scholar] [CrossRef]
- Haanpää, M.; Attal, N.; Backonja, M.; Baron, R.; Bennett, M.; Bouhassira, D.; Cruccu, G.; Hansson, P.; Haythornthwaite, J.A.; Iannetti, G.D. NeuPSIG guidelines on neuropathic pain assessment. PAIN® 2011, 152, 14–27. [Google Scholar] [CrossRef] [PubMed]
- Cleeland, C.; Ryan, K. Pain assessment: Global use of the Brief Pain Inventory. Ann. Acad. Med. Singap. 1994, 23, 129–138. [Google Scholar]
- Sheridan, D.; Foo, I.; O’Shea, H.; Gillanders, D.; Williams, L.; Fallon, M.; Colvin, L. Long-term follow-up of pain and emotional characteristics of women after surgery for breast cancer. J. Pain Symptom Manag. 2012, 44, 608–614. [Google Scholar] [CrossRef]
- Steyaert, A.; Forget, P.; Dubois, V.; Lavand’homme, P.; De Kock, M. Does the perioperative analgesic/anesthetic regimen influence the prevalence of long-term chronic pain after mastectomy? J. Clin. Anesth. 2016, 33, 20–25. [Google Scholar] [CrossRef]
- Bruce, J.; Thornton, A.J.; Powell, R.; Johnston, M.; Wells, M.; Heys, S.D.; Thompson, A.M.; Cairns Smith, W.; Chambers, W.A.; Scott, N.W. Psychological, surgical, and sociodemographic predictors of pain outcomes after breast cancer surgery: A population-based cohort study. PAIN® 2014, 155, 232–243. [Google Scholar] [CrossRef]
- Leysen, L.; Beckwée, D.; Nijs, J.; Pas, R.; Bilterys, T.; Vermeir, S.; Adriaenssens, N. Risk factors of pain in breast cancer survivors: A systematic review and meta-analysis. Support. Care Cancer 2017, 25, 3607–3643. [Google Scholar] [CrossRef]
- Peuckmann, V.; Ekholm, O.; Rasmussen, N.K.; Groenvold, M.; Christiansen, P.; Møller, S.; Eriksen, J.; Sjøgren, P. Chronic pain and other sequelae in long-term breast cancer survivors: Nationwide survey in Denmark. Eur. J. Pain 2009, 13, 478–485. [Google Scholar] [CrossRef]
- Ewertz, M.; Qvortrup, C.; Eckhoff, L. Chemotherapy-induced peripheral neuropathy in patients treated with taxanes and platinum derivatives. Acta Oncol. 2015, 54, 587–591. [Google Scholar] [CrossRef]
- Abdel-Rahman, O. Impact of diabetes comorbidity on the efficacy and safety of FOLFOX first-line chemotherapy among patients with metastatic colorectal cancer: A pooled analysis of two phase-III studies. Clin. Transl. Oncol. 2019, 21, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.C.; Zhang, S.; Ou, F.-S.; Venook, A.P.; Niedzwiecki, D.; Lenz, H.-J.; Innocenti, F.; O’Neil, B.H.; Shaw, J.E.; Polite, B.N. Diabetes and Clinical Outcome in Patients With Metastatic Colorectal Cancer: CALGB 80405 (Alliance). JNCI Cancer Spectr. 2020, 4, pkz078. [Google Scholar] [CrossRef]
- Ramanathan, R.K.; Rothenberg, M.; de Gramont, A.; Tournigand, C.; Goldberg, R.; Gupta, S.; Andre, T. Incidence and evolution of oxaliplatin-induced peripheral sensory neuropathy in diabetic patients with colorectal cancer: A pooled analysis of three phase III studies. Ann. Oncol. 2010, 21, 754–758. [Google Scholar] [CrossRef] [PubMed]
- Kenkhuis, M.-F.; van Duijnhoven, F.J.B.; van Roekel, E.H.; Breedveld-Peters, J.J.L.; Breukink, S.O.; Janssen-Heijnen, M.L.; Keulen, E.T.P.; Mols, F.; Weijenberg, M.P.; Bours, M.J.L. Longitudinal associations of fiber, vegetable, and fruit intake with quality of life and fatigue in colorectal cancer survivors up to 24 months posttreatment. Am. J. Clin. Nutr. 2022, 115, 822–832. [Google Scholar] [CrossRef] [PubMed]
- Molassiotis, A.; Cheng, H.L.; Leung, K.T.; Li, Y.C.; Wong, K.H.; Au, J.S.K.; Sundar, R.; Chan, A.; Ng, T.R.D.; Suen, L.K.P.; et al. Risk factors for chemotherapy-induced peripheral neuropathy in patients receiving taxane- and platinum-based chemotherapy. Brain Behav. 2019, 9, e01312. [Google Scholar] [CrossRef] [PubMed]
- Cooley, S.A.; Heaps, J.M.; Bolzenius, J.D.; Salminen, L.E.; Baker, L.M.; Scott, S.E.; Paul, R.H. Longitudinal Change in Performance on the Montreal Cognitive Assessment in Older Adults. Clin. Neuropsychol. 2015, 29, 824–835. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Lenzi, R.; Theriault, R.L.; Davis, R.N.; Meyers, C.A. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma: Results of a prospective, randomized, longitudinal trial. Cancer 2004, 100, 2292–2299. [Google Scholar] [CrossRef] [PubMed]
- Shibayama, O.; Yoshiuchi, K.; Inagaki, M.; Matsuoka, Y.; Yoshikawa, E.; Sugawara, Y.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K. Long-term influence of adjuvant breast radiotherapy on cognitive function in breast cancer patients treated with conservation therapy. Int. J. Clin. Oncol. 2019, 24, 68–77. [Google Scholar] [CrossRef]
- Lee, P.E.; Tierney, M.C.; Wu, W.; Pritchard, K.I.; Rochon, P.A. Endocrine treatment-associated cognitive impairment in breast cancer survivors: Evidence from published studies. Breast Cancer Res. Treat. 2016, 158, 407–420. [Google Scholar] [CrossRef]
- Lange, M.; Hardy-Léger, I.; Licaj, I.; Pistilli, B.; Rigal, O.; Le Fel, J.; Lévy, C.; Capel, A.; Coutant, C.; Meyer, J.; et al. Cognitive Impairment in Patients with Breast Cancer before Surgery: Results from a CANTO Cohort Subgroup. Cancer Epidemiol. Biomark. Prev. 2020, 29, 1759–1766. [Google Scholar] [CrossRef]
- Wefel, J.S.; Lenzi, R.; Theriault, R.; Buzdar, A.U.; Cruickshank, S.; Meyers, C.A. ‘Chemobrain’ in breast carcinoma?: A prologue. Cancer 2004, 101, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Menning, S.; de Ruiter, M.B.; Kieffer, J.M.; Agelink van Rentergem, J.; Veltman, D.J.; Fruijtier, A.; Oldenburg, H.S.A.; Boven, E.; van der Meij, S.; Lustig, V.; et al. Cognitive Impairment in a Subset of Breast Cancer Patients After Systemic Therapy—Results From a Longitudinal Study. J. Pain Symptom Manag. 2016, 52, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Vardy, J.L.; Stouten-Kemperman, M.M.; Pond, G.; Booth, C.M.; Rourke, S.B.; Dhillon, H.M.; Dodd, A.; Crawley, A.; Tannock, I.F. A mechanistic cohort study evaluating cognitive impairment in women treated for breast cancer. Brain Imaging Behav. 2019, 13, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Wefel, J.S.; Vardy, J.; Ahles, T.; Schagen, S.B. International Cognition and Cancer Task Force recommendations to harmonise studies of cognitive function in patients with cancer. Lancet Oncol. 2011, 12, 703–708. [Google Scholar] [CrossRef] [PubMed]
- Hermelink, K.; Voigt, V.; Kaste, J.; Neufeld, F.; Wuerstlein, R.; Bühner, M.; Münzel, K.; Rjosk-Dendorfer, D.; Grandl, S.; Braun, M.; et al. Elucidating Pretreatment Cognitive Impairment in Breast Cancer Patients: The Impact of Cancer-Related Post-Traumatic Stress. JNCI J. Natl. Cancer Inst. 2015, 107, djv099. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Palmer, C.; Stone, P.C. Evaluation of screening instruments for depression and anxiety in breast cancer survivors. Breast Cancer Res. Treat. 2010, 122, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Gulpers, B.J.; Voshaar, R.C.O.; van Boxtel, M.P.; Verhey, F.R.; Köhler, S. Anxiety as a risk factor for cognitive decline: A 12-year follow-up cohort study. Am. J. Geriatr. Psychiatry 2019, 27, 42–52. [Google Scholar] [CrossRef]
- Sachs-Ericsson, N.; Joiner, T.; Plant, E.A.; Blazer, D.G. The influence of depression on cognitive decline in community-dwelling elderly persons. Am. J. Geriatr. Psychiatry 2005, 13, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Spira, A.P.; Chen-Edinboro, L.P.; Wu, M.N.; Yaffe, K. Impact of sleep on the risk of cognitive decline and dementia. Curr. Opin. Psychiatry 2014, 27, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Diotti, D.; Puce, L.; Mori, L.; Trompetto, C.; Saretti, E.; Contenti, C.; Marinelli, L. The role of physical activity against chemotherapy-induced peripheral neuropathy: A narrative review. Explor. Neuroprot. Ther. 2022, 2, 87–99. [Google Scholar] [CrossRef]
| Participants’ and Cancer Characteristics and Treatments | n | % | |
|---|---|---|---|
| Socio-demographics | |||
| Age | |||
| ≥55 years | 230 | 49.8 | |
| Education (years) | |||
| Primary education (≤4) | 194 | 42.0 | |
| Lower secondary education (5–9) | 133 | 28.8 | |
| At least upper secondary education (≥10) | 135 | 29.2 | |
| Living in Greater Porto Area | 207 | 44.8 | |
| Marital status | |||
| Married/living together | 323 | 69.9 | |
| Single | 49 | 10.6 | |
| Widow/divorced | 90 | 19.5 | |
| Professionally active (n = 460) | |||
| Yes | 242 | 52.6 | |
| Monthly income (n = 454) | |||
| Above EUR 500 a | 204 | 44.9 | |
| Lifestyles | |||
| Alcohol consumption > 10 g/day (n = 461) | 92 | 20.0 | |
| Past or current smoker | 96 | 20.8 | |
| Fruits and vegetables ≥ 5/day (n = 459) | 101 | 22.0 | |
| Playing a sport | 80 | 17.3 | |
| Comorbidities | |||
| Hypertension | 146 | 31.6 | |
| Diabetes | 46 | 10.0 | |
| Body Mass Index (kg/m2) | |||
| <18.5 | 5 | 1.1 | |
| 18.5–24.5 | 198 | 43.0 | |
| 25.0–29.9 | 155 | 33.6 | |
| ≥30 | 103 | 22.3 | |
| Chronic medicines consumption | |||
| None | 165 | 35.7 | |
| One | 77 | 16.7 | |
| Two to four | 149 | 32.3 | |
| More than four | 71 | 15.4 | |
| Cancer stage | |||
| 0 | 31 | 6.71 | |
| I | 223 | 48.27 | |
| II | 140 | 30.3 | |
| III | 68 | 14.7 | |
| Breast cancer subtype (n = 433) | |||
| HR+/HER2- | 333 | 76.9 | |
| HER2+ | 64 | 14.8 | |
| Triple negative | 36 | 8.3 | |
| Treatments in the first year | |||
| Breast surgery | |||
| Breast conserving | 235 | 50.9 | |
| Mastectomy | 227 | 49.1 | |
| Axillary surgery | |||
| Sentinel lymph node biopsy | 295 | 65.9 | |
| Axillary lymph node dissection | 153 | 34.1 | |
| Chemotherapy (n = 279) | |||
| Neoadjuvant | 30 | 10.8 | |
| Adjuvant | 249 | 89.2 | |
| Taxane-based | 198 | 71.0 | |
| Radiotherapy | |||
| Yes | 341 | 73.8 | |
| Endocrine therapy | |||
| Yes | 388 | 84.0 | |
| Targeted therapy | |||
| Yes | 61 | 13.2 | |
| Reference Group Patients Who Never Had CRNP (N = 301) | Patients with CRNP at Least Once (N = 163) | Those with CRNP at Least Once vs. Reference Group | Patients with CRNP at Five-Years (N = 75) | Those with CRNP at Five-Years vs. Reference Group | Patients with CRNP in All Evaluations (N = 36) | Those with CRNP in All Evaluations vs. Reference Group | ||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Adjusted OR [95% CI] | n (%) | Adjusted OR [95% CI] | n (%) | Adjusted OR [95% CI] | ||
| Age (years) | ||||||||
| <55 | 138 (59.5) | 94 (40.5) | ref. | 44 (19.0) | ref. | 18 (7.8) | ref. | |
| ≥55 | 162 (70.4) | 68 (29.6) | 0.62 * [0.42, 0.91] | 31 (13.5) | 0.60 [0.36, 1.00] | 18 (7.8) | 0.85 [0.43, 1.70] | |
| Education (years) | ||||||||
| ≤4 | 122 (62.9) | 72 (37.1) | ref. | 33 (17.0) | ref. | 19 (9.8) | ref. | |
| 5–9 | 87 (65.4) | 46 (34.6) | 0.66 [0.40, 1.10] e | 24 (18.0) | 0.77 [0.40, 1.46] e | 9 (6.8) | 0.56 [0.23, 1.37] e | |
| ≥10 | 91 (67.4) | 44 (32.6) | 0.59 * [0.35, 0.99] e | 18 (13.3) | 0.54 [0.27, 1.08] e | 8 (5.9) | 0.47 [0.19, 1.19] e | |
| Marital status | ||||||||
| Married/living together | 200 (66.7) | 123 (75.9) | ref. | 52 (69.3) | ref. | 25 (69.4) | ref. | |
| Single | 39 (13.0) | 10 (6.2) | 0.45 * [0.22, 0.94] f | 8 (10.7) | 0.87 [0.38, 2.00] f | 4 (11.1) | 0.90 [0.29, 2.77] f | |
| Widower/divorced | 61 (20.3) | 29 (17.9) | 0.84 [0.51, 1.40] f | 15 (20.0) | 1.05 [0.54, 1.78] f | 7 (19.4) | 0.92 [0.37, 2.29] f | |
| Cancer stage | ||||||||
| 0/I | 179 (70.5) | 75 (29.5) | ref. | 32 (12.6) | ref. | 16 (6.3) | ref. | |
| II | 91 (65.0) | 49 (35.0) | 1.29 [0.82, 2.02] f | 26 (18.6) | 1.56 [0.87, 2.81] f | 10 (7.1) | 1.29 [0.55, 2.99] f | |
| III | 30 (44.1) | 38 (55.9) | 2.95 *** [1.68, 5.18] f | 17 (25.0) | 3.04 ** [1.48, 6.20] f | 10 (14.7) | 3.82 ** [1.56, 9.33] f | |
| Breast cancer subtypes a | ||||||||
| HR+/HER2 | 219 (65.8) | 114 (34.2) | ref. | 51 (15.3) | ref. | 22 (6.6) | ref. | |
| HER2+ | 45 (70.3) | 19 (29.7) | 0.82 [0.45, 1.47] f | 7 (10.9) | 0.68 [0.29, 1.61] f | 4 (6.3) | 0.95 [0.31, 2.93] f | |
| Triple negative | 17 (47.2) | 19 (52.8) | 2.02 * [1.00, 4.07] f | 11 (30.6) | 2.60 * [1.14, 5.95] f | 7 (19.4) | 4.04 ** [1.49, 10.97] f | |
| Breast surgery | ||||||||
| Breast-conserving | 161 (68.5) | 74 (31.5) | ref. | 34 (14.5) | ref. | 17 (7.2) | ref. | |
| Mastectomy | 139 (61.2) | 88 (38.8) | 1.19 [0.77, 1.83] g | 41 (18.1) | 1.13 [0.63, 2.02] g | 19 (8.4) | 0.73 [0.32, 1.67] g | |
| Axillary surgery b | ||||||||
| SLNB | 212 (71.9) | 83 (28.1) | ref. | 39 (13.2) | ref. | 18 (6.1) | ref. | |
| ALND | 79 (51.6) | 74 (48.4) | 2.11 * [1.13, 3.93] g | 34 (22.2) | 1.80 [0.81, 4.03] g | 18 (11.8) | 2.67 [0.80, 8.93] g | |
| Chemotherapy | ||||||||
| No | 135 (74.6) | 46 (25.4) | ref. | 19 (10.5) | ref. | 9 (5.0) | ref. | |
| Yes | 165 (58.7) | 116 (41.3) | 2.05 * [1.19, 3.53] g | 56 (19.9) | 2.69 * [1.24, 5.83] g | 27 (9.6) | 3.40 * [1.16, 9.93] g | |
| Radiotherapy | ||||||||
| No | 84 (70.6) | 35 (29.4) | ref. | 19 (16.0) | ref. | 8 (6.7) | ref. | |
| Yes | 216 (63.0) | 127 (37.0) | 1.16 [0.55, 2.42] h | 56 (16.3) | 0.67 [0.26, 1.77] h | 28 (8.2) | 0.61 [0.15, 2.58] h | |
| Anxiety c | ||||||||
| No | 208 (73.5) | 75 (26.5) | ref. | 28 (9.9) | ref. | 11 (3.9) | ref. | |
| Yes | 91 (51.1) | 87 (48.9) | 2.72 *** [1.80, 4.12] i | 47 (26.4) | 3.95 *** [2.26, 6.90] i | 25 (14.0) | 6.02 *** [2.66, 13.6] i | |
| Depression c | ||||||||
| No | 287 (67.7) | 137 (32.3) | ref. | 59 (13.9) | ref. | 27 (6.4) | ref. | |
| Yes | 13 (34.2) | 25 (65.8) | 3.91 *** [1.90, 8.02] i | 16 (42.1) | 6.13 *** [2.67, 14.12] i | 9 (23.7) | 10.95 *** [3.79, 31.65] i | |
| Poor sleep quality d | ||||||||
| No | 112 (75.7) | 36 (24.3) | ref. | 11 (7.4) | ref. | 5 (3.4) | ref. | |
| Yes | 187 (59.7) | 126 (40.3) | 2.24 ** [1.42, 3.54] i | 64 (20.4) | 4.13 *** [2.03, 8.39] i | 31 (9.9) | 4.19 ** [1.54, 11.44] i | |
| Reference Group Patients Who Never Had CIPN (N = 207) | Patients with CIPN at Least Once (N = 74) | Those with CIPN at Least Once vs. Reference Group | Patients with CIPN at Five-Years (N = 45) | Those with CIPN at Five-Years vs. Reference Group | Patients with CIPN at All Evaluations (N = 33) | Those with CIPN at All Evaluations vs. Reference Group | ||
|---|---|---|---|---|---|---|---|---|
| n (%) | n (%) | Adjusted OR [95% CI] | n (%) | Adjusted OR [95% CI] | n (%) | Adjusted OR [95% CI] | ||
| Age (years) | ||||||||
| ≤55 | 123 (74.5) | 42 (25.5) | ref. | 22 (13.3) | ref. | 15 (9.1) | ref. | |
| >55 | 84 (72.4) | 32 (27.6) | 1.12 [0.65, 1.91] | 23 (19.8) | 1.53 [0.80, 2.92] | 18 (15.5) | 1.76 [0.84, 3.68] | |
| Education (years) | ||||||||
| ≤4 | 72 (73.5) | 26 (26.5) | ref. | 14 (14.3) | ref. | 10 (10.2) | ref. | |
| 5–9 | 77 (77.0) | 23 (23.0) | 0.87 [0.44, 1.73] c | 15 (15.0) | 1.26 [0.54, 2.96] c | 11 (11.0) | 1.39 [0.52, 3.67] c | |
| ≥10 | 58 (69.9) | 25 (30.1) | 1.25 [0.63, 2.50] c | 16 (19.3) | 1.75 [0.75, 4.08] c | 12 (14.5) | 1.95 [0.75, 5.10] c | |
| Diabetes at baseline | ||||||||
| No | 190 (72.8) | 71 (27.2) | ref. | 43 (16.5) | ref. | 31 (11.9) | ref. | |
| Yes | 17 (85.0) | 3 (15.0) | 0.41 [0.11, 1.49] d | 2 (10.0) | 0.43 [0.09, 2.03] d | 2 (10.0) | 0.59 [0.12, 2.83] d | |
| Alcohol consumption at baseline | ||||||||
| <10 g/day | 166 (73.5) | 60 (26.5) | ref. | 35 (15.5) | ref. | 24 (10.6) | ref. | |
| ≥10 g/day | 41 (74.5) | 14 (25.5) | 0.99 [0.49, 1.97] d | 10 (18.2) | 1.26 [0.56, 2.84] d | 9 (16.4) | 1.71 [0.71, 4.12] d | |
| Daily consumption of fruits and vegetables | ||||||||
| Less than 5 portions | 155 (74.9) | 61 (82.4) | ref. | 38 (84.4) | ref. | 30 (90.9) | ref. | |
| At least 5 portions | 52 (25.1) | 13 (17.6) | 0.61 [0.31, 1.20] d | 7 (15.6) | 0.48 [0.20, 1.17] d | 3 (9.1) | 0.25 * [0.07, 0.87] d | |
| Cancer stage | ||||||||
| 0/I | 76 (80.9) | 18 (19.1) | ref. | 9 (9.6) | ref. | 4 (4.3) | ref. | |
| II | 95 (77.9) | 27 (22.1) | 1.24 [0.63, 2.44] d | 17 (13.9) | 1.63 [0.68, 3.92] d | 15 (12.3) | 3.32 * [1.04, 10.61] d | |
| III | 36 (55.4) | 29 (44.6) | 3.63 *** [1.76, 7.47] d | 19 (29.2) | 5.07 *** [2.04, 12.63] d | 14 (21.5) | 8.75 *** [2.60, 29.41] d | |
| Breast cancer subtypes | ||||||||
| HR+/HER2 | 145 (77.5) | 42 (22.5) | ref. | 25 (13.4) | ref. | 18 (9.6) | ref. | |
| HER2+ | 41 (65.1) | 22 (34.9) | 1.84 [0.99, 3.44] d | 12 (19.0) | 1.63 [0.75, 3.56] d | 11 (17.5) | 2.10 [0.91, 4.86] d | |
| Triple negative | 21 (67.7) | 10 (32.3) | 1.72 [0.75, 3.96] d | 8 (25.8) | 2.46 [0.97, 6.27] d | 4 (12.9) | 1.70 [0.52, 5.61] d | |
| Taxanes-based chemotherapy | ||||||||
| No taxanes | 74 (96.1) | 3 (3.9) | ref. | 2 (2.6) | ref. | 1 (1.3) | ref. | |
| Taxanes | 133 (65.2) | 71 (34.8) | 12.69 *** [3.45, 46.74] e | 43 (21.1) | 8.79 ** [1.80, 42.97] e | 32 (15.7) | 8.77 * [1.04, 73.60] e | |
| 5-FU-based chemotherapy | ||||||||
| No 5-FU | 71 (78.0) | 20 (22.0) | ref. | 14 (15.4) | ref. | 8 (8.8) | ref. | |
| 5-FU | 136 (71.6) | 54 (28.4) | 1.45 [0.75, 2.80] e | 31 (16.3) | 1.05 [0.48, 2.30] e | 25 (13.2) | 1.31 [0.51, 3.38] e | |
| Anxiety a | ||||||||
| No | 128 (74.9) | 43 (25.1) | ref. | 31 (18.1) | ref. | 22 (12.9) | ref. | |
| Yes | 78 (71.6) | 31 (28.4) | 1.28 [0.73, 2.25] f | 14 (12.8) | 0.79 [0.38, 1.62] f | 11 (10.1) | 0.84 [0.37, 1.89] f | |
| Depression a | ||||||||
| No | 193 (74.2) | 67 (25.8) | ref. | 40 (15.4) | ref. | 30 (11.5) | ref. | |
| Yes | 14 (66.7) | 7 (33.3) | 1.27 [0.47, 3.42] f | 5 (23.8) | 1.40 [0.44, 4.39] f | 3 (14.3) | 0.90 [0.22, 3.63] f | |
| Poor quality of sleep b | ||||||||
| No | 78 (80.4) | 19 (19.6) | ref. | 15 (15.5) | ref. | 11 (11.3) | ref. | |
| Yes | 129 (70.1) | 55 (29.9) | 1.72 [0.94, 3.17] f | 30 (16.3) | 1.16 [0.57, 2.34] f | 22 (12.0) | 1.09 [0.48, 2.45] f | |
| MoCA Value after One Year Minus Baseline Value | MoCA Value after Three Years Minus Baseline Value | MoCA Value after Five Years Minus Baseline Value | |||||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Adjusted β Coefficient [95% CI] | Mean (SD) | Adjusted ꞵ Coefficient [95% CI] | Mean (SD) | Adjusted ꞵ Coefficient [95% CI] | ||
| All participants | 0.6 (2.4) | 0.1 (2.8) | 0.3 (2.9) | ||||
| MoCA score at baseline | −0.20 *** [−0.26, −0.14] | −0.36 *** [−0.46, −0.26] | −0.38 *** [−0.48, −0.28] | ||||
| Age (years) a | |||||||
| <50 | 0.5 (2.2) | ref. | 0.2 (2.7) | ref. | 0.5 (2.3) | ref. | |
| 50–64 | 0.6 (2.5) | −0.29 [−0.80, 0.23] e | 0.0 (2.8) | −0.17 [−0.79, 0.44] e | 0.4 (2.9) | −0.15 [−0.78, 0.47] e | |
| ≥65 | 0.6 (2.6) | −0.74 * [−1.42, −0.06] e | −0.0 (3.1) | −0.19 [−0.96, 0.57] e | −0.3 (3.5) | −0.87 * [−1.65, −0.09] e | |
| Education (years) a | |||||||
| ≤4 | 0.7 (2.6) | ref. | −0.2 (3.3) | ref. | 0.1 (3.4) | ref. | |
| 5–9 | 0.5 (2.3) | 0.91 ** [0.28, 1.53] f | 0.2 (2.5) | 1.65 *** [0.91, 2.39] f | 0.3 (2.7) | 1.25 ** [0.50, 2.01] f | |
| 10–12 | 0.4 (2.4) | 1.33 *** [0.56, 2.10] f | 0.1 (2.5) | 2.06 *** [1.15, 2.97] f | 0.4 (2.2) | 1.94 *** [1.02, 2.87] f | |
| >12 | 0.4 (1.9) | 1.73 *** [0.85, 2.61] f | 0.6 (2.1) | 2.87 *** [1.82, 3.91] f | 0.5 (2.1) | 2.38 *** [1.32, 3.44] f | |
| Cancer-stage | |||||||
| 0/I | 0.6 (2.6) | ref. | 0.1 (2.9) | ref. | 0.1 (3.0) | ref. | |
| II | 0.5 (2.2) | −0.02 [−0.52, 0.49] g | 0.0 (2.5) | −0.21 [−0.80, 0.38] g | 0.5 (2.7) | 0.28 [−0.32, 0.88] g | |
| III | 0.6 (2.4) | 0.08 [−0.58, 0.73] g | −0.2 (3.2) | −0.44 [−1.21, 0.33] g | 0.5 (2.9) | 0.29 [−0.49, 1.08] g | |
| Subtypes b | |||||||
| ER+/HER2 | 0.5 (2.5) | ref. | 0.0 (2.9) | ref. | 0.2 (3.0) | ref. | |
| HER2+ | 0.5 (2.1) | 0.03 [−0.62, 0.68] g | 0.0 (2.6) | −0.02 [−0.79, 0.75] g | 0.6 (2.4) | 0.44 [−0.34, 1.22] g | |
| Triple negative | 0.2 (2.3) | −0.39 [−1.23, 0.45] g | −0.5 (2.9) | −0.60 [−1.59, 0.39] g | 0.0 (2.6) | −0.36 [−1.37, 0.65] g | |
| Chemotherapy | |||||||
| No | 0.8 (2.6) | ref. | 0.2 (2.9) | ref. | 0.2 (3.1) | ref. | |
| Yes | 0.4 (2.3) | −0.32 [−0.78, 0.15] g | −0.0 (2.8) | −0.32 [−0.87, 0.23] g | 0.3 (2.8) | 0.02 [−0.54, 0.58] g | |
| Radiotherapy | |||||||
| No | 0.9 (2.3) | ref. | 0.4 (2.7) | ref. | 0.4 (3.1) | ref. | |
| Yes | 0.4 (2.5) | −0.19 [−0.69, 0.31] g | −0.1 (2.9) | −0.28 [−0.87, 0.31] g | 0.3 (2.8) | 0.01 [−0.59, 0.62] g | |
| Hormone therapy | |||||||
| No | 0.6 (2.0) | ref. | 0.2 (2.7) | ref. | 0.3 (2.6) | ref. | |
| Yes | 0.5 (2.5) | 0.09 [−0.51, 0.68] g | 0.0 (2.9) | −0.02 [−0.73, 0.69] g | 0.3 (3.0) | 0.17 [−0.55, 0.89] g | |
| Anxiety c | |||||||
| No | 1.0 (2.4) | ref. | 0.5 (2.8) | ref. | 0.7 (2.9) | ref. | |
| Yes | 0.3 (2.5) | −0.63 ** [−1.07, −0.19] g | −0.1 (2.9) | −0.48 [−1.01, 0.05] g | 0.0 (2.9) | −0.71 ** [−1.25, −0.18] g | |
| Depression c | |||||||
| No | 0.7 (2.5) | ref. | 0.3 (2.8) | ref. | 0.5 (2.9) | ref. | |
| Yes | 0.5 (2.5) | −0.47 [−1.28, 0.34] g | −0.4 (3.5) | −1.13 * [−2.08, −0.18] g | −0.5 (3.2) | −1.60 ** [−2.55, −0.64] g | |
| Poor quality of sleep d | |||||||
| No | 1.0 (2.2) | ref. | 0.6 (2.8) | ref. | 1.0 (2.6) | ref. | |
| Yes | 0.6 (2.6) | −0.23 [−0.70, 0.23] g | 0.1 (2.9) | −0.49 [−1.04, 0.06] g | 0.2 (3.1) | −0.68 * [−1.23, −0.12] g | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, S.; Araújo, N.; Fontes, F.; Lopes-Conceição, L.; Dias, T.; Ferreira, A.; Morais, S.; Cruz, V.T.; Lunet, N. Cancer-Related Neuropathic Pain, Chemotherapy-Induced Peripheral Neuropathy and Cognitive Decline in a 5-Year Prospective Study of Patients with Breast Cancer—NEON-BC. Healthcare 2023, 11, 3132. https://doi.org/10.3390/healthcare11243132
Pereira S, Araújo N, Fontes F, Lopes-Conceição L, Dias T, Ferreira A, Morais S, Cruz VT, Lunet N. Cancer-Related Neuropathic Pain, Chemotherapy-Induced Peripheral Neuropathy and Cognitive Decline in a 5-Year Prospective Study of Patients with Breast Cancer—NEON-BC. Healthcare. 2023; 11(24):3132. https://doi.org/10.3390/healthcare11243132
Chicago/Turabian StylePereira, Susana, Natália Araújo, Filipa Fontes, Luisa Lopes-Conceição, Teresa Dias, Augusto Ferreira, Samantha Morais, Vítor Tedim Cruz, and Nuno Lunet. 2023. "Cancer-Related Neuropathic Pain, Chemotherapy-Induced Peripheral Neuropathy and Cognitive Decline in a 5-Year Prospective Study of Patients with Breast Cancer—NEON-BC" Healthcare 11, no. 24: 3132. https://doi.org/10.3390/healthcare11243132
APA StylePereira, S., Araújo, N., Fontes, F., Lopes-Conceição, L., Dias, T., Ferreira, A., Morais, S., Cruz, V. T., & Lunet, N. (2023). Cancer-Related Neuropathic Pain, Chemotherapy-Induced Peripheral Neuropathy and Cognitive Decline in a 5-Year Prospective Study of Patients with Breast Cancer—NEON-BC. Healthcare, 11(24), 3132. https://doi.org/10.3390/healthcare11243132









